ABSTRACT
Objective
To evaluate current practice of mechanical ventilation in the ICU and the characteristics and outcomes of patients receiving it.
Design
Pre-planned sub-study of a multicenter, multinational cohort study (SAPS 3).
Patients
13,322 patients admitted to 299 intensive care units (ICUs) from 35 countries.
Interventions
None.
Main measurements and results
Patients were divided into three groups: no mechanical ventilation (MV), noninvasive MV (NIV), and invasive MV. More than half of the patients (53% [CI: 52.2-53.9%]) were mechanically ventilated at ICU admission. FiO2, Vt and PEEP used during invasive MV were on average 50% (40-80%), 8 mL/kg actual body weight (6.9-9.4 mL/kg) and 5 cmH2O (3-6 cmH2O), respectively. Several invMV patients (17.3% (CI:16.4-18.3%)) were ventilated with zero PEEP (ZEEP). These patients exhibited a significantly increased risk-adjusted hospital mortality, compared with patients ventilated with higher PEEP (O/E ratio 1.12 [1.05-1.18]). NIV was used in 4.2% (CI: 3.8-4.5%) of all patients and was associated with an improved risk-adjusted outcome (OR 0.79, [0.69-0.90]).
Conclusion
Ventilation mode and parameter settings for MV varied significantly across ICUs. Our results provide evidence that some ventilatory modes and settings could still be used against current evidence and recommendations. This includes ventilation with tidal volumes >8mL/kg body weight in patients with a low PaO2/FiO2 ratio and ZEEP in invMV patients. Invasive mechanical ventilation with ZEEP was associated with a worse outcome, even after controlling for severity of disease. Since our study did not document indications for MV, the association between MV settings and outcome must be viewed with caution.
Similar content being viewed by others
INTRODUCTION
Mechanical ventilation (MV) is a mainstay of supportive therapy for patients with acute respiratory failure, both to maintain adequate systemic oxygenation and to rest the respiratory muscles. After its introduction into clinical practice (1), physiological studies advanced the basic understanding of MV to the point where randomized controlled trials could be undertaken to evaluate the impact of different ventilator strategies on clinical outcome variables. Such trials usually compare a new treatment with conventional MV therapy. However, the difficulty of defining “conventional” MV has emerged because of the extreme variability in its use. Esteban et al. found that, although indications for ventilation were similar in different countries, profound differences were observed in the percentage of ventilated patients, selection of ventilatory modes, and weaning strategies (2). This marked heterogeneity has been associated with the lack of defined standards of care (2-4) and with the observed gap between the results of clinical trials and clinical practice (2–5). This may be of concern, as ventilatory management has been shown to have an important impact on prognosis (2, 4, 6–8).
A pre-planned secondary analysis of the SAPS 3 study (9) was performed aiming to describe the characteristics of patients receiving MV, the modes of mechanical ventilation and ventilator settings used and the effects of these variables on clinical outcome;
METHODS
The study represents a prospective, multinational pre-planned cohort substudy of the SAPS 3 project (9, 10). Data were collected at ICU admission (± 1 h), and on days 1, 2 and 3 after ICU admission, and in the last 24 h of the ICU stay. Data included socio-demographics, chronic conditions, diagnostic information, physiological derangement at ICU admission, number and severity of organ dysfunctions as evaluated by the Sequential Organ Failure Assessment (SOFA) score [11], and vital status at ICU and hospital discharge. SOFA scores were computed as proposed by Moreno et al. [12]. A complete description of the study protocol and patient and ICU characteristics can be found elsewhere (9).
Database
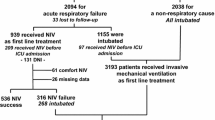
From the SAPS 3 Hospital Outcome Cohort (comprising 16,784 patients from 303 ICUs), 2,946 postoperative patients with planned ICU admission, scheduled surgery, an length of stay (LOS) in the ICU of <=2 days who were discharged alive from the ICU were excluded. Further patients with missing ventilatory support on either all days (n=219) or at admission (n=297) were excluded. Thus, the study cohort comprises of 13,322 patients from 299 ICUs. A list of countries, ICUs and patient numbers can be found in the Appendix of the ESM.
Patients were divided into three mutually exclusive subgroups according to their ventilatory status at ICU admission: patients not receiving any type of MV (noMV; n = 6,261); patients receiving positive-pressure MV via a noninvasive interface (facial, oro-nasal or nasal masks or helmet) (NIV; n = 554), and patients receiving MV via an invasive interface (endotracheal tube or tracheostomy) (invMV; n = 6,507).
Documentation of mechanical ventilation
Ventilatory settings were registered at ICU admission. Ventilatory modes registered during NIV were Continuous Positive Airway Pressure (CPAP), and Pressure Support Ventilation (PSV). Ventilatory modes registered during invMV were CPAP, PSV, Synchronized Intermittent Mandatory Ventilation (SIMV), SIMV+PSV, Pressure Control Ventilation (PCV), Assisted / Controlled Mechanical Ventilation (CMV and A/CMV). Further modalities used during NIV and invMV were captured as “others”. The exact definitions can be found in the Appendix of the ESM.
The following ventilatory settings were registered: tidal volume (Vt) (mL/kg of actual body weight), positive end-expiratory pressure (PEEP) (cm H2O) and fraction of inspired oxygen (FiO2). Unless otherwise indicated, settings from the day of ICU admission were used for analysis.
Data quality
Quality of the recorded data was evaluated for completeness of the documentation and reliability. Inter-rater quality control was performed through rescoring of the data and calculation of kappa coefficients and intra-class correlation coefficients, as appropriate (13). Results of this analysis are presented in detail in the Electronic Supplementary Material (ESM) file of the SAPS 3 primary report [9].
Statistical analysis
Statistical analysis was performed using the SAS system, version 9.1 (SAS Institute Inc., Cary, NC, USA). A P value of < 0.05 was considered significant. Unless otherwise specified, results are expressed as median and quartile ranges. The chi-square test was used for categorical data, and the Wilcoxon rank sum test or the Kruskal-Wallis test was used to compare two or more groups. Missing values (4%) were replaced by imputed values (using SAS procedure MI). For this purpose, imputations were done five times (n=13,322 each) for missing values, thus providing five datasets.
To explain which factors contributed to the use of MV, a stepwise multiple logistic regression was performed in each of the five imputed data sets. Those variables were selected for the final model, which showed a significant contribution at a two-sided level of 0.05 in all five analyses. The final estimates of the coefficients were determined by a weighted average of the coefficients found in the five imputed data sets (using SAS procedure MIANALYZE). In order to adjust for regional differences, regions were entered as fixed effects into the model. To evaluate the impact of imputation on the results, a sensitivity analysis with the complete cases dataset, excluding patients with at least one missing value in the explanatory variables, was performed.
To explain which factors contributed to the use of either NIV or invMV, a stepwise multiple logistic regression was performed in the same way as described above. The list of variables which were entered as independent variables into the models can be found in the ESM (Appendix A).
Moreover, to analyze the association between PEEP level and hospital mortality, a multivariate analysis was done using different PEEP categories as independent and hospital mortality as dependent variables. SAPS 3 was used to adjust for severity of illness.
RESULTS
The SAPS 3 ventilation cohort comprises 13,322 patients admitted to 299 participating ICUs from 35 countries. Median SAPS 3 score was 51 (41-63), and 60.2% of patients were male, with a median age of 63 (48-74) years. More than two thirds of the patients were admitted to the ICU from the same hospital, mostly from emergency departments, operating rooms, and normal wards (Table 1). Since planned surgical admission with a LOS < 2 days were excluded from the study cohort, most admissions were unplanned. Roughly half of the patients (52.7%) were admitted for medical reasons.
Use of Mechanical Ventilation
Of the 13,322 admitted patients, 53% were mechanically ventilated at admission, with the majority being invasively (48.8%), and only a small number of patients (4.2%) noninvasively ventilated. 61.5% (8,196) were mechanically ventilated during the first 3 days of intensive care, with 7.3% being managed by NIV. Of the 5,970 patients still in the ICU by day 3, still 59.1% (3,527) were ventilated. Significant differences between ICUs and regions were observed (ESM, Table E2, Figure E1). Surgical patients had a significantly higher relative risk (RR) of being mechanically ventilated at ICU admission (RR, 1.613; 95% confidence interval [95% CI], 1.546-1.684) compared with medical patients.
The main acute medical diseases responsible for ICU admission were cardiovascular, respiratory and digestive diseases (ESM Table E1). The use of MV (noninvasive or invasive) varied significantly by acute medical disease: Patients with traumatic brain injury had the highest incidence of invMV (72.1%), and pregnant women had the lowest (37.2%) (ESM Table E3). Several organ dysfunctions and failures increased the relative risk of receiving MV (Table 2). Multivariate analysis showed several variables to be associated with the use of MV (Table 3): increasing age, chronic heart failure class NYHA III or IV; basic observational care, neurologic and respiratory reasons for admission; emergency surgery; lower respiratory tract infection; hospital acquired infection; lower GCS score, increasing heart rate, increasing white blood count, decreasing pH;
Noninvasive Mechanical Ventilation
CPAP was the most used mode of NIV (69.7%), followed by PSV (28.3%). 67.3% of non-invasively ventilated patients had a medical reason for admission. Acute exacerbation of COPD was the main indication for NIV (24.7% of NIV patients). However, only a minority of COPD patients received NIV (17.4%). Whether or not hospitals were university affiliated did not seem to influence the use of NIV (4.4% vs. 3.9%; p = 0.136). Multivariate analysis showed several variables to be independently favouring the use of NIV (Table E4, ESM).
Invasive Mechanical Ventilation
In patients receiving invasive MV, A/CMV was the main mode of invasive ventilation (46.4%), followed by PCV (19.7%) and SIMV + PSV in 16.3%. PSV was used in 6.4%, SIMV in 6.3%, “other” types of invMV in 3.3% and CPAP in 1.6%. The use of invMV varied between regions (ESM Table E2, Figure E1), and also between different groups of acute medical diseases (ESM, Table E3).
FiO2, Vt and PEEP used during invasive MV were on average 50% (40-80%), 8 mL/kg body weight (6.9-9.4 mL/kg) and 5 cmH2O (3-6 cmH2O), respectively. More than half (51.6%) of patients were ventilated with a Vt ≥ 8 mL/kg body weight, and still 25% were ventilated with a Vt ≥ 9.4 mL/kg. More than a third (33.4%) with a PEEP of <5 cm H2O, with 17.3% (n=1,128) patients with ZEEP.
For reasons of analysis, patients were grouped into four quartiles of the PaO2/FiO2 ratio: 106.5 (79.3-131.4), 196.9 (175.0-219.6), 294.5 (267.5-320.0) and 430 (386.7-494.0) respectively. With decreasing PaO2/FiO2 ratio, patients were more often ventilated with a PEEP ≥ 5 cm H2O and tidal volumes <8 mL/kg (Figure 1, ESM Table E5). In patients with evidence for severe respiratory failure (lowest PaO2/FiO2 ratio quartile) this difference was statistically significant (both p<0.001). Moreover, in the subgroup of patients with ALI/ARDS (n=998), 15.9% of patients were ventilated with a PEEP < 5cm H2O and 30.5% with a Vt ≥ 8 mL/kg body weight.
Outcome
Mechanically ventilated patients exhibited a significantly increased raw mortality rate compared to noMV patients (36.4 vs. 17.5%, p<0.001). However, after risk adjustment, mortality rates were not different between the two groups (1.01 [0.99-1.04] and 1.00 [0.95-1.05], respectively). Patients assisted with NIV exhibited a significantly reduced risk-adjusted mortality compared to patients assisted with invMV (0.79 [0.69-0.90] vs. 1.03 [1.00-1.06, respectively]. The excluded postsurgical patients (n=2,946) exhibited a hospital mortality of 2.5%.
Hospital mortality was not different whether Vt was higher or lower than 8mL/kg in the whole cohort (35.7 vs. 37.4%; n.s., O/E ratio 1.01 (0.97-1.05) and 1.02 (0.97-1.06), respectively). However, in the least hypoxemic patients a Vt ≥ 8mL/kg was associated with a trend for an increased risk of death, (relative risk 1.08, (95% CI: 0.98 - 1.19).
There was no difference in mortality rates whether PEEP was higher or lower than 5 cm H2O in the whole cohort (36.3 vs. 35.4%, O/E ratio 0.99 (0.96-1.02) and 1.03 (0.98-1.08), respectively) or in the different PaO2/FiO2 quartiles. However, in invMV patients, ZEEP was associated with significantly increased odds to die in the hospital (OR 1.29 [1.08-1.53], ESM Table E7). This resulted also in a significantly increased risk-adjusted mortality for these patients (O/E ratio 1.12 [1.05-1.18], ESM Figure E2].
Sensitivity analysis revealed that that some influence factors which were significant in the imputed data set did not reach statistical significance within the complete cases data set (data not shown).
DISCUSSION
This study is, to the best of our knowledge, one of the largest ever performed on the epidemiology of mechanical ventilation in the ICU. Most of the previous studies involved specific patient typologies (e.g., acute lung injury, acute respiratory distress syndrome or COPD) or were done in fewer centres. On the other hand, this study used a high-quality multinational database that, although not primarily designed for the assessment of the epidemiology of mechanical ventilation, may in fact reflect the heterogeneity of current ICU case mix and typology from 299 ICUs in 35 countries all over the world.
We found that mechanical ventilation is one of the most common interventions employed in intensive care: More than half of the patients were ventilated in the first 24 h after ICU admission, a value greater than that described in some studies (2, 3, 14, 15) but similar to that in others (16, 17). Regarding this figure, it should be noted that 2946 post-surgical patients were excluded, since they represent a cohort that needs rather intermediate then intensive care. The fact that these patients exhibited an extremely low hospital mortality of 2.5% supports this.
Despite the widespread recommendation for the use of NIV in patients with acute exacerbation of COPD and with cardiogenic pulmonary edema, immuno-suppression and specific postoperative conditions (18), our study shows that NIV was used only in a minority of ventilated patients (4.2% at admissi\\\on, 7.3% during the first three days). These values are lower than the 11% reported by Esteban et al. in a worldwide cohort (15) or the 23% of the French survey (17). Consistently we found that use of NIV varied significantly with ICU type and region. The fact, that patients assisted with NIV exhibited a better outcome than invasively ventilated patients confirms the need for a careful selection of possible candidates for NIV (18, 19, 20).
As reported by others, A/CMV was the main invasive ventilatory mode, (3, 15, 19). Our data confirm the widespread use of SIMV as an isolated ventilatory mode. SIMV was used in 6.3% of invasively ventilated patients, which is similar to the 5.8% reported by Esteban et al. in 2000 (3) (see also ESM, Figure E1 + E2).
Ventilation with high Vt has been repeatedly linked to lung injury in ALI/ARDS (4, 24-26) and in normal lungs (6, 7, 27). In our cohort, setting of Vt varied significantly: more than half of invMV patients were ventilated with a Vt ≥ 8 mL/kg actual body weight. Moreover, a third of patients admitted due to ALI/ARDS were ventilated with such a setting. This finding is consistent with data from previous studies (21-23) and might be cause for concern. Especially if we consider that actual body weight usually exceeds predicted body weight by 20% [4], but ventilator settings should rather use the latter one. Unfortunately, there were too many missing data on patients’ height to calculate predicted body weight in our cohort. This means that our median Vt of 8.0 mL/kg of actual body weight might even underestimate the really used Vt – which could be as high as 9.6 mL/kg of predicted body weight, a value similar to that described in previous series [28].
Patients with the lowest PaO2/FiO2 ratios were ventilated more often with tidal volumes <8mL/kg body weight, than were patients with higher PaO2/FiO2 ratios (Figure 1, white bars). This is consistent with results from previous studies showing that lower values of Vt were used in patients with a higher severity of lung injury (21, 23). However, it should be noticed that in our cohort still 45.8% of these patients were ventilated with tidal volumes ≥8 mL/kg. Since a low PaO2/FiO2 might be indicative for ALI, this seems to be at least a reason for concern. Correspondingly, higher PEEP values were used in these patients (Figure 1, grey bars). Again it should be noticed, that still approximately a third of patients with the lowest PaO2/FiO2 ratio (27.8%) and 15.9% of patients with the admission diagnosis ALI/ARDS were ventilated with PEEP values <5 cm H2O (Figure 1, ESM Table E5).
A substantial portion of invasively ventilated patients was managed without any PEEP at all. Invasive mechanical ventilation with ZEEP was independently associated with mortality (ESM Table E6, Figure 2). It is well known that PEEP may prevent alveolar de-recruitment associated with lower Vt, thus reducing shear stress and also reduce the impact of denitrogenation atelectasis (observed in patients ventilated with high oxygen concentrations) (29-32). Moreover, Ferguson et al. (23) have shown an association between low values of PEEP and mortality in ARDS patients. Although an independent association between ZEEP and higher mortality has not been consistently found in all patient typologies, a large amount of data exists which supports this finding (4, 22, 24, 25, 33-37).
Our study has obviously some limitations: First, data on the use of MV were limited to the first 3 days of the ICU stay. Second, due to the epidemiologic nature of our study, no data concerning ventilatory mechanics or specific morbidity potentially associated with MV, such as barotrauma or infection are available. Moreover, data about the eventual use of MV before study enrolment were not available. Also, data about indications for MV were not available. For this reason we cannot fully exclude a potential bias which might confound the association between MV settings and outcome.
With respect to the use of NIV we cannot present data on the use of NIV before ICU admission, nor about those patients who were failing on NIV and therefore later during the ICU stay switched to invMV. Thus, the improved outcome (although risk-adjusted) must be viewed with caution and needs further prospective studies.
Multiple imputations to compensate for missing values were performed in cases of missing values (which made up 4%). However, differences were found between the complete cases data set and the imputed data sets in a sense that some influence factors in the imputed data set did not reach statistical significance within the complete cases data set. This may be explained by the much smaller sample size of the complete cases dataset (n= 7,.050) and is furthersupported by the observation, that most of the non-significant odds ratios in the complete cases data set were further away from one than the corresponding significant odds ratios in the imputed data set. For this reason we decided to report only the imputed values here.
In conclusion, MV is one of the most common interventions employed in the intensive care unit, with more than half of the patients being mechanically ventilated. However, the use of MV is still variable and possibly also a reason for concern: Several patients with evidence for acute lung injury (low PaO2/FiO2 ratios) were ventilated with tidal volumes >8 mL/kg actual body weight. Moreover, ZEEP was found to be used in a high percentage of invasively ventilated patients and this was associated with a worse outcome, even after controlling for severity of disease.
References
Ibsen B (1954) The anaesthetist's viewpoint on the treatment of respiratory complications in poliomyelitis during the epidemic in copenhagen, 1952. Proc R Soc Med 47:72-74
Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguía C, Nightingale P, Arroliga AC, Tobin MJ; Mechanical Ventilation International Study Group (2002) Characteristics and outcomes in adult patients receiving mechanical ventilation: A 28-day international study. JAMA 287:345-355
Esteban A, Anzueto A, Alia I, Gordo F, Apezteguia C, Palizas F, Cide D, Goldwaser R, Soto L, Bugedo G, Rodrigo C, Pimentel J, Raimondi G, Tobin MJ (2000) How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 161:1450-1458
The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med 342:1301-1308
Eichacker PQ, Gerstenberger EP, Banks SM, Cui X, Natanson C (2002) Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. Am J Respir Crit Care Med 166:1510-1514
Gajic O, Frutos-Vivar F, Esteban A, Hubmayr RD, Anzueto A (2005) Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med 31:922-926
Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, Rana R, St Sauver JL, Lymp JF, Afessa B, Hubmayr RD (2004) Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical venti-lation. Crit Care Med 32:1817-1824
Mascia L, Zavala E, Bosma K, Pasero D, Decaroli D, Andrews P, Isnardi D, Davi A, Arguis MJ, Berardino M, Ducati A; Brain IT group (2007) High tidal volume is associated with the development of acute lung injury after severe brain injury: An international observational study. Crit Care Med 35:1815-1820
Metnitz PG, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR; SAPS 3 Investigators (2005) SAPS 3. From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med 31:1336-1344
Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR; SAPS 3 Investigators (2005) SAPS 3. From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at icu admission. Intensive Care Med 31:1345-1355
Vincent J-L, Moreno R, Takala J, Willats S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The sofa (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med 22:707-710
Moreno R, Vincent J-L, Matos R, Mendonça A, Cantraine F, Thijs L, Takala J, Sprung C, Antonelli M, Bruining H, Willatts S (1999) The use of maximum sofa score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Intensive Care Med 25:686-696
Kramer MS, Feinstein AR (1981) Clinical biostatistics. Liv. The biostatistics of concordance. Clin Pharmacol Ther 29:111-123
Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand J-A, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F; ALIVE Study Group (2004) Epidemiology and outcome of acute lung injury in european intensive care units. Results from the alive study. Intensive Care Med 30:51-61
Esteban A, Ferguson ND, Meade MO, Frutos-Vivar F, Apezteguia C, Brochard L, Raymondos K, Nin N, Hurtado J, Tomicic V, González M, Elizalde J, Nightingale P, Abroug F, Pelosi P, Arabi Y, Moreno R, Jibaja M, D'Empaire G, Sandi F, Matamis D, Montañez AM, Anzueto A; VENTILA Group (2008) Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med 177:170-177
Carlucci A, Richard J-C, Wysocki M, E. L, Brochard L, and the SRLF Col-laborative Group on Mechanical Ventilation (2001) Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med 163:874-880
Demoule A, Girou E, Richard JC, Taille S, Brochard L (2006) Increased use of noninvasive ventilation in french intensive care units. Intensive Care Med 32:1747-1755
Evans TW (2001) International consensus conferences in intensive care medicine: Noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 163:283-291
Maheshwari V, Paioli D, Rothaar R, Hill NS (2006) Utilization of noninvasive ventilation in acute care hospitals: A regional survey. Chest 129:1226-1233
Burns KE, Sinuff T, Adhikari NK, Meade MO, Heels-Ansdell D, Martin CM, Cook DJ (2005) Bilevel noninvasive positive pressure ventilation for acute respiratory failure: Survey of ontario practice. Crit Care Med 33:1477-1483
Weinert CR, Gross CR, Marinelli WA (2003) Impact of randomized trials results on acute lung injury ventilator therapy in teaching hospitals. Am J Respir Crit Care Med 167:1304-1309
Wongsurakiat P, Pierson DJ, Rubenfeld GD (2004) Changing pattern of ventilator settings in patients without acute lung injury: Changes over 11 years in a single institution. Chest 126:1281-1291
Ferguson ND, Frutos-Vivar F, Esteban A, Anzueto A, Alía I, Brower RG, Stewart TE, Apezteguía C, González M, Soto L, Abroug F, Brochard L; Me-chanical Ventilation International Study Group (2005) Airway pressures, tidal volumes, and mortality in patients with acute respiratory distress syndrome. Crit Care Med 33:21-30
Amato MB, Barbas CS, Medeiros DM, Schettino GD, Lorenzi Filho G, Kairalla RA, Deheinzelin D, Morais C, Fernandes ED, Takagaki TY (1995) Beneficial effects of the “Open lung approach” With low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am J Respir Crit Care Med 152:1835–1846
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS (1999) Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: A randomized controlled trial. JAMA 282:54–61
Fernández-Pérez ER, Keegan MT, Brown DR, Hubmayr RD, Gajic O (2006) Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomy. Anesthesiology 105:14-18
Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, Gasparetto A, Lemaire F (1994) Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med 150:896-903
Ranieri VM, Mascia L, Fiore T, Bruno F, Brienza A, Giuliani R (1995) Cardiorespiratory effects of positive end-expiratory pressure during progressive tidal volume reduction (permissive hypercapnia) in patients with acute respira-tory distress syndrome. Anesthesiology 83:710-720
Cereda M, Foti G, Musch G, Sparacino ME, Pesenti A (1996) Positive end-expiratory pressure prevents the loss of respiratory compliance during low tidal volume ventilation in acute lung injury patients. Chest 109:480-485
Richard JC, Maggiore SM, Jonson B, Mancebo J, Lemaire F, Brochard L (2001) Influence of tidal volume on alveolar recruitment. Respective role of peep and a recruitment maneuver. Am J Respir Crit Care Med 163:1609-1613.
Richard JC, Brochard L, Vandelet P, Breton L, Maggiore SM, Jonson B, Clabault K, Leroy J, Bonmarchand G (2003) Respective effects of end-expiratory and end-inspiratory pressures on alveolar recruitment in acute lung injury. Crit Care Med 31:89-92
Webb HH, Tierney DF (1974) Experimental pulmonary edema due to in-termittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am J Respir Crit Care Med 110:556-565
Muscedere JG, Mullen JB, Gan K, Slutsky AS (1994) Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149:1327-1334
Corbridge TC, Wood LD, Crawford GP, Chudoba MJ, Yanos J, Sznajder JI (1990) Adverse effects of large tidal volume and low peep in canine acid aspi-ration. Am J Respir Crit Care Med 142:311-315
Stewart TE (2002) Controversies around lung protective mechanical ven-tilation. Am J Respir Crit Care Med 166:1421-1422
de Durante G, del Turco M, Rustichini L, Cosimini P, Giunta F, Hudson LD, Slutsky AS, Ranieri VM (2002) ARDSnet lower tidal volume ventilatory strategy may generate intrinsic positive end-expiratory pressure in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 165:1271-1274
Acknowledgements
Statistical analysis was supported by a grant from the Fund of the Austrian National Bank, Project # 12690 ONB.
Our thanks go to the participants from all over the world, who dedicated a significant amount of their time and effort to this project, proving that it is still possible to conduct a worldwide academic study. A list of these centres can be found in the ESM file of the original SAPS 3 report (9).
Authors Contributions:
Study concept and design: PM, RM, VMR
Acquisition of data: SAPS 3 Participants
Analysis and interpretation of data: BM, PB, PM, RM, VMR
Drafting of the manuscript: PM, RM, VMR
Critical revision of the manuscript: All authors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Statistical analysis was supported by a grant from the Fund of the Austrian National Bank, Project # 12690 ONB.
This article is discussed in the editorial available at: doi:10.1007/s00134-009-1450-3.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Metnitz, P.G.H., Metnitz, B., Moreno, R.P. et al. Epidemiology of Mechanical Ventilation: Analysis of the SAPS 3 Database. Intensive Care Med 35, 816–825 (2009). https://doi.org/10.1007/s00134-009-1449-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1449-9