Abstract
BACKGROUND: Observational studies report that lower driving pressure (ie, the difference between plateau pressure and PEEP) is associated with improved survival in patients with ARDS and may be a key mediator of lung-protective ventilation strategies. The primary objective of this study was to characterize reductions in driving pressure that could be achieved through changes in PEEP.
METHODS: In this prospective physiological pilot study, 10 subjects with ARDS were placed on PEEP according to the ARDS Network Lower PEEP/FIO2 Table. PEEP was adjusted in small increments and decrements above and below this initial PEEP, and driving pressure was measured at each PEEP level. Subsequently, PEEP was set at the level resulting in the lowest driving pressure, and driving pressure was measured after 1, 5, 15, and 30 min to assess stability over time at constant PEEP.
RESULTS: All subjects had ARDS with a median (interquartile range [IQR]) PaO2/FIO2 of 116 (98–132) at enrollment. Median (IQR) driving pressure at baseline was 14 (13–17) cm H2O. After PEEP titration, median driving pressure decreased to 13 (12–14) cm H2O. The largest reduction in driving pressure was 4 cm H2O. Two subjects had no change in driving pressure at multiple PEEP levels. To achieve the lowest driving pressure, final PEEP was increased in 6 subjects and decreased in 4 subjects from the baseline PEEP prescribed by the ARDS Network Lower PEEP/FIO2 Table. Driving pressure reached equilibrium within 1–5 min and remained stable for 30 min following PEEP titration.
CONCLUSIONS: PEEP titration had a variable effect in changing driving pressure across this small sample of ARDS subjects. In some subjects, PEEP was decreased from values given in the ARDS Network Lower PEEP/FIO2 Table to minimize driving pressure. Changes in driving pressure stabilized within a few minutes of PEEP titration.
- driving pressure
- mechanical ventilation
- PEEP
- respiratory system compliance
- ARDS
- acute respiratory failure
Introduction
Mechanical ventilation is a cornerstone of treatment for ARDS, but it may cause ventilator-induced lung injury, which can contribute to mortality. Lung-protective ventilation strategies are critical to reduce the burden of this frequently fatal syndrome. Recent observational studies suggest that reducing driving pressure (ΔP) may be important for optimizing lung-protective ventilation and improving survival in ARDS.1–4
ΔP is determined by the ratio of tidal volume to respiratory system compliance. It is easily calculated as the difference between inspiratory plateau pressure (Pplat) and PEEP. ΔP likely reflects the cyclic stress to the respiratory system generated by mechanical ventilation determined by the underlying lung injury, chest wall mechanics, and the chosen ventilator settings.1,5 In several observational studies, including an individual patient data meta-analysis of > 3,500 ARDS subjects enrolled in 9 randomized controlled trials of lung-protective mechanical ventilation strategies, ΔP was the ventilator variable most strongly associated with mortality.1–4,6,7 Moreover, reductions in ΔP appeared to be a critical mediator of lung-protective ventilation strategies.1
At a fixed tidal volume, changes in ΔP that occur as PEEP is increased or decreased reflect changes in respiratory system compliance, primarily from either lung recruitment, de-recruitment, or overdistention. At the PEEP associated with the lowest ΔP, respiratory system compliance is the highest. This level of PEEP may represent an optimum balance between the goals of preventing ventilator-induced lung injury from overdistention versus tidal opening/closing of small bronchioles and alveoli. Although the rationale for targeting the lowest ΔP in patients with ARDS is strong, the existing evidence is composed primarily of post hoc analyses or observational studies.1–4
The objectives of this prospective pilot study were to: (1) demonstrate the feasibility of a ΔP assessment protocol in which PEEP is adjusted until a minimum ΔP is identified, (2) characterize differences in ΔP-guided PEEP from that prescribed in the ARDS Network Lower PEEP/FIO2 Table, and (3) demonstrate the time required to reach a new steady state in ΔP after changing PEEP.
QUICK LOOK
Current knowledge
In observational studies, lower driving pressure is associated with improved survival among subjects with ARDS. Driving pressure may be reduced by either decreasing tidal volume or increasing respiratory system compliance through PEEP titration. However, standardized approaches to achieve the lowest achievable driving pressure have not been evaluated.
What this paper contributes to our knowledge
Using a standardized approach to PEEP titration in subjects with ARDS, changes in driving pressure were highly variable. In several subjects, the PEEP required to minimize driving pressure was below that prescribed by the ARDS Network Lower PEEP/FIO2 table. Driving pressure stabilized within minutes of PEEP titration.
Methods
The study was conducted in the medical ICU of Johns Hopkins Hospital, a tertiary academic hospital in Baltimore, Maryland. The Johns Hopkins Institutional Review Board approved the study protocol (00079500), and written informed consent was obtained for all research subjects.
Study Population
We screened all patients ≥18 y old admitted to the medical ICU. Patients receiving mechanical ventilation were eligible for participation if they met the following criteria: 1) ARDS based on Berlin criteria,8 2) intubated < 7 d, and 3) PEEP ≥ 8 cm H2O. Patients were excluded if any of the following conditions were present: elevated intracranial pressure, right heart failure, barotrauma within 10 d, severe refractory hypoxemia (defined as SpO2 < 90% on 1.0 FIO2), baseline Pplat ≥ 35 cm H2O, or the physician or patient declined participation.
Study Procedures
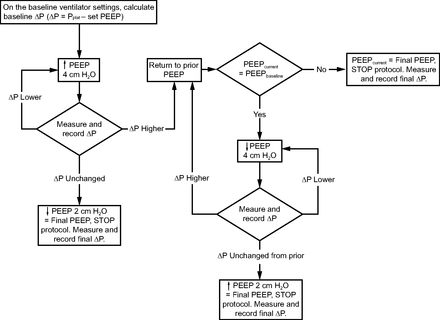
At baseline, all subjects were managed with volume controlled continuous mandatory ventilation and upper body positioned at 30° above supine. Tidal volumes were prescribed by each subject's treating team and based on the ARDS Network recommendation for a goal tidal volume of 6 mL/kg predicted body weight. Tidal volumes were not changed during the course of the study protocol. Baseline PEEP was determined per the ARDS Network Lower PEEP/FIO2 Table, which is usual care for setting PEEP in this ICU.9 Baseline ΔP was calculated after a minimum of 30 min on these settings. Pplat was measured in triplicate using a minimum 0.5-s end-inspiratory pause with at least 10 breaths between each measurement. ΔP was calculated by subtracting the set PEEP from the average Pplat. Subjects then underwent the ΔP assessment protocol in which repeated measurements of ΔP were made following directed adjustments in PEEP by 2–4 cm H2O (Fig. 1). If ΔP increased following a change in PEEP, no further escalations or reductions in PEEP were made in that direction. At each level of PEEP, Pplat was measured and ΔP was calculated at 1 min and 5 min before moving to the next step of the protocol. The PEEP associated with the lowest ΔP was termed the “optimum PEEP.” Visual inspection of ventilator waveforms was performed in real time by a single researcher (SKS) to ensure consistency in the Pplat measurements. The FIO2 could be increased if the SpO2 decreased by 5% from baseline or below 88% and remained low for > 1 min. Following the ΔP assessment protocol, PEEP was set at the “optimum PEEP” level for 30 min with serial assessments of Pplat and ΔP at 1, 5, 15, and 30 min to assess stability of airway pressures. The investigator performing the PEEP titration (SKS) visually monitored heart rate and SpO2 continuously for the duration of the study procedures. This PEEP titration protocol was piloted and refined on 9 subjects prior to this study (reported previously in abstract form).10
Algorithm for driving pressure (ΔP) assessment protocol.
After the 30-min assessment, the ventilator was returned to baseline settings, and the optimum PEEP and lowest ΔP were reported to the clinical team. We recorded if reporting the optimum PEEP to the clinical team changed clinical management by monitoring the electronic health record for 24 h following the protocol. Changes in clinical management were attributed to the ΔP assessment protocol if ventilator settings reflected the use of optimum PEEP and if this was different than the PEEP prescribed by the ARDS Network Lower PEEP/FIO2 Table. Subjects were monitored for 48 h to identify any adverse events potentially related to the protocol, including pneumothorax, pneumomediastinum, hypoxemia requiring rescue therapy (eg, ECMO, recruitment maneuver, inhaled pulmonary vasodilator), acute hypotension, new-onset cardiac arrhythmia, or cardiac arrest.
Statistical Analysis
Continuous data are presented as median and interquartile range (IQR) unless otherwise indicated. Categorical variables are presented as counts and percentages. We evaluated changes in ΔP using the airway pressures measured after 5 min at a given PEEP level. We used repeated measures analysis of variance to evaluate for significant changes in ΔP over 4 time points (ie, 1, 5, 15, and 30 min) during optimum PEEP ventilation. All statistical analyses were performed using STATA 14.0 (StataCorp, College Station, Texas).
Results
Ten subjects were enrolled in the study over 14 months of active enrollment between January 2016 and August 2018 (Table 1). The 10 subjects included 6 men and 4 women. The median (IQR) age was 45 (27–54) y. The ARDS risk factor for 8 subjects was pneumonia; of the remaining 2 subjects, one had non-pulmonary sepsis and the other had pancreatitis. At enrollment, the median (IQR) APACHE II score was 33 (31–37). The median (IQR) PaO2/FIO2 was 116 (98–132), consistent with moderate to severe ARDS at the time of enrollment. Median (IQR) values for baseline ventilator settings included: tidal volume 5.8 (5.0–6.0) mL/kg predicted body weight, breathing frequency 32 (25–35) breaths/min, PEEP 10 (10–10) cm H2O, and FIO2 0.6 (0.5–0.7). Baseline Pplat was 24 (24–27) with a ΔP of 14 (13–17) cm H2O. Two subjects were receiving neuromuscular blockade at the time of enrollment, and no subjects were in a prone position at the time of assessment.
Subject Characteristics at Time of Enrollment
Identifying Optimal PEEP
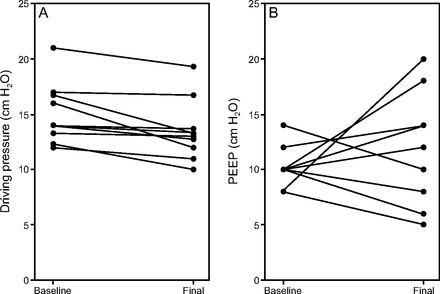
After the ΔP assessment protocol, ΔP was reduced to a median of 13 (12–14) cm H2O. The range of ΔP reductions was 0–4 cm H2O. To minimize ΔP, PEEP was increased in 6 subjects and decreased in 4 subjects. Two subjects had no change in ΔP at multiple PEEP levels, but the final PEEP was changed by 2 cm H2O from baseline based on our protocol (Fig. 1). One of these subjects had a final PEEP 2 cm H2O above baseline, and one subject had a final PEEP 2 cm H2O below baseline. Among subjects in whom ΔP changed with PEEP titration, the change in PEEP required to minimize ΔP ranged from −4 to +12 cm H2O compared to the baseline PEEP level per the ARDS Network Lower PEEP/FIO2 Table (Fig. 2). There were no clinically important changes in heart rate during the PEEP titration protocol. One subject had a decrease in SpO2 from 90% to 88% at 5 min when PEEP was decreased from 10 to 6 cm H2O, with a simultaneous increase in ΔP from 11 to 12 cm H2O. SpO2 returned to > 90% when PEEP was increased back to 10 cm H2O. FIO2 did not require adjustment in any subject during the PEEP titration protocol.
Change in driving pressure and PEEP from baseline to post-PEEP titration. (A) Driving pressure and (B) PEEP for individual subjects (N = 10) at baseline and after performing the PEEP titration protocol.
Changes in ΔP Over Time
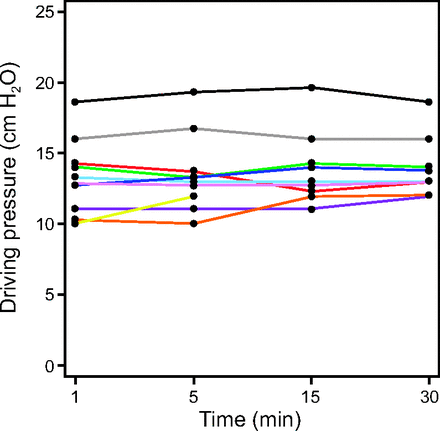
During the 30 min of ventilation using optimal PEEP, there was no significant change in ΔP after the first min (P = .63) (Fig. 3). The 30-min optimal PEEP trial was stopped early (after 15 min) in one subject due to a new cuff leak that precluded reliable assessment of Pplat and ΔP. No incidents of delayed desaturation occurred during the 30-min observation.
Driving pressure over a 30-min observational period at time points 1, 5, 15, and 30 min after setting PEEP at the optimum level. One subject developed a cuff leak at 15 min, after which driving pressure could not be assessed. Each color represents an individual subject.
No protocol-related adverse events were observed in the 48 h following the ΔP assessment protocol. In 7 of 10 subjects, the clinical team chose to use the optimum PEEP following the ΔP assessment protocol rather than return to the value recommended by the ARDS Network Lower PEEP/FIO2 Table.
Discussion
This prospective physiological study used a detailed protocol to target and minimize ΔP through PEEP titration. We demonstrated that changes in ΔP that resulted from changing PEEP were highly variable. To minimize ΔP, several subjects required a higher PEEP, but others required a lower PEEP than that prescribed in the ARDS Network Lower PEEP/FIO2 Table. Our results also suggest that changes in ΔP stabilize within a few minutes of changing PEEP. These results have important implications that will inform the design of future clinical studies of ΔP-targeted mechanical ventilation strategies.
First, the reduction in ΔP achieved from PEEP titration was highly variable among the participants when starting from values given in the ARDS Network Lower PEEP/FIO2 Table. Nevertheless, we observed a reduction in ΔP from baseline in 8 of 10 participants, with the largest reduction being 4 cm H2O. These findings suggest that many patients may benefit from PEEP titration to achieve a lower ΔP, while some subjects may already be at their minimum ΔP while using the ARDS Network Lower PEEP/FIO2 Table. Importantly, even small reductions in ΔP may be important as suggested in a recent, large, individual-patient meta-analysis.1 In that study, a 3 cm H2O reduction in ΔP was associated with an absolute mortality reduction of 5.5%. Hence, even small decreases in ΔP may translate to clinically important improvements in patient outcomes.
Second, ΔP assessments early in a patient's mechanical ventilation course may be a way to identify patients who are more or less likely to benefit from higher PEEP. Ventilator-induced lung injury from alveolar overdistention may occur at high inspiratory pressures and lung volumes.11 Alternatively, injury may occur at lower alveolar pressures and volumes due to the repetitive opening and closing of alveoli or excessive tension at the margins of aerated and non-aerated lung parenchyma.12,13 Although many investigators have recommended using higher levels of PEEP than were traditionally used to support arterial oxygenation,14–17 4 randomized controlled trials of higher versus lower PEEP approaches did not report benefits of higher PEEP.9,18,19 One reason for these negative results is that some patients recruit lung in response to higher PEEP, while others do not. In patients who do not recruit additional alveoli, higher PEEP may only cause overdistention injury to alveoli that are already aerated and thus contribute to adverse hemodynamic consequences.20 Including such patients in the higher PEEP arms of these trials may have biased the results toward the null.
Indeed, in our study, the direction of PEEP change that minimized ΔP differed among our subjects. Four of the 10 subjects actually required a decrease in PEEP from baseline to reduce ΔP, which suggests they were at risk for overdistention injury even while receiving PEEP according to the ARDS Network Lower PEEP/FIO2 Table. These data highlight the potentially problematic nature of a fixed PEEP/FIO2 Table, which deliberately links increases in PEEP to increases in FIO2 and vice versa. For some patients, a lower PEEP strategy may actually be less injurious if the lung is not recruitable.1,21–23 The potential for lung recruitability may be predicted from changes in PaO2/FIO2, respiratory system compliance, and dead space when PEEP is raised.24 However, assessing change in ΔP is a simple and structured approach to distinguish patients who may benefit from increases in PEEP from patients who may benefit from decreases in PEEP relative to the ARDS Network PEEP/FIO2 Table. Future studies investigating PEEP strategies may be improved by including a baseline assessment of PEEP responsiveness to predictively enrich the study population, perhaps by evaluating changes in ΔP or oxygenation following an increase in PEEP.23,25
At a given tidal volume, reductions in ΔP in response to changes in PEEP are related to increases in respiratory system compliance. PEEP titration strategies that optimize respiratory system compliance were associated with improved oxygen delivery, less organ dysfunction, improved oxygenation, and lower levels of inflammatory cytokines.15,26,27 However, many of these strategies are dependent on specialized equipment (eg, super-syringes), software, heavy sedation, and even neuromuscular blockade, which has limited clinical adoption.17,21,28 By comparison, ΔP is measured easily and quickly at the bedside by clinicians and respiratory therapists, without the need for special equipment and usually without paralytics or excessive sedation provided the patient is relaxed.29
Finally, we also observed that ΔP did not change significantly during the 30-min trials of optimum PEEP. This suggests stability of recruited alveoli following increases in PEEP and the absence of delayed de-recruitment following decreases in PEEP. Although changes in ΔP after 30 min were not assessed in this study, the stability we observed is consistent with previous observations. For example, in one study PEEP-related changes in respiratory system compliance at 5 min predicted changes at 60 min.30 It is therefore unlikely that significant changes in physiology, including oxygenation and respiratory system compliance, will occur if optimum PEEP is prescribed following the assessment of ΔP. This stability has important implications with respect to the feasibility of directing respiratory therapists to use the ΔP assessment protocol to guide PEEP titration.
There have been numerous calls for the evaluation of a ΔP-targeted lung-protective ventilation strategy following the publication of several observational studies.1–5,31,32 Our study increases the understanding of ΔP and may inform future studies, but it also has several potential limitations. First, the observed reductions in ΔP were variable and frequently small. One reason for this is that the subjects were ventilated with low tidal volumes at baseline. Reducing tidal volume is a powerful mechanism for reducing ΔP. With a low-baseline tidal volume and a low-baseline ΔP, the potential to decrease ΔP further through PEEP titration was limited. We suggest that future studies evaluating ventilation strategies guided by ΔP utilize a combination of tidal volume reduction and PEEP titration to reduce ΔP. In these future studies, careful attention should be paid to the effects of reducing ΔP, via manipulating tidal volume and PEEP, on other important mechanical parameters such as minute ventilation, dead space, and mechanical power. Another reason for the variable reductions in ΔP may be related to the potential for altered chest wall compliance within our patient population. At a fixed tidal volume, changes in ΔP represent changes in respiratory system compliance (lung and chest wall combined). Our subjects had a high median (IQR) body mass index of 38 (29–40) kg/m2 and many had cirrhosis, which may contribute to altered chest wall compliance.33 Transpulmonary ΔP better reflects the potential for injury to the lung alone but requires the measurement of pleural pressure via esophageal manometry. Retrospective studies indicate ΔP is an adequate surrogate for transpulmonary ΔP, however, this should be confirmed with prospective studies.3
Second, many physiologic studies establish a standardized lung volume history by using recruitment maneuvers at the beginning of a PEEP titration protocol. However, we deliberately chose not to incorporate a recruitment maneuver into our protocol. The effects of recruitment maneuvers are typically transient and temporarily change respiratory system compliance, thus the optimal PEEP immediately after a recruitment maneuver would likely not be the same PEEP without a recruitment maneuver.34 Pragmatically, because recruitment maneuvers are not used routinely in our clinical practice, we wanted to identify the changes in ΔP and PEEP that would result when starting from usual care, not from a transiently more compliant lung. Although the short period of observation prevents us from reporting the extent to which delayed recruitment or de-recruitment may occur beyond 30 min after PEEP titration, the results are more generalizable to usual care because we chose not to utilize an initial recruitment maneuver. Third, the short-term nature of the study limits our ability to correlate clinical outcomes related to lowering ΔP. Fourth, this prospective physiological pilot study included only 10 subjects from a single center, which may limit the precision and generalizability of the results. Finally, there is no direct evidence that reducing ΔP is associated with lower mortality. Randomized clinical trials will be essential to establish whether the risks of increasing or decreasing PEEP to achieve lower ΔP will translate to improved clinical outcomes.
Conclusions
In our prospective physiological study of 10 subjects with moderate to severe ARDS, we found that reductions in ΔP could be achieved through PEEP titration, although the magnitude of ΔP reductions and the direction of PEEP change needed to reduce ΔP were variable. In almost half of our participants, ΔP was reduced by lowering PEEP from its starting level on the ARDS Network Lower PEEP/FIO2 Table. Future investigation is needed to confirm the clinical feasibility and acceptability of a ΔP targeted mechanical ventilation strategy, to identify specific goals and safety limits when targeting a low ΔP, and to determine whether patient outcomes can be improved by targeting ΔP compared to usual care ventilator strategies for patients with ARDS.
Footnotes
- Correspondence: Sarina K Sahetya MD MHS, 1830 E Monument St, Suite 503, Baltimore, MD 21287. E-mail: ssahety1{at}jhmi.edu.
Dr Sahetya presented a version of this paper at the American Thoracic Society International Conference, held May 18-23, 2018, in San Diego, California.
Dr Sahetya is supported by grants funded by the NIH NHLBI (T32HL007534) and by the Pearl M Stetler Fellowship Award.
See the Related Editorial on Page 722
- Copyright © 2020 by Daedalus Enterprises