Abstract
BACKGROUND: ARDS mortality is lower among subjects participating in randomized controlled trials (RCTs) compared to subjects in observational studies. Excluding potential subjects with inordinately high mortality risk is necessary to prevent masking the impact of potentially effective treatments. We inquired whether observed mortality differed between RCT-eligible and RCT-ineligible subjects managed with varying degrees of lung-protective ventilation in a non-research setting.
METHODS: This single-center, retrospective, observational study utilized quality assurance data for monitoring lung-protective ventilation practices based upon National Institutes of Health ARDS Network (ARDSNet) protocols. Between 2002 and 2017, 1,975 subjects meeting the 1994 consensus criteria for acute lung injury/ARDS (later reclassified by the Berlin definition) were prospectively identified and classified as RCT-eligible or RCT-ineligible on the basis of the original ARDSNet exclusion criteria for comorbidities or moribund condition. Demographic and physiologic data from the day of ARDS onset and outcome data were studied. Survival was modeled with a mixed-effect Cox proportional hazard model adjusted for age, both illness and lung injury severity plateau pressure, and formal use of the ARDSNet ventilator protocol. The primary outcome of interest was all-cause mortality during the first 90 d following onset of ARDS.
RESULTS: Day 90 mortality was 27.6% in RCT-eligible subjects versus 50.4% in RCT-ineligible subjects (hazard ratio 0.47 [95% CI 0.41–0.54], P < .001). Regardless of eligibility or ineligibility, achieving a plateau pressure ≤ 30 cm H2O was associated with lower mortality. Overall, mortality risk was lower in subjects managed by protocol versus clinician-directed lung-protective ventilation (hazard ratio 0.60 [95% CI 0.52–0.69], P < .001), even among those in whom plateau pressure was ≤ 30 cm H2O (hazard ratio 0.64 [95% CI 0.54–0.76], P < .001).
CONCLUSIONS: Mortality in non-research, RCT-eligible subjects was substantially lower compared to RCT-ineligible subjects. Managing non-research patients with ARDS by keeping plateau pressure ≤ 30 cm H2O and formal use of a lung-protective ventilation protocol significantly reduces mortality risk.
Introduction
Since publication of the seminal trial on low tidal volume (VT) ventilation by the National Institutes of Health ARDS Clinical Trials Network (ARDSNet),1 studies have reported higher mortality in the general ARDS population managed with lung-protective ventilation compared to those in randomized controlled trials (RCT).2,3 This was largely attributed to exclusion criteria used in the latter to prevent masking the effects of potential useful treatments due to subjects with exceptionally high mortality risk. In addition, rigorous adherence to treatment protocols in RCTs are speculated to enhance mortality reduction.3,4 Furthermore, delayed recognition of ARDS in observational studies along with the small fraction of screened subjects enrolled into RCTs are cited as additional factors that limit generalizing beneficial RCT results to clinical practice.3
We previously reported that adopting the ARDSNet lung-protective ventilation protocol for clinical management of ARDS significantly reduced mortality in patients meeting RCT-eligibility as well as in patients meeting RCT-ineligibility criteria compared to traditional mechanical ventilation practices.5 The current study reexamines in more detail, and with a larger sample, how mortality and other patient-centered outcomes are influenced by RCT-eligibility and ineligibility criteria.1
Quick Look
Current Knowledge
Mortality reported in the general ARDS population managed with lung-protective ventilation is higher than that reported in randomized controlled trials (RCTs) because of the need to exclude patients with comorbid conditions and excessive mortality risk. This limits the ability to generalize these findings to the ARDS population at large.
What This Paper Contributes to Our Knowledge
In a non-research setting, early identification of patients with ARDS and use of the National Institutes of Health ARDSNet ventilator protocol produced mortality rates in those who would meet RCT eligibility criteria that were similar to mortality rates reported in several ARDSNet trials. These RCT-eligible subjects had a > 50% reduction in mortality risk compared to RCT-ineligible subjects.
Methods
Population
Consecutive subjects treated at San Francisco General Hospital for acute lung injury or ARDS on the basis of the American-European Consensus Conference criteria6 (and subsequently reclassified according to the Berlin definition7) were entered into a quality assurance database used to monitor adoption of the ARDSNet ARMA ventilator protocol.1 Beginning in 2005 the PEEP/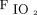 grid from the ARDSNet ALVEOLI trial protocol was incorporated as an option for 91% of our subjects managed by protocol.8 Continuous mandatory ventilation was the primary ventilator mode used when implementing these protocols. Protocolized management was at the ICU attending’s discretion and, over a 16-y period, protocol usage averaged 74%, ranging annually between 61% and 85%. Patients undergoing clinician-directed lung-protective ventilation typically received a VT of 7–8 mL/kg. By policy, VT was set according to predicted body weight and corrected for compressible volume loss in the circuit.
grid from the ARDSNet ALVEOLI trial protocol was incorporated as an option for 91% of our subjects managed by protocol.8 Continuous mandatory ventilation was the primary ventilator mode used when implementing these protocols. Protocolized management was at the ICU attending’s discretion and, over a 16-y period, protocol usage averaged 74%, ranging annually between 61% and 85%. Patients undergoing clinician-directed lung-protective ventilation typically received a VT of 7–8 mL/kg. By policy, VT was set according to predicted body weight and corrected for compressible volume loss in the circuit.
RCT Eligibility
One investigator (RHK) who was site clinical coordinator for the ARDSNet clinical trials group (1996–2007) screened and entered each subject into the database according to the primary source of lung injury, as well as sepsis as a co-diagnosis. Subjects were classified as either meeting or not meeting RCT-eligibility criteria as defined in the ARDSNet ARMA trial.1 Ineligibility criteria used for quality-assurance purposes were restricted to comorbid conditions likely to increase mortality, duration of mechanical ventilation, or ICU length of stay (LOS) (see the supplementary materials at http://www.rcjournal.com). No patients had been co-enrolled into any ongoing ARDSNet clinical trials between 2002 and 2008.
Measurements
The quality assurance database consisted primarily of information gathered from the day of ARDS onset including mechanical ventilation and gas exchange data, initial illness severity scores, use of ancillary ARDS therapies, as well as other demographic and outcome data. In the subset of 1,230 subjects managed with the ARDSNet protocol, additional ventilator data were collected ∼ 24 h after protocol initiation to assess protocol adherence.
Acute Physiology and Chronic Health Evaluation score (APACHE II),9 Simplified Acute Physiology score (SAPS II),10 and lung injury score11 were calculated on the day of ARDS onset. Ventilator systems status checks with contemporaneous arterial blood gas data were collected within 4 h after ARDS onset. Measurements included respiratory system compliance (CRS) calculated as VT divided by the difference between end-inspiratory plateau pressure and PEEP (Pplat – PEEP), which also was recorded as elastic driving pressure.12
Oxygenation was assessed both as the ratio of  to
to 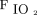 (
(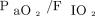 ) and as the oxygenation index, calculated as the product of mean airway pressure and the percent of inspired oxygen divided by
) and as the oxygenation index, calculated as the product of mean airway pressure and the percent of inspired oxygen divided by 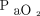 .13 Ventilation efficiency was assessed using the ventilatory ratio, calculated as
.13 Ventilation efficiency was assessed using the ventilatory ratio, calculated as 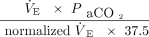 , where
, where  is minute ventilation.14
is minute ventilation.14
Temporal measurements included days from ICU admission to initiation of invasive mechanical ventilation, and from its initiation to ARDS onset. Duration of mechanical ventilation, ICU LOS, and hospital LOS from ARDS onset were calculated for survivors only. Approval to use our quality assurance data was granted by the University of California, San Francisco institutional review board (#268589).
Statistical Analysis
Statistical analysis was done using PRISM 8.2.3 (Graphpad Software, La Jolla, California) and R package 3.2-10 (Available at: https://CRAN.R-project.org/package=survival). Continuous variables were expressed as either mean ± SD or median and interquartile range (IQR) and were compared using either unpaired t test or the Mann-Whitney test. Paired comparisons were made using either paired t test or Wilcoxon sign-rank test. Categorical variables were compared using the chi square test with Yates correction. The Kruskall-Wallis test was used to compare > 2 groups.
The primary outcome of interest was all-cause mortality during the first 90 d following ARDS onset. The primary comparison was between RCT-eligible subjects and RCT-ineligible subjects. Additional prospectively planned comparisons included Pplat below or above 30 cm H2O, Berlin classes, protocol-based versus clinician-based ventilator management, and use of ancillary therapies to support gas exchange.
Actuarial survival was displayed using Kaplan-Meier plots and compared using the log rank test. Survival was modeled using a mixed-effect Cox proportional hazard model adjusted for age, APACHE II score, Berlin class, lung injury score, Pplat, ARDS etiology, concomitant sepsis, type of ICU (medical, neurologic, or surgical-trauma), number of ancillary therapies employed as fixed effect, and the year of hospitalization as random intercept. Secondary outcomes focused on the duration of mechanical ventilation and ICU and hospital LOS in survivors from the onset of ARDS. Alpha was set at 0.05.
Results
Population Characteristics
This study analyzed data from 1,975 consecutive subjects between July 2002 and December 2017. This sample comprised 1,136 (58%) RCT-eligible subjects and 839 (42%) RCT-ineligible subjects. The most frequent reasons for RCT ineligibility were acute brain injury (n = 292; 34.8%), end-stage liver disease (ie, Child’s Class C) (n = 153; 18.2%), and perceived moribund condition at the time of initial assessment (ie, apparent refractory shock not based upon pre-hoc formal criteria) (n = 124; 14.8%), of whom 35 also had end-stage liver disease. Significantly more RCT-eligible subjects received care in either the medical or surgical-trauma ICU setting, whereas significantly more RCT-ineligible subjects were managed in the neurocritical care setting and also were older (Table 1). Neither gender nor racial-ethnic background were different between groups.
Subject Characteristics on Day of ARDS Onset and Outcomes
Although RCT-eligible subjects had significantly lower APACHE II and SAPS II scores, ARDS severity at onset was not different between eligibility groups by Berlin category, lung injury score, or those with a lung injury score > 3 (ie, eligibility criteria for extracorporeal membrane oxygenation).15 RCT-eligible subjects had a higher incidence of pancreatitis, nonpulmonary sepsis, and sepsis as a co-diagnosis (Table 1).
Respiratory Mechanics and Quality of Lung-Protective Ventilation
RCT-eligible subjects had significantly higher 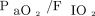 and lower oxygenation index, weight-adjusted VT, and CRS compared to RCT-ineligible subjects. However, PEEP,
and lower oxygenation index, weight-adjusted VT, and CRS compared to RCT-ineligible subjects. However, PEEP,  , mean airway pressure, Pplat, and ventilatory ratio did not differ (Table 2). Use of the ARDSNet ventilator protocol was significantly higher in the RCT-eligible group than in the RCT-ineligible group (73% vs 58.2%; P < .001). ARDSNet ventilator protocol was initiated either on the day of ARDS onset or on the following day in 92.8% of RCT-eligible subjects and 90.6% of RCT-ineligible subjects (P = .20).
, mean airway pressure, Pplat, and ventilatory ratio did not differ (Table 2). Use of the ARDSNet ventilator protocol was significantly higher in the RCT-eligible group than in the RCT-ineligible group (73% vs 58.2%; P < .001). ARDSNet ventilator protocol was initiated either on the day of ARDS onset or on the following day in 92.8% of RCT-eligible subjects and 90.6% of RCT-ineligible subjects (P = .20).
Mechanical Ventilation and Gas Exchange Variables on Day of ARDS Onset
There was a small, significant difference in PEEP among RCT eligible subjects managed with the ARDSNet protocol between the study period prior to the ALVEOLI study and the study period after the ALVEOLI study (ie, 2002–2004 vs 2005–2017): 8 (IQR 5–10) vs 10 (IQR 5–10), respectively (P < .001). A similar trend also was observed in RCT-ineligible subjects managed with the protocol: 8 (IQR 5–10) vs 10 (IQR 5–12), respectively (P = .06).
The quality of lung-protective ventilation was not different between RCT-eligible and RCT-ineligible groups in terms of achieving Pplat and VT targets (see the supplementary materials at http://www.rcjournal.com). In addition, there was no difference between groups at ARDS onset in incidents when VT, Pplat, or elastic driving pressure reached levels believed to substantially increase ventilator-induced lung injury risk (ie, VT ≥ 12 mL/kg, Pplat ≥ 35 cm H2O, and elastic driving pressure > 20 cm H2O).1,12,16,17 In both study cohorts, subjects managed by protocol experienced further significant reductions in VT, elastic driving pressure, and 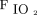 as well as increased PEEP over the first 24 h following protocol initiation (see the supplementary materials at http://www.rcjournal.com). There was no difference in the frequency or number of ancillary therapies used to support gas exchange between RCT-eligible subjects and RCT-ineligible subjects (see the supplementary materials at http://www.rcjournal.com).
as well as increased PEEP over the first 24 h following protocol initiation (see the supplementary materials at http://www.rcjournal.com). There was no difference in the frequency or number of ancillary therapies used to support gas exchange between RCT-eligible subjects and RCT-ineligible subjects (see the supplementary materials at http://www.rcjournal.com).
Primary Outcome
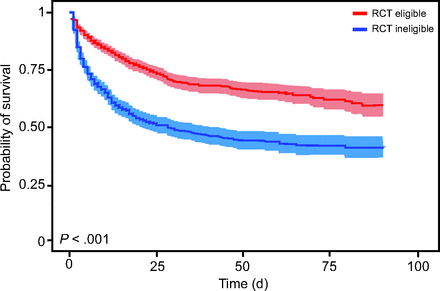
The predicted mortality based on the APACHE II scores was 40% and 55% for RCT-eligible subjects and RCT-ineligible subjects, respectively. Observed mortality was markedly lower in RCT-eligible subjects compared to RCT-ineligible subjects at day 90: 27.6% versus 50.4% (hazard ratio 0.47 [95% CI 0.41–0.54], P < .001) (Fig. 1). This was the case across both Berlin classifications and ARDS etiology, with one exception: those with pneumonia (see the supplementary materials at http://www.rcjournal.com). Among the 15% of RCT-ineligible subjects considered moribund at ARDS onset, the mortality was 83%, which was significantly greater than other RCT-ineligible subjects (44.8%, P < .001) (see the supplementary materials at http://www.rcjournal.com). Nonetheless, after excluding moribund subjects from the analysis, the mortality risk between RCT-eligible subjects and RCT-ineligible subjects remained significant (hazard ratio 0.57 [95% CI 0.49–0.67], P < .001). In addition, there was no pattern suggesting a consistent reduction in mortality over the 16-y study period (see the supplementary materials at http://www.rcjournal.com).
Kaplan-Meier plots of the probability of 90-d survival with 95% CI between patients meeting randomized controlled trial (RCT) eligibility versus ineligibility criteria.
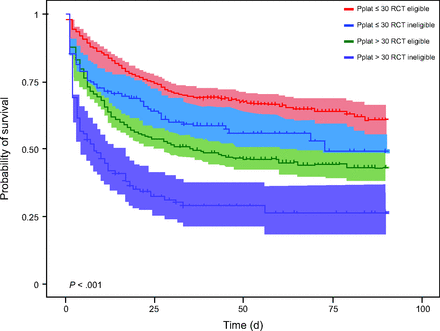
Regardless of RCT eligibility or ineligibility, achieving a Pplat ≤ 30 cm H2O was associated with lower mortality (Fig. 2). However, even when stratified by Pplat, RCT eligibility remained strongly associated with 90-d mortality. Overall, mortality risk was lower in subjects managed with protocol-driven versus clinician-driven lung-protective ventilation (hazard ratio 0.60 [95% CI 0.52–0.69], P < .001), even among subjects whose Pplat was ≤ 30 cm H2O (hazard ratio 0.64 [95% CI 0.54–0.76], P < .001). Among RCT-ineligible subjects, mortality also was significantly lower among protocol-managed subjects with Pplat ≤ 30 cm H2O versus clinician-driven management with a Pplat ≤ 30 cm H2O (hazard ratio 0.67 [95% CI 0.54–0.83], P < .001).
Kaplan-Meier plots of the probability of 90-d survival with 95% CI between subjects meeting randomized controlled trial (RCT) eligibility versus ineligibility criteria and a plateau pressure (Pplat) cutoff of 30 cm H2O.
Factors contributing to 90-d mortality in multivariate analysis included age, APACHE II score, RCT ineligibility, Berlin classification severity, and number of ancillary therapies (Table 3). In contrast, a Pplat ≤ 30 cm H2O and development of ARDS in either a surgical or neurocritical care setting were associated with decreased 90-d mortality.
Adjusted Cox Proportional Hazard Model for 90-d Mortality
Secondary Outcomes
Among survivors, mechanical ventilation duration, ICU LOS, and hospital LOS (following ARDS onset) were significantly shorter in the RCT-eligible group (Table 1). However, these differences may be attributable to the inclusion of subjects with acute brain injury, whose mechanical ventilation duration, ICU LOS, and hospital LOS were significantly longer than non-brain-injured RCT-ineligible subjects, as well as RCT-eligible subjects (see the supplementary materials at http://www.rcjournal.com). When acute brain injury subjects were removed from the analysis, there were no differences in any of these variables between other RCT-ineligible and RCT-eligible subjects.
Discussion
Our main finding was that RCT-eligible subjects managed with lung-protective ventilation had markedly lower mortality compared to RCT-ineligible subjects at day 90 despite having similar severity of acute lung injury and benefiting from similar quality of lung-protective ventilation. This remained true after excluding subjects deemed moribund because of apparent refractory shock. Consistent with other studies, our results indicate that RCT-ineligible subjects had significantly higher illness severity scores at ARDS onset. In those who survived to hospital discharge, RCT-eligible subjects had significantly fewer days of mechanical ventilation and shorter ICU LOS and hospital LOS compared to RCT-ineligible subjects. These particular findings may be explained in part by the presence of subjects with acute brain injury, who accounted for ∼ 35% of the RCT-ineligible study cohort.
Mortality was extraordinarily high among RCT-ineligible subjects in specific subsets, namely Berlin classification of severe ARDS (62%), nonpulmonary sepsis (72%), other less common etiologies (67%), end-stage liver disease (73%), and those deemed moribund (83%). In contrast, subgroups of RCT-ineligible subjects with acute brain injury, pneumonia, and trauma had lower mortality rates of 43%, 37%, and 36%, respectively, which were similar to crude mortality rates reported in the general ARDS population.18-22 Overall mortality risk among RCT-ineligible subjects was more than twice that of RCT-eligible subjects (hazard ratio 2.26 [95% CI 1.95–2.63], P < .001).
A systematic review and meta-analysis of studies between 1994 and 2006 reported significantly lower pooled mortality among subjects enrolled into RCTs versus observational studies (36.2% vs 44.0%, respectively); observational studies were associated with a substantially higher mortality risk (odds ratio 1.36 [95% CI 1.08–1.73].2 Observational study subjects included both those who would have met RCT-eligibility criteria and those who would have been ineligible for RCTs. Moreover, approximately half of the time period covered by these studies was prior to publication of the seminal ARDSNet study1 and more widespread adoption of lung-protective ventilation.
In contrast, our study sample spanned ∼ 16 y at an original ARDSNet study site that quickly adopted the ventilator protocol for clinical management,5 in which the majority of subjects (58%) were RCT-eligible. These factors likely account for our lower crude mortality rate of 37% for the entire study sample. Despite less rigid adherence to the ARDSNet protocol, mortality among our RCT-eligible subjects (with some exceptions) was similar to that reported in several ARDSNet studies, with the exception being the aerosolized albuterol trial (see the supplementary materials at http://www.rcjournal.com).1,8,23-26
The generalizability of our findings is limited because of 3 factors, some of which may be unique to our institution. First, we approach ARDS surveillance consistent with our participation as an ARDSNet clinical trials site from 1996 to 2008. This includes daily screening for early identification of at-risk patients, rapidly detecting ARDS onset, and strongly advocating use of the ARDSNet protocol. Second, our highly skilled respiratory therapists were individually trained in (and had used) the ARDSNet ventilator protocols continuously since 1996. Third, these efforts were facilitated by consistent, strong cross-disciplinary physician support for lung-protective ventilation.
Others have speculated that mortality rates between therapeutic RCTs and observational studies might be reduced by more stringent adherence to lung-protective ventilation protocols.3 Our study supports this notion. Within hours of ARDS recognition, 73% of our subjects were ventilated at a VT ≤ 8 mL/kg and 92% at VT ≤ 9 mL/kg. Pplat was universally monitored, and 85% of subjects had a Pplat ≤ 30 cm H2O within that time frame. Furthermore, 74% of subjects managed by protocol experienced additional reductions in VT, Pplat, and elastic driving pressure within 24 h of protocol initiation.
In contrast, a 50-nation observational study reported that < 67% of ARDS subjects were managed with a VT ≤ 8 mL/kg, while Pplat was monitored in < 40% with corresponding hospital mortality rates of 34.9% (95% CI 31.4–38.5%) for mild ARDS, 40.3% (95% CI 37.4–43.3%) for moderate ARDS, and 46.1% (95% CI 41.9–50.4%) for severe ARDS.18 When comparing our RCT-eligible data to multi-center RCT data used by Force et al,7 our hospital mortality was below the 95% CI for mild ARDS (19.5% vs 24–30%), moderate ARDS (24.4% vs 29–34%), and severe ARDS (38.3% vs 42–48%).7
Our study addresses some previously cited limitations of applying RCT study results to the general ARDS population, namely the prevalence of higher nonenrollment into RCTs among public hospitals caring for vulnerable populations.3 San Francisco General Hospital provides care primarily to this patient population. Early identification and enrollment of RCT-eligible subjects is another factor that limits the ability to generalize of lung-protective ventilation RCT results to the ARDS population at large.3 Following announcement of the seminal ARDSNet study results in the spring of 1999, we made a concerted effort to identify patients with ARDS quickly and to encourage implementation of the ARDSNet ventilator protocol.5
We previously reported that our formal adoption of the ARDSNet ventilator protocol (2000–2003) reduced hospital mortality compared to clinical practice (1998–1999) both in RCT-eligible (from 40% to 23%, P = .02) and RCT-ineligible subjects (from 78% to 48%, P = .031), which indicates that the ARDSNet protocol improved survival regardless of mortality risk categorization.5 Our current study extends these findings and suggests that even initiating less structured lung-protective ventilation soon after ARDS onset reduces mortality risk.
Our study is limited by our reliance upon data gathered for quality assurance purposes. That data had to be abstracted by hand necessitated practical limitations on the amount of data that could be collected (eg, our inability to collect Sequential Organ Failure Assessment scores, fluid balance, ventilator settings over an extended time period, sedative use). Therefore, our data lack much of the “granularity” that could provide greater control of important confounders and, therefore, a more refined interpretation of our results. Although our impression was that day-to-day ventilator management was reasonably constant, we have no data to support it.
Conclusions
Among subjects with ARDS who were identified early and managed primarily using the ARDSNet ventilator protocol, those who met RCT enrollment eligibility criteria had a mortality rate similar to that reported in several ARDSNet trials. Moreover, RCT-eligible subjects had a > 50% reduction in mortality risk compared to RCT-ineligible subjects.
Footnotes
- Correspondence: Richard H Kallet MSc RRT FAARC, 2070 Fell St #1, San Francisco, CA. 94117. E-mail: richkallet{at}gmail.com
See the Related Editorial on Page 1498
A version of this paper was presented by Mr Kallet at AARC Congress 2018, held December 4–7, 2018, in Las Vegas, Nevada.
Supplementary material related to this paper is available at http://www.rcjournal.com.
Mr Kallet has disclosed a relationship with Nihon Kohden. Dr Pirracchio has disclosed a relationship with General Electric HealthCare. Dr Lipnick has disclosed a relationship the US Agency for International Development.
- Copyright © 2021 by Daedalus Enterprises