Abstract
Objective
Lack of evidence that some monitoring systems can improve outcomes has raised doubts about their use in the intensive care unit (ICU). The objective of this study was to determine which monitoring techniques have been shown to improve outcomes in ICU patients.
Design
Comprehensive literature review.
Methods
We conducted a highly sensitive search, up to June 2006, in the Cochrane Central Register of Controlled Trials (CENTRAL) and MedLine, for prospective, randomized controlled trials (RCTs) conducted in adult patients in the ICU and the operating room (major surgical procedures) and focusing on the impact of monitoring on outcome.
Measurements and results
Of 4,175 potential articles, 67 evaluated the impact of monitoring in acutely ill adult patients. There were 40 studies related to hemodynamic monitoring, 17 to respiratory monitoring, and10 to neurological monitoring. Seven studies were classified in two different categories. Positive non-mortality outcomes were observed in 17 of 40 hemodynamic studies, 11 of 17 respiratory, and in all 10 neurological studies. Mortality was evaluated in 31 hemodynamic studies, but a beneficial impact was demonstrated in only 10. For respiratory monitoring, 7 studies evaluated mortality, but only 3 of them showed an improved outcome. We found no neurological monitoring studies that assessed mortality.
Conclusion
There is no broad evidence that any form of monitoring improves outcomes in the ICU and most commonly used devices have not been evaluated by RCT. This review puts into perspective the recent negative studies on the use of the pulmonary artery catheter in the acutely ill.
Similar content being viewed by others
Introduction
Monitoring techniques in the intensive care unit (ICU) are primarily used to identify disease patterns and titrate therapies. Understanding of pathophysiological processes may help limit disease progression and promote recovery, so early detection of physiological alterations to guide therapeutic interventions should improve outcomes; however, doubts have been raised about the utility of some monitoring systems, e.g., the pulmonary artery catheter (PAC) [1–3], in the ICU.
In evidence-based medicine, the randomized controlled trial (RCT) represents the ideal study design to validate an intervention; however, demonstration of utility of a monitoring system may be difficult and few monitoring systems have been evaluated by RCT. We performed a systematic review of the literature for RCTs that have evaluated the impact of monitoring systems on outcomes in critically ill patients and in perioperative patients undergoing major procedures.
Methods
We conducted a comprehensive search for all publications of prospective RCTs that focused on the impact of monitoring systems in adult critically ill patients and adult perioperative patients undergoing major procedures. A highly sensitive search strategy was carried out in the Cochrane Central Register of Controlled Trials (CENTRAL) and in MedLine using the approach shown in the electronic supplement. Eligibility assessment and data abstraction were performed independently in an unblinded standardized manner by two reviewers (G.A.O and R.L.C). Interrater reliability between the abstractors for RCT selection was evaluated using the κ statistic. Discrepancies about classification and outcome evaluation were resolved by author consensus.
A study was selected when it compared a monitored group of patients with a non-monitored control group or when the health-care team was unaware of monitoring measurements and so was unable to use them to guide therapy. We defined outcome in general, including morbidity and mortality, optimization of therapeutic strategies, complications, costs, and quality of life. We excluded animal and laboratory studies, non-randomized trials, reviews, letters, meta-analyses, guidelines or commentaries, trials comparing monitoring methods without a non-monitored control group, trials evaluating the impact of medicaments or therapies on measurements obtained by a monitoring system, and pediatric studies.
Selected studies were separated into those evaluating hemodynamic (or tissue perfusion), respiratory, neurological, or metabolic variables and, according to the results obtained for each of their outcome objectives, were classified as positive, neutral, or detrimental. Additionally, we classified studies evaluating mortality as primary or secondary outcome studies.
Results
Study selection
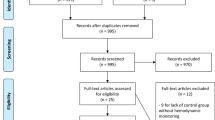
A total of 4175 articles were identified and 67 were included (Fig. 1). Interobserver agreement for selection and final classification of the studies was high: κ statistic 0.90. Seven studies [4–10], which assessed two monitoring devices in the same protocol, were enrolled in two different categories.
Hemodynamic and perfusion monitoring (Fig. 2)
Arterial pressure
No RCTs evaluating the impact of arterial pressure monitoring on outcomes when used in the ICU or operating room were identified (Table 1).
Central venous pressure
Two RCTs evaluated the impact of CVP monitoring on outcome in the ICU [8, 11]. One RCT compared conventional intraoperative fluid management with transesophageal Doppler or CVP monitoring to optimize intraoperative fluid therapy in patients with hip fracture [8]. Although in the conventional group a catheter was placed and CVP recorded by the investigator, the clinician was unaware of these measurements and so unable to use them to guide therapy. Patients in the CVP and transesophageal Doppler groups had a significantly higher intraoperative fluid balance; however, there were no differences in mortality or morbidity (p = 0.24), although fluid administration guided by CVP monitoring shortened the time before patients were fit for discharge. In the other RCT, fluid challenge targeted at keeping the CVP > 5 mmHg during renal transplant surgery resulted in a greater frequency of onset of graft function within the first three postoperative days than in a control group without CVP monitoring [11].
Pulmonary artery catheter
Sixteen studies assessed a possible influence of the PAC on ICU outcomes: seven in patients undergoing cardiac or major peripheral vascular surgery [6, 9–16]; three in high-risk surgical patients [4, 10, 17]; three in mixed-ICU populations [1, 18, 19]; two in patients with acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) [3, 20]; and one in patients with congestive cardiac failure [2]. Of 15 studies evaluating mortality as a primary or secondary outcome when a PAC was placed [1, 2–4, 6, 9, 10, 12–15, 17–20], nine used predefined hemodynamic targets [2–4, 9, 10, 14–17]; of these, only two [4, 17] demonstrated a positive effect on mortality. Mortality outcomes were not improved in studies that did not include a hemodynamic goal protocol.
In high-risk surgical patients, Shoemaker et al. [4] demonstrated lower mortality when a PAC-protocol group was compared with a PAC control group (4 vs. 33%, p < 0.01), as well as with a CVP+PAC-control group (4 vs. 28%, p < 0.02). In this study, the PAC was inserted early (preoperatively) and the PAC protocol established before the development of organ dysfunction. There were no differences in mortality between a CVP group and the PAC control group. Complications, duration of mechanical ventilation, as well as ICU and hospital stays, were less in the PAC-protocol group when compared with the CVP+PAC control group. Again in high-risk surgical patients, Sandham et al. [10] reported no significant differences in hospital mortality among groups managed with or without a PAC (7.8 vs. 7.7%) despite having a PAC-directed protocol. Pearson et al. [6] showed no significant differences in morbidity or mortality, but increased costs, in patients undergoing cardiac surgery when compared with management with a CVP catheter, but no predefined hemodynamic targets were established.
Studies on PAC monitoring in patients undergoing abdominal aortic surgery [12, 13] did not show improved complication rates, duration of intensive care, postoperative hospital stay, or mortality, but no preoperative optimization was conducted and predefined hemodynamic goals were not established. In a study by Joyce et al. [13], all the serious postoperative cardiac complications occurred in patients with impaired left ventricular function, independent of PAC use.
Preoperative hemodynamic optimization with a PAC has been evaluated in four RCTs in patients undergoing major vascular surgery [9, 14–16] and one in patients with proximal hip-fracture repair [17]. Two of these RCTs demonstrated a positive impact on ICU outcomes [14, 17]. Berlauk et al. [14] showed fewer intra- and postoperative adverse events, less early graft thrombosis, and lower mortality rates than in the control group. Likewise, Shultz et al. [17] showed a lower mortality rate in patients undergoing surgical repair of proximal hip fracture. In contrast, three RCTs [9, 15, 16] showed no improvement in morbidity, intraoperative events, ICU stay, hospital stay, or mortality when preoperative optimization was guided by a PAC.
In ALI/ARDS, Richard et al. [20] found no differences in morbidity or mortality in patients randomized to PAC or no PAC. The ARDS Network recently reported similar mortality and organ function measures in patients with ALI managed by PAC-guided compared with CVP-guided therapy, but higher complication rates [3]. In a mixed ICU population, Rhodes et al. [19] also found similar mortality rates, organ dysfunction, and ICU or hospital stays, in patients managed with or without a PAC. Likewise, Harvey et al. [1] found no significant differences in hospital mortality or length of ICU and hospital stays in patients managed with or without a PAC in a mixed ICU population. In these studies, no predefined hemodynamic goals were proposed in the PAC groups.
In patients with severe symptomatic and recurrent heart failure [2], use of a PAC did not significantly affect days alive and out-of-hospital in the first 6 months, mortality, number of days hospitalization, or quality of life.
Extravascular lung water
Eisenberg et al. [21] evaluated the impact of extravascular lung water (EVLW) monitoring to guide therapy in a mixed ICU population. In the routine management group, EVLW was measured, but results were unknown to the primary care physicians, while the protocol group used an EVLW-guided algorithm. In-hospital mortality was not significantly lower in the protocol group (54 vs. 65%). The ICU mortality was similar in all hemodynamic subsets, except for patients with an initially elevated EVLW and pulmonary artery occlusion pressure (PAOP) < 18 mmHg, where the protocol group had lower mortality rates.
Mitchell et al. [22] showed a significant decrease in ventilator days and ICU stay in critically ill patients managed with EVLW monitoring; however, differences in ICU (35 vs. 47%) and hospital (56 vs. 65%) mortality rates did not reach statistical significance.
Transesophageal echocardiography and esophageal Doppler
Six RCTs evaluated the impact of transesophageal echocardiography (TEE) or esophageal Doppler in surgical patients: two [8, 23] in patients undergoing proximal femoral fracture repair; two in patients undergoing colon surgery [24, 25]; one in patients undergoing major surgery [26]; and one in patients undergoing cardiac surgery [27]. All the studies used a protocol guided by TEE/esophageal Doppler to optimize fluid loading. A reduction in postoperative complications was reported in all the studies, with a significant reduction in in-hospital length of stay in four studies [8, 23, 26, 27]. None of the studies evaluated mortality.
Gastric tonometry
Although a number of observational studies have demonstrated that intramucosal pH (pHi) is a good indicator of morbidity [28, 29] and mortality [30], we found only five RCTs that assessed the influence of gastric tonometry on outcomes [31, 32–35]. Gutierrez et al. [31] studied 260 medical, postoperative, and trauma patients admitted to the ICU. Patients were randomized to a gastric tonometry-guided protocol or conventional treatment in which pHi values were recorded but not reported to the health-care team. In patients admitted with a low pHi, survival was similar (37 vs. 36%), whereas for those admitted with a normal pHi, it was significantly greater in the protocol than in the control group (58 vs. 42%, p < 0.01). Ivatury et al. [32] evaluated optimization of therapy in post-resuscitated trauma patients using a pHi-guided protocol: normalization of pHi in the first 24 h was associated with greater survival compared with the control group; however, in another RCT including trauma patients [35], resuscitation guided by gastric tonometry did not produce significant differences in mortality rates, ventilator days, organ dysfunction, or length of stay.
In a study by Pargger et al. [33], patients were randomized to pHi-guided therapy or control (pHi was registered but treating physicians were blinded to pHi values) after elective repair of infrarenal abdominal aneurysm. Low pHi values (< 7.32) and their persistence were predictors of major complications, but treatment aimed at correcting low pHi values did not improve postoperative outcomes. In resuscitated acutely ill adult patients admitted to the ICU, Gomersall et al. [34] showed no clinically or statistically significant differences in ICU or hospital survival, organ function, or duration of stay in an intent-to-treat analysis for patients randomized to pHi-guided therapy (pHi measurements were obtained in the control group, but not used to guide treatment).
Mixed (SvO2) and central (ScvO2) venous oxygen saturation
Only six studies have evaluated the impact of these monitoring variables on outcomes [6, 36–40].
Jastremski et al. [36] analyzed the impact and cost-effectiveness of SvO2 monitoring in medical ICU patients. There was no difference in the number of ICU days, decrease in potentially adverse hemodynamic events, or survival between the groups, although the authors still considered that SvO2 measurement could have a reasonable cost-effectiveness ratio. In a mixed population of critically ill patients, Gattinoni et al. [37] showed no differences in morbidity or mortality when comparing a normal SvO2 or supranormal cardiac index (CI) with a normal CI control group.
Three studies evaluated SvO2 monitoring in cardiac and vascular surgery patients [6, 38, 39]. Preoperative optimization of cardiovascular function using a target SvO2 > 65% did not reduce intra- or postoperative complications in patients undergoing elective peripheral vascular surgery [38]. Pearson et al. [6] found no significant differences in length of ICU stay, morbidity, or mortality among patients monitored by conventional PAC, SvO2 – PAC, or CVP; however, Polonen et al. [39] reported a reduction in hospital stay and postsurgical morbidity in cardiac surgery patients randomized to SvO2 and lactate monitoring in the first 8 postoperative hours. A post-hoc analysis showed improved survival at 6-months and 1-year after randomization in patients who achieved the target SvO2 and lactate values.
In an RCT evaluating early goal-directed therapy in the treatment of severe sepsis and septic shock, Rivers et al. [40] observed that patients randomized to a protocol group who received treatment guided by ScvO2, in addition to other variables, had less in-hospital mortality than a control group (30.5 vs. 46.5%; p < 0.009) and major improvements in organ function.
Oxygen delivery (DO2) and consumption (VO2)
We identified eight studies on DO2 and VO2 monitoring in ICU patients in which, for the control group, treating physicians were blinded to measurements or the measurements were not used to guide some form of therapy. Four studies in high-risk surgical and severe trauma patients [4, 41–43] showed that a deliberate perioperative increase in CI, DO2, and VO2 reduced mortality and complications; however, two studies in mixed ICU populations [44, 45] and another in high-risk surgical patients [10] showed no improvement in organ failure or mortality rates.
Other perfusion monitoring
We identified no RCT that evaluated the influence of other hemodynamic or perfusion monitoring techniques, including hepatic venous oxygen saturation, abdominal pressure monitoring, near-infrared spectroscopy, and mucosal laser Doppler flowmetry, on outcomes.
Respiratory monitoring (Fig. 3)
Pulse oximetry
We identified seven RCTs that evaluated outcomes with pulse oximetry: four in the operating or postoperative room [46–49], two in the ICU [5, 7], and one on a specialized, postsurgical hospital floor [50] (Table 2).
Several studies indicated that pulse oximetry could help to detect hypoxemic events [46, 47]. Moller et al. [48] demonstrated no significant differences in late cognitive dysfunction when perioperative monitoring with pulse oximetry was employed. A large RCT evaluating pulse oximetry in 20,802 surgical patients [49] reported no differences in cardiovascular, neurological, or infectious complications, or in-hospital deaths. Niehoff et al. [5] showed the potential utility of pulse oximetry and capnography in postoperative weaning from mechanical ventilation in a subset of only 24 post-cardiac surgery patients. Finally, Ochroch et al. [50] demonstrated that continuous pulse oximetry did not prevent readmissions to the ICU when cardiac and thoracic postoperative patients were monitored in a specialized postsurgical care floor.
Capnography
We identified three studies of capnography monitoring [5, 7, 51], including two also dealing with pulse oximetry monitoring [5, 7]. In the third study in this group, Helm et al. [51] used capnography to monitor ventilation in prehospital trauma victims, and found a higher proportion of patients who were “normoventilated” at hospital admission in the monitor group than in the monitor-blind group (63 vs. 20%, p < 0.001), but final in-hospital outcomes were not reported.
Respiratory mechanics
A number of studies showed that respiratory mechanics monitoring can guide ventilator adjustments as part of protective ventilation strategies in acute respiratory failure [52–56]. Amato et al. demonstrated that a protective ventilation strategy adjusting the positive end-expiratory pressure (PEEP) to above the lower inflection point (LIP) of the static pressure-volume curve and maintaining end-expiratory plateau pressure and peak-inspiratory pressures below 20 and 40 cmH2O, respectively, improved lung function in patients with ARDS [52], increased the chance of early weaning, and reduced mortality [54]. The control groups in these two studies did not consider airway pressure vigilance.
Ranieri et al. [55] reported a decreased inflammatory response in patients undergoing a protective ventilation strategy using tidal volumes of 5–8 ml/kg and PEEP adjusted above the LIP of the static pressure-volume curve. Recently, Villar et al. [56] showed a reduction in ICU mortality (53 vs. 32%), in-hospital mortality (55 vs. 34%), and ventilator-free days at day 28 (6 vs. 11) with a strategy using low-tidal volume and PEEP adjusted according to the LIP of the static pressure-volume curve.
Other studies did not show a significant difference in mortality rates associated with limited airway pressures [57–59]. An ARDS network study [60] showed that the reduction in mortality rates in patients with ARDS was related to a limitation in tidal volumes more than plateau pressures, questioning the importance of the monitoring of airway pressures.
Neurological monitoring (Fig. 4)
Intracranial pressure
We identified no RCT evaluating the effects of intracranial pressure (ICP) monitoring on outcomes in acutely ill patients.
Depth of anesthesiaand continuous electroencephalogram
No RCTs evaluating the impact of continuous depth of anesthesia and continuous electroencephalogram (EEG) monitoring devices on outcomes in the ICU were identified.
Ten studies evaluated the effects of bispectral index (BIS) monitoring during major surgical procedures, including one in patients undergoing abdominal surgery [61], two in mixed major and minor procedures [62, 63], three in cardiac surgery [64–66], two in major surgery [67, 68], one in neurosurgery [69], and one in the emergency department [70] (Table 3). All these studies showed a reduction in anesthetic recovery times and in adverse effects of the anesthetic agent.
Other neurological monitoring
We found no RCTs assessing the effects of jugular venous bulb saturation, transcranial Doppler, near-infrared spectroscopy, or cerebral microdialysis.
Metabolic monitoring
We identified no RCTs that assessed the impact of indirect calorimetry in the ICU.
Discussion
Therapy based only on bedside clinical observations is often subjective, sometimes inadequate, and potentially harmful [71]. A wide range of clinical and technological tools allows multiple variables to be assessed at the bedside in an invasive or non-invasive, continuous, or intermittent manner. As monitoring of important physiological variables can guide a number of therapeutic interventions, one could anticipate a strong body of evidence demonstrating that monitoring improves outcomes; however, our literature review illustrates that few RCTs document a positive impact of any monitoring system on outcome.
Multiple limitations can prevent the accurate study of the impact of monitoring on outcomes. Firstly, the need for the monitoring system may be so obvious that it has never been tested, or to test it would be considered unethical. This is true for direct information about so-called vital signs. An RCT on electrocardiogram monitoring in patients with acute myocardial infarction, or on arterial pressure monitoring in shock states, would not be acceptable. Sometimes, also, the benefit/risk profile of a monitoring system may clearly be very good, e.g., for pulse oximetry monitoring; however, it is interesting that even very large RCTs with pulse oximetry could not show significant differences in late cognitive dysfunction, morbidity, or mortality rates [48, 49]. In addition, monitoring devices may never have been subjected to an RCT because they evaluate variables considered to be a sound basis for therapeutic interventions. ICP monitoring is a good example of this. ICP monitoring has not been subjected to an RCT evaluating its impact on outcomes; however, the prognostic value of ICP monitoring has been clearly established [72, 73]. Some observational cohort studies in patients with severe head trauma have demonstrated favorable effects of aggressive ICP-guided treatment on mortality when compared with historic controls [72, 74] or with patients managed in hospitals that did not use this type of monitoring [75]. ICP monitoring has been shown to reveal episodes of intracranial hypertension in severe head trauma patients without tomographic signs of intracranial hypertension [73]. Likewise, observational studies have suggested that monitoring of jugular bulb oxygen saturation (SjO2) could enable early identification of transitory episodes of cerebral ischemia which would otherwise go undetected and untreated [76, 77] resulting in poorer neurological outcomes [78]. On the other hand, one could apply the same rationale to the PAC as the monitored cardiovascular variables have prognostic value, are less accessible by other techniques, and can be influenced by therapy.
Secondly, the extreme heterogeneity of the populations studied and the multiple therapeutic interventions that may be used can create excessive noise and limit study interpretation.
Thirdly, the way in which the monitoring system is used, and the accuracy with which monitoring data are collected and interpreted, can influence the final results. Monitoring systems cannot improve outcomes per se. The impact of a monitoring system will depend on its correct usage. There may also be significant variability in the interpretation of measured variables by physicians, perhaps particularly true for the PAC [79]. It is difficult to differentiate between poor technology and good technology used poorly. On some occasions, the frontier between a direct monitoring benefit and the benefit of a monitoring-guided therapy is difficult to determine. This is the case in some studies using CI, DO2, and VO2 as resuscitation end points in critically ill patients [80–86]. All these studies used a “supranormal” hemodynamic target in the protocol group, whereas the control groups were treated to achieve lesser hemodynamic goals; hence, these studies were not included in our review, although other studies on “supranormal” hemodynamic targets, which included a control group that received standard treatment (when the clinician was unaware of monitoring measurements or when no effort was made to correct the measures obtained through the monitoring device), were included [37, 41–45].
The benefits of monitoring can also be influenced by the timing of the interventions used to correct the altered measurements. Early goal-directed therapy guided by ScvO2 in the first 6 h after admission to an emergency department may improve mortality in severe sepsis and septic shock [40], but restoring SvO2 in critically ill patients may not show the same benefit when this is achieved later in the course of disease [37]. Likewise, increasing DO2 to supranormal levels may improve outcome in the early perioperative period [14, 17, 38, 39] but not later [37]; however, in these conditions, the question can be raised as to whether the clinical benefit may have been the result of more attentive and more aggressive therapy, with or without monitoring systems.
The safety and utility of invasive hemodynamic monitoring devices, including the PAC, have been debated for years. The PAC debate was highlighted in 1996 when the results of a prospective cohort study [87] suggested an increased 30-day mortality, greater intensity of care, and longer ICU stays in patients managed with a PAC. A recent European study, using the same methodology (based on a propensity score), could not reproduce these observations [88]. RCTs have not shown increased mortality or morbidity in patients managed with a PAC, except for one study that showed an increase in adverse in-hospital events attributable to higher rates of PAC infection [2]. The lack of demonstrated benefit with the use of PACs could be due to the lack of standardized therapeutic protocols for managing patients in the PAC groups. The same may or may not apply to other monitoring studies.
This literature review has its limitations. Firstly, monitoring is difficult to define; in particular, the distinction between monitoring and diagnostic techniques is often blurred. In addition, monitoring systems may not always reliably measure what they are supposed to. Secondly, one cannot define a homogenous patient population or setting where the monitoring system was assessed. As it is sometimes difficult to establish a distinction between the operating room and the ICU, we extended our review to patients undergoing major procedures, even though the data obtained may not apply fully to critically ill patients in the ICU; for example, data obtained by esophageal Doppler during major surgery [8, 23–26] may not be applicable to guiding fluid challenge in the ICU. Thirdly, due to the range of monitoring systems studied and the limited number and small size of available studies in several groups, we did not perform a meta-analysis, but results of meta-analyses for several individual monitoring systems, e.g., the PAC [89], have recently been published.
Conclusion
In conclusion, although it is often argued that monitoring systems should be subjected to objective evaluation, the value of conducting an RCT to demonstrate an impact of monitoring systems on outcomes may be questioned. Our literature review revealed that monitoring systems have not been well evaluated in RCTs, and that, of the few studies available, most have not yielded positive results. The literature search also revealed somewhat puzzling results, including the lack of evidence supporting the use of pulse oximetry, a widely accepted technique, but some evidence supporting the use of gastric tonometry, a technique which has not really stood the test of time. Finally, the PAC has been subjected to more evaluation than any other monitoring technique in acutely ill patients.
References
Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, Brampton W, Williams D, Young D, Rowan K (2005) Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet 366:472–477
Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW (2005) Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. J Am Med Assoc 294:1625–1633
Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, DeBoisblanc B, Connors AF Jr, Hite RD, Harabin AL (2006) Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 354:2213–2224
Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS (1988) Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest 94:1176–1186
Niehoff J, DelGuercio C, LaMorte W, Hughes-Grasberger SL, Heard S, Dennis R, Yeston N (1988) Efficacy of pulse oximetry and capnometry in postoperative ventilatory weaning. Crit Care Med 16:701–705
Pearson KS, Gomez MN, Moyers JR, Carter JG, Tinker JH (1989) A cost/benefit analysis of randomized invasive monitoring for patients undergoing cardiac surgery. Anesth Analg 69:336–341
Ruckoldt H, Marx G, Leuwer M, Panning B, Piepenbrock S (1998) Pulse oximetry and capnography in intensive care transportation: combined use reduces transportation risks. Anasthesiol Intensivmed Notfallmed Schmerzther 33:32–36 [in German]
Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P (2002) Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth 88:65–71
Bonazzi M, Gentile F, Biasi GM, Migliavacca S, Esposti D, Cipolla M, Marsicano M, Prampolini F, Ornaghi M, Sternjakob S, Tshomba Y (2002) Impact of perioperative haemodynamic monitoring on cardiac morbidity after major vascular surgery in low risk patients. A randomised pilot trial. Eur J Vasc Endovasc Surg 23:445–451
Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, Kirby A, Jacka M (2003) A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 348:5–14
Thomsen HS, Lokkegaard H, Munck O (1987) Influence of normal central venous pressure on onset of function in renal allografts. Scand J Urol Nephrol 21:143–145
Isaacson IJ, Lowdon JD, Berry AJ, Smith RB III, Knos GB, Weitz FI, Ryan K (1990) The value of pulmonary artery and central venous monitoring in patients undergoing abdominal aortic reconstructive surgery: a comparative study of two selected, randomized groups. J Vasc Surg 12:754–760
Joyce WP, Provan JL, Ameli FM, McEwan MM, Jelenich S, Jones DP (1990) The role of central haemodynamic monitoring in abdominal aortic surgery. A prospective randomised study. Eur J Vasc Surg 4:633–636
Berlauk JF, Abrams JH, Gilmour IJ, O'Connor SR, Knighton DR, Cerra FB (1991) Preoperative optimization of cardiovascular hemodynamics improves outcome in peripheral vascular surgery. Ann Surg 214:289–299
Bender JS, Smith-Meek MA, Jones CE (1997) Routine pulmonary artery catheterization does not reduce morbidity and mortality of elective vascular surgery: results of a prospective, randomized trial. Ann Surg 226:229–236
Valentine RJ, Duke ML, Inman MH, Grayburn PA, Hagino RT, Kakish HB, Clagett GP (1998) Effectiveness of pulmonary artery catheters in aortic surgery: a randomized trial. J Vasc Surg 27:203–211
Schultz RJ, Whitfield GF, LaMura JJ, Raciti A, Krishnamurthy S (1985) The role of physiologic monitoring in patients with fractures of the hip. J Trauma 25:309–316
Guyatt G, Ontario Intensive Care Study Group (1991) A randomized control trial of right heart catheterization in critically ill patients. J Intensive Care Med 6:91–95
Rhodes A, Cusack RJ, Newman PJ, Grounds M, Bennett D (2002) A randomised, controlled trial of the pulmonary artery catheter in critically ill patients. Intensive Care Med 28:256–264
Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F, Boyer A, Brochard L, Teboul JL (2003) Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. J Am Med Assoc 290:2713–2720
Eisenberg PR, Hansrough JR, Anderson D, Schuster DP (1987) A prospective study of lung water measurements during patient management in an intensive care unit. Am Rev Respir Dis 136:662–668
Mitchell JP, Schuller D, Calandrino FS, Schuster DP (1992) Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis 145:990–998
Sinclair S, James S, Singer M (1997) Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. Br Med J 315:909–912
Conway DH, Mayall R, Abdul-Latif MS, Gilligan S, Tackaberry C (2002) Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery. Anaesthesia 57:845–849
Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR, Fleming SC (2005) Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 95:634–642
Gan TJ, Soppitt A, Maroof M, el Moalem H, Robertson KM, Moretti E, Dwane P, Glass PS (2002) Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 97:820–826
Mythen MG, Webb AR (1995) Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg 130:423–429
Mohsenifar Z, Hay A, Hay J (1993) Gastric intramural pH as a predictor of success or failure in weaning patients from mechanical ventilation. Ann Intern Med 119:794–798
Roumen RM, Vreugde JP, Goris RJ (1994) Gastric tonometry in multiple trauma patients. J Trauma 36:313–316
Doglio GR, Pusajo JF, Egurrola MA, Bonfigli GC, Parra C, Vetere L, Hernandez MS, Fernandez S, Palizas F, Gutierrez G (1991) Gastric mucosal pH as a prognostic index of mortality in critically ill patients. Crit Care Med 19:1037–1040
Gutierrez G, Palizas F, Doglio G, Wainsztein N, Gallesio A, Pacin J, Dubin A, Schiavi E, Jorge M, Pusajo J, Klein F, San Roman E, Dorfman B, Shottlender J, Giniger R (1992) Gastric intramucosal pH as a therapeutic index of tissue oxygenation in critically ill patients. Lancet 339:195–199
Ivatury RR, Simon RJ, Islam S, Fueg A, Rohman M, Stahl WM (1996) A prospective randomized study of end points of resuscitation after major trauma: global oxygen transport indices versus organ-specific gastric mucosal pH. J Am Coll Surg 183:145–154
Pargger H, Hampl KF, Christen P, Staender S, Scheidegger D (1998) Gastric intramucosal pH-guided therapy in patients after elective repair of infrarenal abdominal aneurysms: Is it beneficial? Intensive Care Med 24:769–776
Gomersall CD, Joynt GM, Freebairn RC, Hung V, Buckley TA, Oh TE (2000) Resuscitation of critically ill patients based on the results of gastric tonometry: a prospective, randomized, controlled trial. Crit Care Med 28:607–614
Miami Trauma Clinical Trials Group (2005) Splanchnic hypoperfusion-directed therapies in trauma: a prospective, randomized trial. Am Surg 71:252–260
Jastremski MS, Chelluri L, Beney KM, Bailly RT (1989) Analysis of the effects of continuous on-line monitoring of mixed venous oxygen saturation on patient outcome and cost-effectiveness. Crit Care Med 17:148–153
Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R (1995) A trial of goal-oriented hemodynamic therapy in critically ill patients. N Engl J Med 333:1025–1032
Ziegler DW, Wright JG, Choban PS, Flancbaum L (1997) A prospective randomized trial of preoperative “optimization” of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery 122:584–592
Polonen P, Ruokonen E, Hippelainen M, Poyhonen M, Takala J (2000) A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg 90:1052–1059
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Boyd O, Grounds M, Bennett ED (1993) A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. J Am Med Assoc 270:2699–2707
Bishop MH, Shoemaker WC, Appel PL, Meade P, Ordog GJ, Wasserberger J, Wo CJ, Rimle DA, Kram HB, Umali R et al. (1995) Prospective, randomized trial of survivor values of cardiac index, oxygen delivery, and oxygen consumption as resuscitation endpoints in severe trauma. J Trauma 38:780–787
Wilson J, Woods I, Fawcett J, Whall R, Dibb W, Morris C, McManus E (1999) Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. Br Med J 318:1099–1103
Durham RM, Neunaber K, Mazuski JE, Shapiro MJ, Baue AE (1996) The use of oxygen consumption and delivery as endpoints for resuscitation in critically ill patients. J Trauma 41:32–39
Velmahos GC, Demetriades D, Shoemaker WC, Chan LS, Tatevossian R, Wo CC, Vassiliu P, Cornwell EE III, Murray JA, Roth B, Belzberg H, Asensio JA, Berne TV (2000) Endpoints of resuscitation of critically injured patients: Normal or supranormal? A prospective randomized trial. Ann Surg 232:409–418
Moller JT, Jensen PF, Johannessen NW, Espersen K (1992) Hypoxaemia is reduced by pulse oximetry monitoring in the operating theatre and in the recovery room. Br J Anaesth 68:146–150
Bierman MI, Stein KL, Snyder JV (1992) Pulse oximetry in the postoperative care of cardiac surgical patients. A randomized controlled trial. Chest 102:1367–1370
Moller JT, Svennild I, Johannessen NW, Jensen PF, Espersen K, Gravenstein JS, Cooper JB, Djernes M, Johansen SH (1993) Perioperative monitoring with pulse oximetry and late postoperative cognitive dysfunction. Br J Anaesth 71:340–347
Moller JT, Pedersen T, Rasmussen LS, Jensen PF, Pedersen BD, Ravlo O, Rasmussen NH, Espersen K, Johannessen NW, Cooper JB (1993) Randomized evaluation of pulse oximetry in 20,802 patients: I. Design, demography, pulse oximetry failure rate, and overall complication rate. Anesthesiology 78:436–444
Ochroch EA, Russell MW, Hanson WC III, Devine GA, Cucchiara AJ, Weiner MG, Schwartz SJ (2006) The impact of continuous pulse oximetry monitoring on intensive care unit admissions from a postsurgical care floor. Anesth Analg 102:868–875
Helm M, Schuster R, Hauke J, Lampl L (2003) Tight control of prehospital ventilation by capnography in major trauma victims. Br J Anaesth 90:327–332
Amato MB, Barbas CS, Medeiros DM, Schettino GP, Lorenzi FG, Kairalla RA, Deheinzelin D, Morais C, Fernandes EO, Takagaki TY (1995) Beneficial effects of the “open lung approach” with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am J Respir Crit Care Med 152:1835–1846
Carvalho CR, Barbas CS, Medeiros DM, Magaldi RB, Lorenzi FG, Kairalla RA, Deheinzelin D, Munhoz C, Kaufmann M, Ferreira M, Takagaki TY, Amato MB (1997) Temporal hemodynamic effects of permissive hypercapnia associated with ideal PEEP in ARDS. Am J Respir Crit Care Med 156:1458–1466
Amato MB, Barbas CS, Medeiro DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Ranieri VM, Suter PM, Tortorella C, Tullio R de, Dayer JM, Brienza A, Bruno F, Slutsky AS (1999) Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. J Am Med Assoc 282:54–61
Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A (2006) A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 34:1311–1318
Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, Mazer CD, McLean RF, Rogovein TS, Schouten BD, Todd TR, Slutsky AS, Pressure- and Volume-Limited Ventilation Strategy Group (1998) Evaluation of a ventilation strategy to prevent borotrauma in patients at high risk for acute respiratory distress syndrome. N Engl J Med 338:355–361
Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondejar E, Clementi E, Mancebo J, Factor P, Matamis D, Ranieri M, Blanch L, Rodi G, Mentec H, Dreyfuss D, Ferrer M, Brun-Buisson C, Tobin M, Lemaire F (1998) Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med 158:1831–1838
Brower RG, Shanholtz CB, Fessler HE, Shade DM, White P Jr, Wiener CM, Teeter JG, Dodd-o JM, Almog Y, Piantadosi S (1999) Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med 27:1492–1498
The ARDS Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Paventi S, Santevecchi A, Metta E, Annetta MG, Perilli V, Sollazzi L, Ranieri R (2001) Bispectral index monitoring in sevoflurane and remifentanil anesthesia. Analysis of drugs management and immediate recovery. Minerva Anestesiol 67:435–439
Yli-Hankala A, Vakkuri A, Annila P, Korttila K (1999) EEG bispectral index monitoring in sevoflurane or propofol anaesthesia: analysis of direct costs and immediate recovery. Acta Anaesthesiol Scand 43:545–549
Leslie K, Myles PS, Forbes A, Chan MT, Short TG, Swallow SK (2005) Recovery from bispectral index-guided anaesthesia in a large randomized controlled trial of patients at high risk of awareness. Anaesth Intensive Care 33:443–451
Puri GD, Murthy SS (2003) Bispectral index monitoring in patients undergoing cardiac surgery under cardiopulmonary bypass. Eur J Anaesthesiol 20:451–456
Bauer M, Wilhelm W, Kraemer T, Kreuer S, Brandt A, Adams HA, Hoff G, Larsen R (2004) Impact of bispectral index monitoring on stress response and propofol consumption in patients undergoing coronary artery bypass surgery. Anesthesiology 101:1096–1104
Vretzakis G, Ferdi E, Argiriadou H, Papaziogas B, Mikroulis D, Lazarides M, Bitzikas G, Bougioukas G (2005) Influence of bispectral index monitoring on decision making during cardiac anesthesia. J Clin Anesth 17:509–516
Hachero A, Alamo F, Caba F, Echevarria M, Merino S, Gomez P, Martinez A, Rodriguez R (2001) Influence of bispectral index monitoring on fentanyl requirements during total intravenous anesthesia for major gynecological surgery. Rev Esp Anestesiol Reanim 48:364–369
Myles PS, Leslie K, McNeil J, Forbes A, Chan MT (2004) Bispectral index monitoring to prevent awareness during anaesthesia: the B-Aware randomised controlled trial. Lancet 363:1757–1763
Boztug N, Bigat Z, Akyuz M, Demir S, Ertok E (2006) Does using the bispectral index (BIS) during craniotomy affect the quality of recovery? J Neurosurg Anesthesiol 18:1–4
Miner JR, Biros MH, Seigel T, Ross K (2005) The utility of the bispectral index in procedural sedation with propofol in the emergency department. Acad Emerg Med 12:190–196
Eisenberg PR, Jaffe AS, Schuster DP (1984) Clinical evaluation compared to pulmonary artery catheterization in the hemodynamic assessment of critically ill patients. Crit Care Med 12:549–553
Saul TG, Ducker TB (1982) Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J Neurosurg 56:498–503
O'Sullivan MG, Statham PF, Jones PA, Miller JD, Dearden NM, Piper IR, Anderson SI, Housley A, Andrews PJ, Midgley S (1994) Role of intracranial pressure monitoring in severely head-injured patients without signs of intracranial hypertension on initial computerized tomography. J Neurosurg 80:46–50
Stuart GG, Merry GS, Smith JA, Yelland JD (1983) Severe head injury managed without intracranial pressure monitoring. J Neurosurg 59:601–605
Colohan AR, Alves WM, Gross CR, Torner JC, Mehta VS, Tandon PN, Jane JA (1989) Head injury mortality in two centers with different emergency medical services and intensive care. J Neurosurg 71:202–207
Garlick R, Bihari D (1987) The use of intermittent and continuous recordings of jugular venous bulb oxygen saturation in the unconscious patient. Scand J Clin Lab Invest (Suppl) 188:47–52
Cruz J, Miner ME, Allen SJ, Alves WM, Gennarelli TA (1991) Continuous monitoring of cerebral oxygenation in acute brain injury: assessment of cerebral hemodynamic reserve. Neurosurgery 29:743–749
Gopinath SP, Robertson CS, Contant CF, Hayes C, Feldman Z, Narayan RK, Grossman RG (1994) Jugular venous desaturation and outcome after head injury. J Neurol Neurosurg Psychiatry 57:717–723
Iberti TJ, Fischer EP, Leibowitz AB, Panacek EA, Silverstein JH, Albertson TE (1990) A multicenter study of physician's knowledge of the pulmonary artery catheter. J Am Med Assoc 264:2928–2932
Fleming A, Bishop M, Shoemaker WC, Appel P, Sufficool W, Kuvhenguwha A, Kennedy F, Wo CJ (1992) Prospective trial of supranormal values as goals of resuscitation in severe trauma. Arch Surg 127:1175–1181
Tuchschmidt J, Fried J, Astiz M, Rackow E (1992) Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest 102:216–220
Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D (1994) Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 330:1717–1722
Yu M, Levy MM, Smith P, Takiguchi SA, Miyasaki A, Myers SA (1993) Effect of maximizing oxygen delivery on morbidity and mortality rates in critically ill patients: a prospective, randomized, controlled study. Crit Care Med 21:830–838
Ueno S, Tanabe G, Yamada H, Kusano C, Yoshidome S, Nuruki K, Yamamoto S, Aikou T (1998) Response of patients with cirrhosis who have undergone partial hepatectomy to treatment aimed at achieving supranormal oxygen delivery and consumption. Surgery 123:278–286
Alia I, Esteban A, Gordo F, Lorente JA, Diaz C, Rodriguez JA, Frutos F (1999) A randomized and controlled trial of the effect of treatment aimed at maximizing oxygen delivery in patients with severe sepsis or septic shock. Chest 115:453–461
Lobo SM, Salgado PF, Castillo VG, Borim AA, Polachini CA, Palchetti JC, Brienzi SL, de Oliveira GG (2000) Effects of maximizing oxygen delivery on morbidity and mortality in high-risk surgical patients. Crit Care Med 28:3396–3404
Connors AF, Speroff T, Dawson NV, Thomas C, Harrell FE, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ, Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus W, SUPPORT Investigators (1996) The effectiveness of right heart catheterization in the initial care of critically ill patients. J Am Med Assoc 276:889–897
Sakr Y, Vincent JL, Reinhart K, Payen D, Wiedermann CJ, Zandstra DF, Sprung CL (2005) Use of the pulmonary artery catheter is not associated with worse outcome in the intensive care unit. Chest 128:2722–2731
Shah MR, Hasselblad V, Stevenson LW, Binanay C, O'Connor CM, Sopko G, Califf RM (2005) Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. J Am Med Assoc 294:1664–1670
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ospina-Tascón, G.A., Cordioli, R.L. & Vincent, JL. What type of monitoring has been shownto improve outcomes in acutely ill patients?. Intensive Care Med 34, 800–820 (2008). https://doi.org/10.1007/s00134-007-0967-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0967-6