Abstract
Objective
To determine the effect of inspiratory time and inspiratory flow pattern on albuterol delivery by aerosol during mechanical ventilation.
Design
A ventilator was connected to a lung model and set to deliver a tidal volume of 0.6 l, PEEP 5 cmH2O, and respiratory rate of 15/min. We evaluated inspiratory times of 1 and 2 s, lung mechanics of 0.05 l/cmH2O compliance and 50 cmH2O/l/s resistance, or 0.02 l/cmH2O compliance and 5 cmH2O/l/s resistance, and three inspiratory flow patterns (constant flow volume-controlled ventilation, descending ramp flow volume-controlled ventilation, and pressure-controlled ventilation). Albuterol was delivered into the ventilator circuit by a nebulizer containing 5 mg (4 ml) albuterol or a pMDI and spacer (four actuations; 360 µg). A filter between the Y-piece and the lung model collected the aerosol, which was analyzed for albuterol by spectrophotometry.
Results
For the nebulizer there were significant differences in albuterol delivered for inspiratory time, flow pattern, and lung mechanics. For the pMDI there were no significant differences for the amount of albuterol delivered for inspiratory time, flow pattern, or lung mechanics.
Conclusions
Albuterol delivery by nebulizer is affected by inspiratory time and inspiratory flow pattern. When a pMDI is used, the amount of albuterol delivered is not affected by inspiratory flow pattern or inspiratory time.
Similar content being viewed by others
Introduction
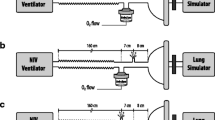
Inhaled β-agonist therapy is commonly used in mechanically ventilated patients. Either nebulizers or pressurized metered-dose inhalers (pMDI) are effective for this therapy, as has been reviewed in detail elsewhere [1, 2, 3, 4, 5]. Aerosol delivery from a small-volume nebulizer during mechanical ventilation is influenced by numerous factors and may be less efficient than delivery by pMDI [6]. The effect of inspiratory flow pattern on aerosol delivery by pMDI has been reported [7], but to our knowledge there have been no studies comparing aerosol delivery by nebulizer during pressure-controlled ventilation (PCV) and volume-controlled ventilation (VCV). Although the effect of lung mechanics and inspiratory flow rates on pulmonary aerosol deposition is well known, the effect of inspiratory flow pattern on aerosol delivery during mechanical ventilation has not been reported. Because the inspiratory flow waveforms differ for various breath delivery types available during mechanical ventilation (Fig. 1), we hypothesized that aerosol delivery by nebulizer would vary with breath delivery type. Because lung mechanics affect inspiratory flow pattern during PCV but not VCV (Fig. 2), we hypothesized that lung mechanics would affect aerosol delivery during PCV. For example, with PVC the inspiratory flow pattern depends on lung mechanics. For some combinations of lung mechanics and inspiratory time flow may decrease to zero before the end of the inspiratory phase, and no aerosol is delivered to the lungs during this zero-flow time. Because a pMDI delivers the aerosol only at the beginning of the inspiratory phase, we further hypothesized that aerosol delivery with this device would not be affected by breath delivery type. We conducted this bench study to evaluate aerosol delivery by nebulizer and pMDI using VCV and PCV.
Inspiratory flow waveforms for pressure-controlled ventilation, volume-controlled ventilation with constant inspiratory flow, and volume-controlled ventilation with a descending ramp inspiratory flow. The conditions of measurement for these waveforms were an inspiratory time of 2 s, resistance 5 cmH2O/l/s, and compliance of 20 ml/cmH2O
Methods
Lung model
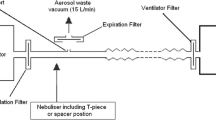
Figure 3 shows the experimental set-up. A Puritan-Bennett 7200 ventilator (Tyco, Carlsbad, Calif., USA) was used to ventilate one chamber of a lung model (Adult TTL Model 1600, Michigan Instruments, Grand Rapids, Mich.,USA). The lung model was set at one of two combinations of simulated lung mechanics: compliance of 0.05 l/cmH2O and resistance of 50 cmH2O/l/s or 0.02 l/cmH2O and 5 cmH2O/l/s. These mechanics settings were chosen to simulate two extremes of time constants; in other words, a short time constant (0.02 l/cmH2O and 5 cmH2O/l s) and a long time constant (0.05 l/cmH2O and 50 cmH2O/l/s). Compliance was set using the adjustable springs on the lung model and confirmed with a calibration syringe and calibrated manometer. Resistance to the lung model was set with a parabolic airway resistor (Pneuflo resistor Rp5 or Rp20, Michigan Instruments). An endotracheal tube was not incorporated into the model for several reasons. First, this makes the study results applicable to noninvasive as well as invasive ventilation. Second, a recent review concluded that that aerosol delivery through an endotracheal tube may approach that seen with the nonintubated patient with careful attention to technique [8].
Study design
The Puritan-Bennett 7200 was set in continuous mandatory ventilation mode with either VCV or PCV. The ventilator was adjusted to deliver a tidal volume of 0.6 l, a respiratory rate of 15/min, and a positive end-expiratory pressure (PEEP) of 5 cmH2O. Inspiratory times of 1 and 2 s were evaluated. Three inspiratory flow patterns were evaluated: constant flow VCV, descending ramp flow VCV, and PCV. The ventilator circuit (Universal Ventilator Circuit, Hudson RCI, Temecula, Calif., USA) was operated dry for all analysis. Combinations of all conditions were conducted in duplicate for nebulizer and pMDI experiments.
For nebulizer therapy a 1 ml solution of 0.5% albuterol sulfate (Warrick Pharmaceuticals, Reno, Nev., USA) was diluted with 3 ml normal saline in a small-volume jet nebulizer (Micromist, Hudson RCI). The performance characteristics of this nebulizer have been previously studied in our laboratory [9]. The nebulizer was driven with the nebulizer control of the ventilator, which is active only during the inspiratory phase. The duration of aerosol delivery by nebulizer was 30 min. The nebulizer was placed into the inspiratory circuit immediately proximal to the Y-piece and maintained in the vertical position.
For pMDI, a metered dose inhaler (Proventil, Schering, Kenilworth, N.J., USA) was used. A spacer (Aerovent, Monaghan Medical, Plattsburgh, N.Y., USA) was inserted into the inspiratory limb immediately proximal to the Y-piece. Four albuterol actuations (total 0.36 mg) were administered via the Aerovent spacer with 15 s between actuations. The pMDI was actuated immediately at the onset of inhalation. The pMDI was shaken between actuations. The metering chambers of new pMDI were primed by wasting several actuations before use in the study.
Albuterol measurement
After aerosol delivery 20 ml 0.9% saline was used to wash the aerosol collected on the filter. The filter was shaken for 1 min to ensure that the aerosol mixed well with the saline. The light absorption of the solution washed from the filter was measured with a spectrophotometer DU Series 500 (Beckman Instruments, Fullerton, Calif., USA) using a 1-ml quartz cuvette at a wavelength of 276 nm. The amount of albuterol captured on the filter was calculated from the absorption-concentration standard curve generated by plotting light absorption as a function of albuterol concentration. There was a linear relationship between absorption and concentration of albuterol between 0.005–0.05 mg/ml with a slope of 0.1426 (R 2=0.99).
We tested the ability of the filters to trap aerosol by placing two filters in series and found that there was no albuterol detected in the second filter. Additionally, we tested the specificity of our analytic technique by nebulization of saline, for which we found that there was no absorption. A known amount of albuterol was mixed in the filter with saline to determine whether all albuterol was recovered, and we found that all albuterol was detected when the filter was shaken for at least 1 min.
Statistical analysis
All data were expressed as mean ± standard deviation. Univariate analysis-of-variance was performed using albuterol delivery as the dependent variable and inspiratory time (two levels), inspiratory flow waveform (three levels), and lung mechanics (two levels) as independent variables. Major effects and two-way interactions were considered. Separate analyses were conducted for nebulizer and pMDI data. Post-hoc analysis was conducted using Scheffe's method. All statistical analysis was conducted using commercially available software (SPSS version 10.1.0, Chicago, Ill., USA). Statistical significance was set at p<0.05.
Results
Summary results for albuterol delivery by nebulizer are shown in Table 1. For the nebulizer there were significant differences for the amount of albuterol delivered to the filter between the two inspiratory times (p<0.001), the three flow patterns (p=0.03), and the two lung mechanics (p<0.001). There were significant interactions between inspiratory time and flow pattern (p<0.001) and lung mechanics and flow pattern (p<0.001) but no significant interaction between time and lung mechanics (p=0.49). These interaction effects are illustrated in Fig. 4. Albuterol delivery during PCV was significantly less than that delivered with constant flow VCV (p=0.03). There was no significant difference between albuterol delivered by VCV with constant flow and with descending ramp flow (p=0.46). Summary results for albuterol delivery by pMDI are shown in Table 2. For the pMDI there were no significant differences for the amount of albuterol delivered to the filter between the two inspiratory times (p=0.37), the three flow patterns (p=0.37), and the two lung mechanics (p=0.50).
Albuterol delivery with the conditions in this study. Note the significant interaction between flow pattern and inspiratory time (p<0.001), and between flow pattern and lung mechanics (p<0.001). Albuterol delivery is better for pressure controlled ventilation (PCV) with obstructive than restrictive conditions. Lengthening the inspiratory flow time increases albuterol delivery. However, lengthening flow time does not always occur with a lengthened inspiratory time. When PCV was used in the restrictive lung model, the longer inspiratory time did not increase inspiratory flow time, and albuterol delivery did not change. Albuterol delivery by pMDI is not dependent on flow pattern or inspiratory flow time. VCV-C Volume-controlled ventilation with a constant inspiratory time; VCV-R volume-controlled ventilation with a descending ramp flow pattern
Discussion
The primary finding of this study is that albuterol delivery using a nebulizer is affected by the inspiratory time and inspiratory flow pattern. During PCV lung mechanics also affect aerosol delivery by nebulizer, with a higher amount of aerosol delivered with a combination of high compliance and high resistance. When a pMDI is used, the amount of aerosol delivered is not affected by inspiratory flow pattern, inspiratory time, or lung mechanics.
A number of factors are known to affect aerosol delivery by nebulizer during mechanical ventilation. These include the position where the nebulizer is placed in the circuit [10, 11], the presence of a spacer device [11, 12], the nebulizer brand and its fill volume [11, 13], humidification of the inspired gas [14], treatment time [14], inspiratory time (duty cycle) [11, 14], intermittent vs. continuous nebulization [10], the ventilator brand [15], and the density of the carrier gas [16]. We found that longer inspiratory time increases the amount of albuterol aerosol delivered, consistent with the findings of others. We did not study the effect of other factors on aerosol delivery as this was beyond the scope of this study.
Although previous studies have shown improved aerosol delivery if the nebulizer is placed closer to the ventilator [10, 11], we placed the nebulizer near the Y-piece of the ventilator for a very practical reason. We used a circuit with heating wires (a common clinical practice) that allows the nebulizer to be inserted only near the Y-piece. For the practical purposes of this study we used a dry circuit that likely resulted in a higher aerosol delivery than would be achieved if the gas were humidified [14]. The nebulizer that we used has been shown to have a satisfactory performance in a previous study conducted in our laboratory [9]. The ventilator that we used provides a flow greater than 7.5 l/min for a duration of 30 min [15]. Moreover, it powers the nebulizer only during the inspiratory phase and cycles off when the flow decreases to 10 l/min [15].
The effect of PCV and lung mechanics on aerosol delivery by nebulizer is worthy of further discussion. During PCV inspiratory flow decreases as a function of lung mechanics [17]. If the resistance and compliance are low, the flow decreases rapidly, and depending on the inspiratory time setting there may be a period of zero flow at end-inhalation. If the resistance and compliance are high, the flow decreases slowly, and unless the inspiratory time is set very long, flow continues throughout inspiration. These characteristics of PCV explain our results. When the resistance and compliance are low, flow may decrease to 10 l/min (the flow at which the nebulizer cycles off) before end-inhalation, at which point no further aerosol is delivered. When the resistance and compliance are high, flow (and hence aerosol delivery) continues to the end of inhalation.
It has been reported that albuterol delivery by pMDI to mechanically ventilated patients with chronic obstructive pulmonary disease is not affected by inspiratory flow pattern (PCV vs. VCV) [7], the level of inspiratory flow (0.6 vs. 1.2 l/s) [18], tidal volume (0.6 l vs. 0.9 l) [19], or the addition of an end-inspiratory pause [20]. Our results support these findings. Because the pMDI delivers the dose at the onset of inhalation, it is affected less by events occurring later in the inspiratory phase. Thus a more reliable dose is delivered by pMDI than by nebulizer, consistent with clinical data supporting a more efficient albuterol delivery by pMDI than by nebulizer [6]. It is possible that the ventilator flow pattern and lung mechanics had a greater influence on albuterol delivery by nebulizer because of the lower delivered fraction. In contrast, delivery from the pMDI was probably maximal with all settings investigated in this study. For pMDI use during mechanical ventilation the inline actuator is an important determinant of the dose delivered. We used a spacer device that has been shown to be more effective than other types of inline actuators [21]. We also actuated the pMDI at the onset of inhalation, which has been shown to improve aerosol delivery from a pMDI [22]. Because a humidified gas decreases the amount of aerosol delivered from a pMDI during mechanical ventilation, our data likely overestimate in vivo delivery [23, 24].
Different from our results, Fink et al. [23] also reported changes in aerosol delivery from a pMDI with changes in inspiratory flow. As with Mouloudi et al. [18], however, we found that aerosol delivery by pMDI is not affected by the inspiratory flow setting. However, there are several important differences between our study and these. Fink et al. [23] incorporated an endotracheal tube into their model, and inspiratory flow may impact deposition in the endotracheal tube. Mouloudi et al. [18] studied albuterol response in intubated patients with chronic obstructive pulmonary disease and many factors may affect bronchodilator response in these patients.
Clinical implications
Issues related to aerosol delivery by nebulizer include contamination of the nebulizer cup [25], decreased ability of the patient to trigger the ventilator [26], and contamination of the expiratory flow sensor of the ventilator [27]. The use of a pMDI overcomes these limitations of nebulizer use during mechanical ventilation. The results of this study suggest that another advantage for the use of a pMDI during mechanical ventilation is the delivery of a consistent dose, regardless of the settings on the ventilator and the lung mechanics of the patient.
Limitations
Our study used a bench model and thus reports only aerosol delivery to the circuit outlet. Because this was an in vitro study, the results need to be confirmed clinically. We cannot comment on how much the aerosol deposits in the respiratory tract. We did not evaluate the amount of aerosol delivered through the endotracheal tube, as has been done in other in vitro studies [28]. However, our objective was not to assess aerosol delivery through the endotracheal tube and, moreover, our results are applicable to both invasive and noninvasive ventilation. Moreover, a recent review concluded that that aerosol delivery through an endotracheal tube may approach that seen with the nonintubated patient with careful attention to technique [8].
Conclusions
Albuterol delivery using a nebulizer is affected by the inspiratory time and inspiratory flow pattern. During PCV lung mechanics affect aerosol delivery by nebulizer, with a higher amount of aerosol delivered with a combination of high compliance and high resistance. When a pMDI is used, the amount of aerosol delivered is not affected by inspiratory flow pattern, inspiratory time, or lung mechanics.
References
Hess D (2000) Nebulizers: principles and performance. Respir Care 45:609–622
Dhand R, Tobin MJ (1997) Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med 156:3–10
Fink JB, Dhand R (1999) Bronchodilator therapy in mechanically ventilated patients. Respir Care 44:53–69
Dhand R, Tobin MJ (1996) Bronchodilator delivery with metered-dose inhalers in mechanically ventilated patients. Eur Respir J 9:585–595
Duarte AG, Fink JB, Dhand R (2001) Inhalation therapy during mechanical ventilation. Respir Care Clin N Am 7:233–260
Marik P, Hogan J, Krikorian J (1999) A comparison of bronchodilator therapy delivered by nebulization and metered-dose inhaler in mechanically ventilated patients. Chest 115:1653–1657
Mouloudi E, Prinianakis G, Kondili E, Georgopoulos D (2000) Bronchodilator delivery by metered dose inhaler in mechanically ventilated patients: influence of flow pattern. Eur Respir J 16:263–268
MacIntyre NR (2002) Aerosol delivery through an artificial airway. Respir Care 47:1279–1285
Hess D, Fisher D, Williams P, Pooler S, Kacmarek RM (1996) Medication nebulizer performance. Effects of diluent volume, nebulizer flow, and nebulizer brand. Chest 110:498–505
Hughes JM, Saez J (1987) Effects of nebulizer mode and position in a mechanical ventilator circuit on dose efficiency. Respir Care 32:1131–1135
O'Doherty MJ, Thomas SH, Page CJ, Treacher DF, Nunan TO (1992) Delivery of a nebulized aerosol to a lung model during mechanical ventilation. Effect of ventilator settings and nebulizer type, position, and volume of fill. Am Rev Respir Dis 146:383–388
Harvey CJ, O'Doherty MJ, Page CJ, Thomas SH, Nunan TO, Treacher DF (1995) Effect of a spacer on pulmonary aerosol deposition from a jet nebulizer during mechanical ventilation. Thorax 50:50–53
Hess D, Fisher D, Williams P, Pooler S, Kacmarek RM (1996) Medication nebulizer performance. Effects of diluent volume, nebulizer flow, and nebulizer brand. Chest 110:498–505
O'Riordan TG, Greco MJ, Perry RJ, Smaldone GC (1992) Nebulizer function during mechanical ventilation. Am Rev Respir Dis 145:1117–1122
McPeck M, O'Riordan TG, Smaldone GC (1993) Choice of mechanical ventilator: influence on nebulizer performance. Respir Care 38:887–895
Good M, Fink JB, Dhand R, Tobin MJ (2001) Improvement in aerosol delivery with helium-oxygene mixtures during mechanical ventilation. Am J Respir Crit Care Med 163:109–114
Hess DR, Medoff MD, Fessler MB (1999) Pulmonary mechanics and graphics during positive pressure ventilation. Int Anesthesiol Clin 37:15–34
Mouloudi E, Prinianakis G, Kondili E, Georgopoulos D (2001) Effect of inspiratory flow rate on beta2-agonist induced bronchodilation in mechanically ventilated COPD patients. Intensive Care Med 27:42–46
Mouloudi E, Katsanoulas K, Anastasaki M, Hoing S, Georgopoulos D (1999) Bronchodilator delivery by metered-dose inhaler in mechanically ventilated COPD patients: influence of tidal volume. Intensive Care Med 25:1215–1221
Mouloudi E, Katsanoulas K, Anastasaki M, Askitopoulou E, Georgopoulos D (1998) Bronchodilator delivery by metered-dose inhaler in mechanically ventilated COPD patients: influence of end-inspiratory pause. Eur Respir J 12:165–169
Fuller HD, Dolovich MB, Turpie FH, Newhouse MT (1994) Efficiency of bronchodilator aerosol delivery to the lungs from the metered dose inhaler in mechanically ventilated patients: a study comparing four different actuator devices. Chest 105:214–218
Diot P, Morra L, Smaldone GC (1995) Albuterol delivery in a model of mechanical ventilation: comparison of metered-dose inhaler and nebulizer efficiency. Am J Respir Crit Care Med 152:1391–1394
Fink JB, Dhand R, Duarte AG, Jenne JW, Tobin MJ (1996) Deposition of aerosol from metered-dose inhaler during mechanical ventilation: an in vitro model. Am J Respir Crit Care Med 154:382–387
Fink, JB, Dhand R, Grychowski J, Fahey PJ, Tobin MJ (1999) Reconciling in vitro and in vivo measurements of aerosol delivery from a metered-dose inhaler during mechanical ventilation and defining efficiency-enhancing factors. Am J Respir Crit Care Med 159:63–68
Craven DE, Lichtenberg DA, Goularte TA, Make BJ, McCabe WR (1984) Contaminated medication nebulizers in mechanical ventilator circuits: a source of bacterial aerosols. Am J Med 77:834–838
Beatty CD, Ritz RH, Benson MS (1989) Continuous in-line nebulizers complicate pressure support ventilation. Chest 96:1360–1363
Hess D (2002) Aerosol delivery during mechanical ventilation. Minerva Anestesiol 68:321–325
Fink JB, Dhand R (2001) Laboratory evaluation of metered-dose inhalers with models that simulate interaction with the patient. Respir Care Clin N Am 7:303–317
Author information
Authors and Affiliations
Corresponding author
Additional information
An editorial regarding this article can be found in the same issue (http://dx.doi.org/10.1007/s00134-003-1791-2)
Rights and permissions
About this article
Cite this article
Hess, D.R., Dillman, C. & Kacmarek, R.M. In vitro evaluation of aerosol bronchodilator delivery during mechanical ventilation: pressure-control vs. volume control ventilation. Intensive Care Med 29, 1145–1150 (2003). https://doi.org/10.1007/s00134-003-1792-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1792-1