Abstract
BACKGROUND: Advanced stages of Duchenne muscular dystrophy (DMD) result in severe lung volume decline and are associated with high respiratory morbidity and mortality. The aim of this study was to investigate whether lung volume decline in subjects with DMD is associated with ventilation inhomogeneity measured with the multiple-breath washout technique.
METHODS: This cross-sectional study of lung function included 45 subjects with DMD and 16 healthy controls using multiple-breath washout, spirometry, and cough peak flow.
RESULTS: Subjects with DMD exhibited an elevated lung clearance index (> 7.0) defined as the cumulative exhaled volume divided by the functional residual capacity to lower the sulfur hexafluoride concentration below 2.5% compared with controls (8.16 ± 2.55 vs 6.23 ± 0.46, P < .001). Lung clearance index elevation was negatively correlated with vital capacity (% predicted: r = −0.79, P < .001) and cough peak flow (L/min: r = −0.41, P = .005). Furthermore, dead-space ventilation (dead-space-to-tidal-volume ratio) and functional residual capacity showed a positive correlation with lung clearance index elevation (r = 0.81 and 0.48, P < .001). An FVC of < 24% predicted lung clearance index elevation with a sensitivity of 96% and a specificity of 80%.
CONCLUSIONS: Moderate-to-severe lung volume decline in subjects with DMD is associated with ventilation inhomogeneity. Lung clearance index elevation may be the result of altered ventilation geometry or retention of airway secretions in the infection-free DMD subject.
Introduction
Respiratory involvement contributes significantly to morbidity and mortality in patients with neuromuscular disorders. Duchenne muscular dystrophy (DMD) is the most common neuromuscular disorder, with an incidence of 3.5 cases/10,000 people and a characteristic course of progressive skeletal and respiratory muscle weakness. The progression of respiratory muscle weakness in DMD is measured by a decline in peak inspiratory pressure, resulting in restricted FVC.1 The degree of lung volume decline is monitored annually by spirometry, as it is the strongest predictor of mortality for DMD patients.2,3 Progressive loss of lung volume and weakness of expiratory muscles result in declining peak expiratory pressure and cough peak flow (CPF), leading to impaired clearance of airway secretions, recurrent respiratory tract infection, and atelectasis.4 Lung volume decline manifests as progressive hypoventilation,5 that is treated effectively with noninvasive ventilation. Morbidity and mortality2,6 can be substantially reduced by a structured proactive approach including the use of noninvasive ventilation and assisted cough. The current concept for the respiratory morbidity in neuromuscular disorders assumes that DMD does not involve primary lung disease, but is due mainly to lung volume decline. However, chronic aspiration or recurrent respiratory tract infection7 might cause secondary lung disease.
The peripheral airways have been termed the silent lung zone due to the insensitivity of conventional lung function tests to detect their involvement in disease process.8 Information about peripheral airway function can be obtained by the multiple-breath washout (MBW) lung function test, recently reintroduced into medical practice. MBW generates lung volume data (functional residual capacity [FRC]) and allows calculation of ventilation inhomogeneity markers, such as the lung clearance index (LCI), which are independent of tidal volume (VT).8 Validated systems employing an ultrasound flow meter, as an alternative to complex and expensive measurement by mass spectrometry, have recently become available.9 LCI elevation has been demonstrated by MBW in a variety of structural lung diseases, including cystic fibrosis,10 asthma,11 infant chronic lung disease,12 and bronchiolitis obliterans.13 LCI is currently thought to detect small airway disease with higher sensitivity and specificity than spirometry. This study aimed to describe MBW in subjects with DMD and lung volume decline.
QUICK LOOK
Current knowledge
Respiratory muscle dysfunction contributes importantly to morbidity and mortality in patients with neuromuscular disorders. Duchenne muscular dystrophy (DMD) is the most common neuromuscular disorder characterized by progressive skeletal and respiratory muscle weakness, and is monitored using FVC and maximum inspiratory pressure. Progressive loss of lung volume and muscle weakness leads to impaired secretion clearance, recurrent infections, and atelectasis. The current concept for the respiratory morbidity in neuromuscular disorders assumes that DMD does not involve lung disease and that respiratory morbidity is secondary to lung volume decline.
What this paper contributes to our knowledge
In a group of children and young adult subjects with DMD, moderate-to-severe lung volume decline was associated with ventilation inhomogeneity. An elevation in the lung clearance index appeared to be the result of altered ventilation geometry or retention of airway secretions in subjects with DMD without active pulmonary infection. The multiple-breath washout technique proved effective in detecting ventilation inhomogeneity.
Methods
Subjects
All subjects with DMD were diagnosed by molecular genetic testing or muscle biopsy and recruited from the Departments of Pediatric Pulmonology and Pediatric Neurology of University Hospital Essen in Germany. Subjects did not receive medications likely to influence lung function measurements. All subjects were clinically stable without respiratory tract infections for 4 weeks before assessment of lung function. Healthy children and young adults without a history of asthma or significant respiratory tract disease served as the control group. All lung function measurements were done in the morning. The ethics committee of the University Hospital Essen approved this study, and parents and/or subjects provided informed written consent before their involvement.
Spirometry and CPF
All measurements were obtained while the subject was seated and wearing a nose clip. FVC and FEV1 were measured with a handheld spirometer (ZAN 100, ZAN Meßgeräte, Obertulba, Germany). The highest values from 3 recordings that met American Thoracic Society criteria for FVC and FEV1 were used,14 and predicted values were calculated from published data.15 CPF was measured when the subject was seated, wearing a nose clip, and performing a maximum cough after inspiration from FRC into a pocket flow meter (Pocket Peak, Ferraris Medical, Enfield, United Kingdom).
MBW Lung Function
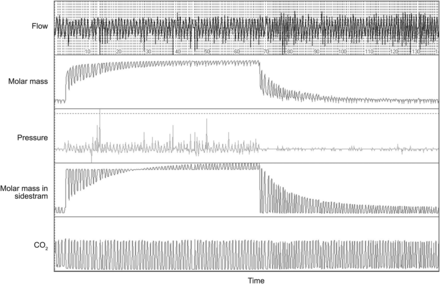
All measurements were obtained while the subject was seated, wearing a nose clip during tidal breathing through a mouthpiece, and watching a video. Measurements were taken using a sidestream ultrasonic flow sensor system as described previously9 and according to American Thoracic Society criteria.16 In brief, the system consisted of a Spiroson ultrasonic flow sensor (ndd Medical Technologies, Zurich, Switzerland) that detected the transit times of moving air, from which the molar mass was determined. The tracer gas was delivered from a premixed gas cylinder (Linde, Munich, Germany) and contained 4% sulfur hexafluoride, 21% oxygen, and 75% nitrogen. Molar mass was measured in the sidestream sensor, where exhaled air passed through a Nafion tube and equilibrated to ambient temperature and pressure-saturated conditions. An infrared CO2 analyzer (Duet end-tidal CO2 module, Welch Allyn, Beaverton, Oregon) allowed breath-by-breath correction of the molar mass for exhaled CO2. WBreath 3.22.0.0 (ndd Medical Technologies) was used for data acquisition, storage, and analysis. WBreath software calculates dead space (VD) according to Fowler's method using the sulfur hexafluoride signal. The LCI was calculated as the cumulative exhaled volume divided by the FRC that was needed to lower the sulfur hexafluoride concentration to < 2.5%. Normative data for LCI in healthy controls using the same equipment have been published previously.17 At least 2 measurements with an inter-test variation of < 10% were recorded, and an example of online monitoring is shown in Figure 1.
Representative trace from multiple-breath washout lung function in a subject with Duchenne muscular dystrophy and severe lung volume decline. This subject had an FVC of 0.81 L, equivalent to a predicted FVC of 17%, which resulted in a significant elevation of the lung clearance index (9.9).
Analysis
Analysis was performed using SPSS 17 (SPSS, Chicago, Illinois). Data are presented as mean ± SD. LCI scores are independent of height, age, and sex of healthy controls.18 The normal upper limit for LCI in children is reported to be 7.0.19 The Kolmogorov-Smirnov test was used to test for normal data distribution. Comparisons between groups were performed using the unpaired t test, Mann-Whitney test, or chi-square test. Correlations of LCI were calculated using Spearman's correlation, as LCI was not normally distributed, and the correlation coefficient (r) is presented. All tests were considered statistically significant at P < .05. Multiple-regression analysis was performed to identify the major determinant of LCI elevation, with LCI as the dependent variable and age, FVC, CPF, and dead-space ventilation (VD/VT) as independent variables. Receiver operating characteristic curves were constructed for FVC, and cutoff points separating subjects with an LCI above or below 7.0 were calculated using bidimensional analysis, setting the sensitivity and specificity at equal values (1:1 ratio).
Results
Elevated LCI in Subjects With DMD
The study enrolled a total of 51 children and young adults with DMD between November 2009 and October 2010. Six subjects were excluded because they were unable to perform MBW lung function testing: 4 were excluded due to irregular breathing patterns, and 2 were excluded due to insufficient mouth closure, resulting in leakage. All 16 healthy control subjects were able to adequately perform lung function tests. Characteristics of the study and control groups are reported in Table 1. Table 2 summarizes the results from lung function tests. As expected, static and dynamic lung volumes, VT, FVC, and FEV1 were significantly lower in subjects with DMD compared with healthy controls. Our data show that the LCI indicative of ventilation inhomogeneity was significantly higher in subjects with DMD versus healthy controls.
Anthropometric Characteristics of Subject With DMD and Healthy Controls
Pulmonary Function Variables for Subjects With DMD and Healthy Controls
LCI Elevation and Lung Volume Decline in Subjects With DMD
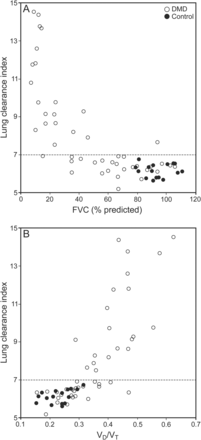
The distribution of the LCI in the DMD group ranged from normal (< 7.0) to highly pathological, with a maximum of 14.5 without normal distribution. We applied Spearman's correlation test to investigate the connections between LCI and subject characteristics, lung volumes, and CPF (Table 3). In healthy controls, LCI showed a positive correlation with higher lung volumes (FVC, FEV1, and VD) and age (r = 0.51, 0.49, and 0.61, respectively; P < .001). Most of the lung function parameters correlated with LCI in the DMD group. FVC and percent-of-predicted FEV1 showed a strong negative correlation (r = −0.79 and −0.80, P < .001) (Fig. 2A and Table 3). VD/VT and FRC showed a strong positive correlation (r = 0.81 and 0.48, respectively; P < .001) (Fig. 2B and Table 3). After we adjusted FRC by VT (VT/FRC), the correlation to LCI significantly increased (r = −0.66, P < .001) (Table 3). The association between LCI elevation and CPF in subjects with DMD was weaker (r = −0.41, P = .005) than the degree of lung volume decline (r = −0.79, P < .001), which might be the result of suboptimal cooperation doing the CPF measurements. Age positively correlated with LCI in the DMD group (r = 0.67 y, P < .001) (Table 3). LCI was significantly elevated in subjects with DMD during noninvasive ventilation (10.2 ± 2.7 vs 6.7 ± 0.9, P < .001) or mechanically assisted cough (10.7 ± 2.6 vs 6.8 ± 0.9, P < .001), which was started based on clinical decisions (data not shown).
Association Between LCI and Pulmonary Function Variables
Relationship between lung clearance index and percent-of-predicted FVC (A) and dead-space ventilation (dead-space-to-tidal-volume ratio [VD/VT]) (B) in subjects with Duchenne muscular dystrophy (DMD) and healthy controls. The horizontal dashed lines represent the normal upper limit for the lung clearance index.
In multiple-regression analyses with LCI as the dependent variable and CPF, VD/VT, and FVC as independent variables (adjusted R2 = 0.65, P < .05), VD/VT was the major determinant for LCI (Table 4).
Multiple-Regression Analysis of the DMD Group Evaluating Lung Function Parameters as Explanatory Variables of Lung Clearance Index
Prediction of LCI Elevation From FVC in Subjects With DMD
Receiver operating characteristic curves revealed that a pathological LCI above 7.0 in subjects with DMD was predicted by an FVC below 24% of predicted (96% sensitivity and 80% specificity).
Discussion
We report that progressive declines in lung volume are associated with increasing ventilation inhomogeneity in subjects with DMD. Standard spirometry is of limited value in neuromuscular disorders, as it underestimates expiratory air flow due to respiratory muscle weakness.20 The MBW technique measures lung function parameters during tidal breathing and allows detection of ventilation inhomogeneity independently of respiratory muscle strength or chest wall geometry. The appearance of ventilation inhomogeneity in subjects with DMD alters the current paradigm that chronic respiratory failure is exclusively the result of respiratory muscle weakness, but supports the hypothesis that severe lung volume decline is associated with secondary lung disease.7
To date, LCI elevation has been reported for subjects with confirmed structural lung diseases, including cystic fibrosis,10,21 asthma,11 and bronchiolitis obliterans,13 but has not been previously assessed for patients with neuromuscular disorders. Lung volume decline in our cohort of subjects with DMD was closely associated with elevated LCI. Little has been published concerning the involvement of ventilation inhomogeneity or airway disease in neuromuscular disorders. A previous report estimating ventilation inhomogeneity by electrical impedance tomography showed that hyperinsufflation maneuvers can improve ventilation in subjects with neuromuscular disorders.22 These data offer indirect evidence for ventilation inhomogeneity in subjects with neuromuscular disorders using a method other than MBW. Older reports measuring lung compliance via an esophageal catheter showed reduced lung compliance in subjects with neuromuscular disorders, which has been attributed to the stiffening of unstretched lung tissues24 and the presence of microatelectasis.23 Cough insufficiency correlates with the degree of lung volume decline25 and impairs the clearance of airway secretions.26 Respiratory tract infections occur more frequently27 and with a more complicated course28 in cough-insufficient subjects with DMD. In our study, the association between LCI and CPF was weaker than the association between LCI and FVC in subjects with DMD, which might be the result of a broad interindividual distribution of normal values for CPF.29 Furthermore, some subjects with normal LCI had significant abnormal CPF (and normal FVC; data not shown), which might be the result of suboptimal cooperation. However, cough insufficiency causes retention of pulmonary secretions and possibly airway plugging, resulting in ventilation inhomogeneity. To date, only one small study assessed the lung parenchyma in infection-free subjects with neuromuscular disorders using high-resolution chest computer tomography.30 In 14 adult subjects with different neuromuscular disorders and varying degrees of lung volume decline with a mean FVC of 60% of predicted, computer tomography detected microatelectasis that was not shown by conventional chest radiograph in 2 subjects.30 The authors concluded that this microatelectasis was the result of airway plugging of pulmonary secretions, but no correlation between microatelectasis and the degree of lung volume decline was detected. We showed that elevated LCI was optimally predicted by an FVC below 24% of predicted, but not in subjects with DMD and an FVC above 50% of predicted. Home mechanical ventilation in subjects with DMD is usually initiated at the threshold FVC of 25% of predicted.31 In our cohort, all subjects on noninvasive ventilation had LCI elevation. Age was significantly and positively correlated with LCI, reflecting the advanced disease state of lung volume decline in subjects with DMD, as LCI is known to decrease with age and height.32 The ventilation inhomogeneity that we found in severe lung volume decline might represent retention of pulmonary secretions and microatelectasis.
The monitoring of respiratory function in DMD relies on spirometry, CPF measurements, and polysomnography. Spirometry is a validated method to provide lung volumes and flows in patients with normal respiratory muscle strength and normal chest wall geometry. Chest wall mechanics in patients with DMD are often complicated by progressive scoliosis.1 After the loss of ambulation, FVC declines by 200 mL/y.33 As the mobility of patients with DMD and lung volume decline is limited, measurements of residual volume by whole-body plethysmography are rarely performed, and data on the course of FRC or residual volume in patients with DMD are lacking. In patients with idiopathic scoliosis, residual volume increases as FVC decreases.34 We propose a similar situation in the course of DMD and lung volume decline, as FRC was relatively conserved in our study population. When residual volume is increased to the disadvantage of FVC, the diffusion-convection front of the airways8 is assumed to move higher. In this situation, the volume that oxygen and carbon dioxide has to pass by diffusion for gas exchange increases. We used 4% sulfur hexafluoride for the MBW studies, which has a higher molar mass compared with air. This heavy gas might deposit in the large residual volume and barely be cleared by the low FVC and low VT in patients with DMD. Unfortunately, we were not able to do a phase III slope analysis to differentiate between the diffusion- and convection-dependent inhomogeneity, as the slope III plateau was not reached in subjects with DMD and severe lung volume decline. No subject had diurnal hypoventilation, and the expired CO2 did not increase during the measurement (data not shown). Therefore, VD/VT cannot be the main factor for the LCI elevation. Thus, beside secondary airway disease due to retention of airway secretions, altered ventilation geometry with a large residual capacity and a low FVC may provide an additional mechanism for the increasing ventilation inhomogeneity in DMD.
Conclusions
Our study shows that lung volume decline in DMD is associated with ventilation inhomogeneity. We believe that 2 factors contribute to this phenomenon: retention of airway secretions even in the infection-free status and altered ventilation geometry.
Acknowledgments
We thank all of the families who participated in this study, Manuela Groch-Seidler and Nadine Kordt (both from the Department of Pediatric Pulmonology and Sleep Medicine, University Children's Hospital Essen, Essen, Germany) for technical assistance, and Kathy Astrahantseff PhD (Department of Pediatric Oncology and Hematology, University Children's Hospital Essen) for editing the manuscript.
Footnotes
- Correspondence: Florian Stehling MD, Department of Pediatric Pulmonology and Sleep Medicine, University Children's Hospital Essen, Hufelandstrasse 55, 45122 Essen, Germany. E-mail: florian.stehling{at}uk-essen.de.
The authors have disclosed no conflicts of interest.
- Copyright © 2015 by Daedalus Enterprises