Abstract
BACKGROUND: The use of prone position (PP) has been widespread during the COVID-19 pandemic. Whereas it has demonstrated benefits, including improved oxygenation and lung aeration, the factors influencing the response in terms of gas exchange to PP remain unclear. In particular, the association between baseline quantitative computed tomography (CT) scan results and gas exchange response to PP in invasively ventilated subjects with COVID-19 ARDS is unknown. The present study aimed to compare baseline quantitative CT results between subjects responding to PP in terms of oxygenation or CO2 clearance and those who did not.
METHODS: This was a single-center, retrospective observational study including critically ill, invasively ventilated subjects with COVID-19–related ARDS admitted to the ICUs of Niguarda Hospital between March 2020–November 2021. Blood gas samples were collected before and after PP. Subjects in whom the PaO2/FIO2 increase was ≥ 20 mm Hg after PP were defined as oxygen responders. CO2 responders were defined when the ventilatory ratio (VR) decreased during PP. Automated quantitative CT analyses were performed to obtain tissue mass and density of the lungs.
RESULTS: One hundred twenty-five subjects were enrolled, of which 116 (93%) were O2 responders and 51 (41%) CO2 responders. No difference in quantitative CT characteristics and oxygen were observed between responders and non-responders (tissue mass 1,532 ± 396 g vs 1,654 ± 304 g, P = .28; density −544 ± 109 HU vs −562 ± 58 HU P = .42). Similar findings were observed when dividing the population according to CO2 response (tissue mass 1,551 ± 412 g vs 1,534 ± 377 g, P = .89; density −545 ± 123 HU vs −546 ± 94 HU, P = .99).
CONCLUSIONS: Most subjects with COVID-19–related ARDS improved their oxygenation at the first pronation cycle. The study suggests that baseline quantitative CT scan data were not associated with the response to PP in oxygenation or CO2 in mechanically ventilated subjects with COVID-19–related ARDS.
- computed tomography
- quantitative CT scan analysis
- prone position
- COVID-19 ARDS
- coronavirus SARS
- pulmonary gas exchange
- lung compliance
- ventilation/perfusion scan
Introduction
Prone position (PP) has been extensively used during the COVID-19 pandemic in invasively ventilated subjects.1 The benefits reported from the use of this position in classic ARDS were also confirmed in COVID-19–associated ARDS.2 This strategy, requiring highly trained personnel and not devoid of possible complications,3,-,5 has thus been included in the guidelines for the treatment of moderate and severe COVID-19–associated ARDS.6 Indeed, whereas results from randomized controlled trials in this specific population are lacking, placing subjects with COVID-19–associated ARDS in PP decreases alveolar collapse, hyperinflation, and improves the homogeneity of lung aeration and ventilation.2,7 Moreover, whereas not the primary target of PP, several studies reported a variable (ie, between 30–80%) improvement in oxygenation during PP of mechanically ventilated subjects with COVID-19–associated ARDS.1,2 However, it is currently unknown which factors contribute to and how to predict the response in terms of oxygenation in patients with ARDS placed in PP.
Chest computed tomography (CT) was broadly used in patients with COVID-19 to facilitate diagnosis and quantify the degree of disease extension.8,9 Several radiological patterns could be observed at different times throughout the disease course, showing diffuse lung alterations ranging from ground-glass opacities to parenchymal consolidations.10,11 In addition, quantitative CT results, cornerstones for the understanding of classic ARDS,12 have been analyzed to investigate the pathophysiology of COVID-19–associated ARDS and the lung response to PP in selected groups of subjects.2,13 Previous studies have suggested that in supine position the amount of non-aerated lung tissue in the dependent lung regions was associated with more recruitable lung volume when PP was used,14,-,16 and recently, a relationship between the dorsal non-aerated tissue quantified at the CT scan and the gas exchange response to PP was recorded in classic ARDS.17 These studies, however, did not focus on the association between quantitative CT results and the oxygenation response to PP in subjects with COVID-19–related ARDS.
Recently, Raimondi et al18 studied awake, noninvasively ventilated subjects with COVID-19 and were not able to find any association between the distribution of CT lung lesions and the response in oxygenation to PP. Information regarding the association of baseline quantitative CT results and the response to PP in invasively ventilated patients with COVID-19–associated ARDS is currently lacking. We hypothesized that the quantitative CT results of scans performed prior to the first PP would differ significantly between responders and non-responders in terms of oxygenation and CO2 clearance. The present retrospective study was conducted to test this hypothesis.
QUICK LOOK
Current knowledge
Chest computed tomography (CT) has been broadly used in COVID-19 pandemic to facilitate diagnosis and quantify the degree of disease extension. Recently, a relationship between the dorsal non-aerated tissue quantified at the CT scan and the gas exchange response to prone position (PP) was demonstrated in classic ARDS, but clinical studies have not confirmed these findings in COVID-19–related ARDS.
What this paper contributes to our knowledge
No relationship between dorsal non-aerated tissue quantified at the CT scan analysis and either oxygen or CO2 response to pronation was found. Quantitative CT imaging should not be accounted for when deciding whether to use PP in intubated patients with COVID-19–related ARDS.
Methods
Study Design
This was a single-center, retrospective, observational study performed at the Grande Ospedale Metropolitano Niguarda in Milan, Italy. The retrospective access to clinical data was approved by the ethical committee Milano Area B (approval number: 593–06102020), and the need for informed consent from individual subjects was waived.
All patients admitted between March 1, 2020–November 30, 2021, to the COVID-19 ICUs were screened for eligibility. Inclusion criteria were as follows: (1) age > 18 y, (2) laboratory-confirmed SARS-CoV-2 infection, (3) ARDS diagnosis according to Berlin criteria at ICU admission,19 (4) tracheal intubation and invasive mechanical ventilation, (5) use of PP, and (6) performance of a chest CT scan within the 72 h prior the first PP. Exclusion criteria were missing clinical data regarding blood gas analysis performed during the first PP cycle. Intubated subjects with COVID-19–related ARDS were maintained sedated and paralyzed. Subjects were ventilated using a lung-protective ventilatory strategy: low tidal volume (VT; 6–8 mL/predicted body weight), medium-high levels (8–12 cm H2O) of PEEP, breathing frequency between 15–25 breaths/min, maintaining a plateau pressure < 28 cm H2O and a driving pressure < 12 cm H2O, and with a target SpO2 of 92–95%. A PaO2/FIO2 < 100 mm Hg was used as a criterion for PP.20
Clinical management, including the decision to use PP and perform a chest CT scan, was at the discretion of the attending physicians. The final date of follow-up for subject outcomes was July 14, 2022. For study purposes linked to the regional research network,21 an extensive set of information was prospectively recorded from the day of ICU admission on an electronic report form (REDCap electronic data capture tools). This information included anthropometric and clinical data, severity scores, vital signs, laboratory tests, radiological information, ICU and hospital length of stay (LOS), and ICU and hospital survival.
To assess the physiologic effects of pronation, subjects’ ventilatory settings were prospectively recorded at 3 different time points: (1) within 2 h before the pronation (baseline); (2) during the last 4 h of the pronation cycle (prone); and (3) within 2–5 h after turning the subjects back to the supine position (supine). At each time point, end-inspiratory and end-expiratory airway occlusion maneuvers were performed to calculate driving pressure and respiratory system compliance (CRS).22 At the same time points, arterial blood gases were drawn to calculate the PaO2/FIO2 and the ventilatory ratio (VR).22,23
Definitions
Subjects were defined as oxygen responders to PP according to 2 different definitions previously applied in the literature: (1) the PaO2/FIO2 increased by ≥ 20 mm Hg during prone ventilation as compared to baseline values in supine position;1 similarly, oxygen non-responders were defined as those subjects in whom this condition was not satisfied; and (2) the median PaO2/FIO2 increase observed during prone ventilation was used as cutoff, defining responders with a PaO2/FIO2 higher than the median value and non-responders with a PaO2/FIO2 below.24 The change in the VR was used to define the response in terms of CO2 clearance. Subjects were defined as CO2 responders when the VR decreased during pronation as compared to supine, while CO2 non-responder when the VR increased or did not change.
CT Scan Acquisition and Image Analysis
All CT images were acquired on 4 scanners of a single vendor (Siemens, Munich, Germany) and with the same acquisition protocol for chest examinations, employing an automatic exposure control and an automatic selection of the tube voltage and sharp reconstruction algorithm.
All CT series were exported from the picture archiving communication system to a dedicated workstation for automatic image analysis. A dedicated processing software developed in Python language was used, as previously described.25,-,27 Briefly, (1) the pipeline rescales CT images to a slice thickness of 3 mm; (2) performs, for each slice, automatic segmentation of the left and right lungs; and (3) calculates the relative distribution of Hounsfield units (HU) of the segmented regions of interest.
In this work, the following metrics were considered: volume (Vlung [mL]), Hounsfield unit related to the lung density (ρ [HU]), and mass (m [g]). The volume and the density were calculated, respectively, as the number of voxels multiplied by the physical voxel dimension and their average HU value of the selected region of interest. The mass was calculated using the following formula:
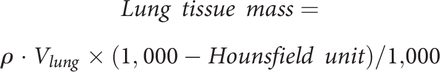
For each CT image, all these metrics were calculated for both lungs, obtained as the sum of the segmentations of the right and left lungs, and in 4 different density regions according to classical aeration thresholds:12 hyperinflated lung [−1,000 to −900] HU, well-aerated lung [−900 to −500] HU, poorly aerated lung [−500 to −100] HU, and non-aerated lung [−100 to +100] HU.28
Furthermore, a geometric subdivision of the entire (both lungs) region of interest was performed. The masks were divided into 10 different regions equally spaced along the sternovertebral axis, and for each subregion, the previous metrics were calculated.
Statistical Analysis
No sample size calculation was performed a priori, and the sample size is equal to the number of patients treated in our hospital during the study period. Comparison between continuous variables was performed via Student t test using Welsh correction for unequal variance, Mann-Whitney rank-sum test, analysis of variance, or Kruskal-Wallis test as appropriate. Differences between categorical variables were assessed using the chi-square or Fisher exact test. The continuous relationship between quantitative variables was investigated using linear regression. Data were expressed as mean ± SD or median and interquartile range. Statistical significance was defined as P < .050. Analyses were performed with Stata statistical software (release 16, StataCorp, College Station, Texas), and graphs were drawn using SigmaPlot v.12.0 (Systat Software, San Jose, California). The Standards of Reporting of Observational Studies in Epidemiology checklist for observational studies was used.
Results
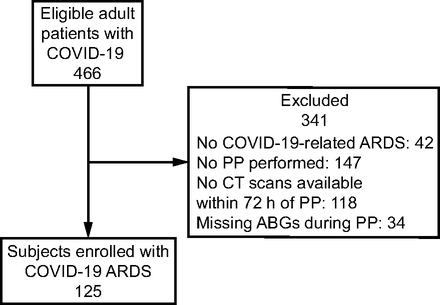
During the study period, 466 patients with COVID-19 were admitted to the ICU (Fig. 1). One hundred twenty-five subjects with a median Simplified Acute Physiology Score II score at ICU admission of 38 [33–43] were enrolled in the study. Baseline demographic characteristics are summarized in Table 1.
Study flow chart. PP = prone position; CT = computed tomography; ABG = arterial blood gas.
Population Demographic Characteristics at ICU Admission Divided by Oxygen Response to Pronation
Oxygen Response to Pronation and Quantitative CT Scan Parameters
According to the PaO2/FIO2 increased by ≥ 20 mm Hg definition, 116 subjects (93%) were O2 responders, while 9 (7%) were non-responders. O2 responders had a higher body mass index (P =.009) and prevalence of hypertension (P = .001) compared to nonresponders. The use of noninvasive respiratory support prior to intubation (72% vs 100%, P = .063), its duration (1 [0–3] d vs 1 [1–4] d, P = .08), and the use of awake PP prior to intubation (37% vs 44% P =.44) were similar between O2 responders and non-responders. No difference in ARDS severity, ventilatory settings, and blood gas parameters was recorded (Table 2).
Ventilatory Parameters of the Population Divided by Oxygen Response to Pronation in Supine Position Before the First Pronation
O2 responders were characterized by higher baseline CRS (42 ± 115 mL/cm H2O vs 32 ± 5 mL/cm H2O, P ≤ .001). The length of the first pronation performed in the ICU was similar in O2 responders and non-responders (21 [18–24] h vs 24 [22–32] h, P = .08).
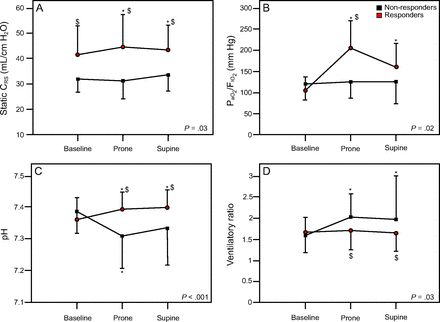
During the first PP, O2 responders improved, as per the definition, the PaO2/FIO2. Moreover, arterial pH and CRS increased, while the VR did not change significantly. On the contrary, PaO2/FIO2 did not change in O2 non-responders, while PaCO2 increased in PP from 48 ± 10 mm Hg to 59 ± 15 mm Hg (P = .01). Consequently, the VR and pH worsened significantly in PP in this subgroup of subjects (Fig. 2).
Variation of clinical parameters during the first pronation cycle of oxygen responders and oxygen non-responders to pronation. Responders were defined as the subjects whose PaO2/FIO2 increased by ≥ 20 mm Hg during prone ventilation as compared to baseline values in supine position. Panel A represents the variations of respiratory system compliance. Panel B represents the ratio variations between arterial PaO2 and inspiratory fraction of oxygen. Panel C represents the variations in pH. Panel D represents the variations in respiratory ratio. * P < .050 vs baseline; $ P < .050 vs non-responders. CRS = respiratory system compliance.
Clinical outcomes divided by O2 responders and non-responders are summarized in Table 3. During the ICU LOS, subjects received 4 [2–6] cycles of pronation for a total amount of 80 [46–146] h spent in PP. No differences in ICU LOS (P = .94) and survival (P = .52) were found between the 2 groups. Bilateral quantitative CT scan analysis did not reveal any difference both in mass and density of hyperinflated, well-aerated, poorly aerated, and non-aerated lung tissue when the subjects were divided into O2 responders and non-responders (Table 4).
Clinical Outcomes of the Population Divided by Oxygen Response to Pronation
Baseline Quantitative Computed Tomography Parameters of the Population Divided for the Oxygen Response to Pronation
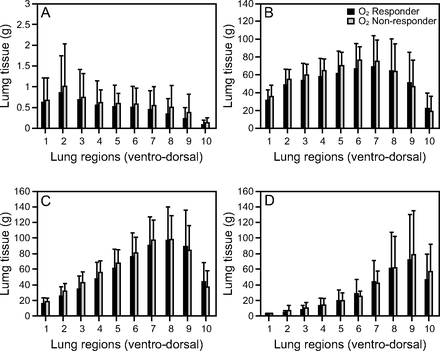
Lastly, the analysis performed on 10 ventral-dorsal lung segments also did not identify any difference between the 2 groups (Fig. 3). For example, the amount of hyperinflated tissue of the ventral region (3.2 ± 2.9 g vs 3.6 ± 2.3 g, P = .66) and non-aerated tissue of dorsal regions (254 ± 161 g vs 263 ± 144 g, P = .85) were similar between O2 responders and non-responders. Similar findings were observed when subjects were divided according to the median increase in PaO2/FIO2 (87 mm Hg). Results can be found in Tables S1, S2, S3, and S4 (see related supplementary materials at http://www.rcjournal.com).
Ventral-dorsal (1 to 10 segment) regional lung tissue distribution subjects divided by oxygen responders and non-responders to pronation. O2 responders defined as the subjects whose PaO2/FIO2 increased by ≥ 20 mm Hg during prone ventilation as compared to baseline values in supine position. A: Hyperinflated tissue, B: well aerated tissue, C: poorly aerated tissue, and D: non-aerated tissue.
CO2 Response to Pronation
Fifty-one (41%) of 125 subjects improved their VR during PP and were thus defined as CO2 responders, while the remaining 74 subjects were defined as CO2 non-responders. Baseline demographic characteristics are summarized in Table S5 (see related supplementary materials at http://www.rcjournal.com). Before PP, CO2 responders were characterized by higher VT (6.9 ± 1.0 mL/kg vs 6.6 ± 0.8 mL/kg, P = .02), breathing frequency (21 ± 4 breaths/min vs 18 ± 3 breaths/min, P < .001), and VR (1.9 ± 0.5 vs 1.5 ± 0.4, P < .001) to maintain a similar arterial PCO2 (49 ± 9 mm Hg vs 46 ± 9 mm Hg, P = .09) as compared to CO2 non-responders (Table S6, see related supplementary materials at http://www.rcjournal.com). No differences in days of ventilation (P = .94), ICU LOS (P = .43), or ICU mortality (P = .46) were found between the 2 groups (Table S7, see related supplementary materials at http://www.rcjournal.com). Similarly, no differences in quantitative CT results were found between CO2 responders and non-responders (Table 5).
Baseline Quantitative Computed Tomography Parameters of the Population Divided for the CO2 Response to Pronation
Discussion
The use of chest CT has permanently changed our understanding of ARDS through its morphological assessment and quantitative analysis of density distribution.12 For these reasons, this radiological examination is extensively used in some centers to evaluate lung structure, extent of disease, response to lung recruitment,15 and evolution of disease. In the context of the outbreak of a novel infectious disease leading to pneumonia and respiratory failure, these concepts were broadly applied. This allowed us to study retrospectively a large number of CT scans of critically ill, mechanically ventilated subjects with COVID-19–related ARDS undergoing PP. Our aim was to evaluate whether the different responses in terms of gas exchange during the first PP were associated with different baseline quantitative CT scan characteristics. In the 125 subjects studied, 93% improved their oxygenation during the first PP, and 41% improved their VR. No relationship was found between quantitative CT scan parameters and either oxygen or CO2 response to PP. Similar results were observed when dividing the population according to the median increase in PaO2/FIO2. Moreover, we confirmed that the O2 responders were characterized by higher baseline compliance and lower driving pressure as compared to non-responders. Of note, the time spent in PP did not differ between the 2 groups.
During the COVID-19 pandemic, pronation was broadly used in mechanically ventilated patients.1,29,-,31 In line with previous data,1 most of the studied subjects improved their oxygenation during the first PP. Aalinezhad and colleagues32 identified in subjects with COVID-19 a relationship between the severity of lung involvement measured at CT scan and blood oxygenation.32 Moreover, the possibility of predicting lung recruitment from a single static baseline CT scan using a machine learning approach has recently been described.33 In classic ARDS, lung perfusion is similar in prone and supine position, being slightly unbalanced toward dorsal lung regions.34,35 According to this characteristic, the improvement in PaO2/FIO2 in PP should parallel the variation in density distribution, corresponding to an increase in well-aerated lung tissue in the dorsal areas of the lungs. Despite these premises, in classic ARDS, Papazian and colleagues36 found no correlation between baseline quantitative CT data and PaO2/FIO2 response to pronation. In addition, similarly to what has been observed in non-intubated subjects with COVID-19,18 we were not able to identify a correlation between baseline quantitative CT-scan characteristics and PaO2/FIO2 response during PP.
The negative findings of these studies might have several explanations. CT data accurately describe lung parenchymal density, while they do not assess pulmonary perfusion. This aspect might be of utmost importance in patients with COVID-19–associated ARDS. Indeed, this disease is characterized by (1) impairment of hypoxic vasoconstriction leading to a marked ventilation/perfusion mismatch37,-,39 and (2) the diffuse presence of pulmonary microthrombosis.40 Since both of these vascular defects can be diffused to all the lungs, irrespective of gravitational distribution (dependent vs non-dependent), and regardless of the parenchymal aspect assessed with CT scan, a dissociation between aeration/ventilation and gas exchange has been described in subjects with COVID-19–associated ARDS.38 It is thus conceivable that, in addition to the unknown potential for lung recruitment, the variable and unpredictable lung perfusion changes further hinder the prediction of the response solely based on baseline quantitative CT information. In addition, the varying potential involvement of pulmonary vasculature could justify the broad spectrum of oxygen response to pronation reported, ranging from 35% to 93%.1,2,13,18,24,41 Interestingly, and in line with this reasoning, in our population no difference in quantitative CT characteristics was observed between subjects with moderate vs severe COVID-19–associated ARDS (Table S8, see related supplementary materials at http://www.rcjournal.com).
A second possible explanation of the different responses to pronation might be the disease time course. Indeed, despite the lack of statistical significance, the time between symptom onset and first pronation was longer in O2 non-responders, possibly resulting in a more severe disease stage, as suggested by the lower CRS. In line with this hypothesis, a decreasing response to PP (in terms of oxygenation) has been described as a consequence of lung consolidation toward organizing fibrotic pneumonia.13,42,43 Regardless of this potential explanation, no relevant differences in gas exchange, lung weight, or non-aerated lung tissue were noted between O2 responders and non-responders.
Response to Pronation
In response to PP, O2 responders increased slightly, but significantly their CRS, which remained above baseline after re-supination. The improvement of PaO2/FIO2 paralleled CRS, except for a slight decrease after re-supination. Notably, these variations were not mirrored by the VR, which did not change significantly.
Fossali et al44 performed a physiological study exploring the early changes after pronation in subjects with COVID-19–associated ARDS, performing CT scans both in supine and PPs. They demonstrated that PP significantly decreased the weight of non-aerated and hyperinflated lung tissue and increased the amount of normally aerated lung. Moreover, the regional response to PP was not homogenous, as demonstrated by the remarkable recruitment in the dorsal regions and de-recruitment in the ventral. However, in our population, O2 responders and non-responders, despite having similar baseline amounts of hyperinflated ventral tissue and non-aerated dorsal tissue, demonstrated markedly different responses in terms of lung mechanics and gas exchanges during PP. Notably, also in Fossali’s44 work, no association between the amount of ventral de-recruitment or dorsal recruitment and the oxygen response was found. Taken together, our results and the findings of this author foster two considerations: First, in patients with COVID-19, the oxygen response to pronation is most likely not predictable from a static baseline CT scan; and second, the observed increase in oxygenation is possibly due mainly to the improvement of the ventilation/perfusion matching related to a persistency of perfusion in the vertebral part of the thorax and a reopening of the dorsal collapsed lung.45,-,48 This second hypothesis is corroborated by the study of Richter et al,46 who demonstrated that the oxygenation response to pronation in subjects with ARDS was consequent to an improvement in ventilation/perfusion match due to the unchanged perfusion in the dorsal part of the thorax associated with a reopening of the dorsal collapsed lung. This mechanism, also described using the ventilation/perfusion tools of electrical impedance tomography in classic subjects with ARDS,49 was confirmed in COVID-19–associated ARDS.50,51
In O2 non-responders, no variations in CRS or PaO2/FIO2 were observed. Furthermore, differently from responders, they experienced an increase in VR and PaCO2 that persisted after the re-supination. This observation points toward an increased dead space. As an increase in hyperinflation is unlikely the underlying mechanism, we think that also in this case the worsening of ventilation/perfusion might be the cause. Of note, also when dividing the population according to their response in terms of CO2 clearance (ie, variation in VR) no difference in baseline quantitative CT data was observed.
Automated CT Scan Segmentation
Despite the absence of association between CT scan characteristics and response to PP, CT exams represent the accepted standard for evaluating the alterations of lung parenchyma, even in the early stages of disease, when the patient has few or no symptoms. Moreover, it is also a useful tool for monitoring diesase progression.11,52
In addition to the classic qualitative visual image interpretation, the automated and integrated workflow of image analysis allows to extract several objective metadata quantitative information retained in the image, such as parenchymal density and volume, and permits the definition of lung compartments based on the different degrees of aeration.12 Although CT image analysis could be extremely informative, some aspects need to be considered for its use in clinical practice. In this work, an algorithm for automatic segmentation of lungs in CT images was employed,25 which drastically reduces analysis time and enables real-time quantitative results through the use of dedicated in-house software. Avoiding the time-consuming task of drawing the lung boundaries (selection of regions of interest), the physician can thus focus more on interpreting the results of the obtained quantitative metrics.
Limitations
Several limitations have to be addressed for this study. First, due to the retrospective nature of the study and the low number of O2 non-responders, our analyses could be underpowered to identify any difference in quantitative CT characteristics; a controlled methodology and more homogeneous groups may produce different results. Second, the CT scans were performed for clinical purposes and retrospectively used for the analyses. Thus, no standardization of ventilation mode nor respiratory phase (eg, inspiratory pause) was performed during CT scan acquisition. Third, no data regarding perfusion of the lung were available. Consequently, the pathophysiological role of ventilation/perfusion matching in explaining oxygen and CO2 responses to pronation can only be hypothesized. Finally, the trunk inclination angle used during the respiratory mechanics measurement was not standardized.53,-,55
Conclusions
Most subjects with COVID-19–related ARDS improved their oxygenation at the first pronation cycle. Our study performed on a large population of critically ill, mechanically ventilated subjects with COVID-19–related ARDS suggests that quantitative data obtained from a baseline CT scan are neither associated with the oxygen response nor with the response in terms of CO2 elimination.
Acknowledgments
The authors are deeply grateful to all the physicians and nurses of the COVID-19 ICUs of the Niguarda Hospital.
Footnotes
- Correspondence: Thomas Langer MD, Department of Medicine and Surgery, University of Milan-Bicocca, Monza, Italy. E-mail: Thomas.Langer{at}unimib.it
The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 1482
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2024 by Daedalus Enterprises