Abstract
Purpose
To evaluate whether extracorporeal carbon dioxide removal by means of a pumpless extracorporeal lung-assist (PECLA) device could be an effective and safe alternative to invasive mechanical ventilation in patients with chronic pulmonary disease and acute hypercapnic ventilatory failure not responding to noninvasive ventilation (NIV).
Methods
In this multicentre, retrospective study, 21 PECLA patients were compared with respect to survival and procedural outcomes to 21 matched controls with conventional invasive mechanical ventilation. Matching criteria were underlying diagnosis, age, Simplified Acute Physiology Score II and pH at ICU admission.
Results
Of the 21 patients treated with PECLA, 19 (90 %) did not require intubation. Median PaCO2 levels and pH in arterial blood prior to PECLA were 84.0 mmHg (54.2–131.0) and 7.28 (7.10–7.41), respectively. Within 24 h, median PaCO2 levels and pH had significantly improved to 52.1 (33.0–70.1; p < 0.001) and 7.44 (7.27–7.56; p < 0.001), respectively. Two major and seven minor bleeding complications related to the device occurred. Further complications were one pseudoaneurysm and one heparin-induced thrombocytopenia type 2. Compared to the matched control group, there was a trend toward a shorter hospital length of stay in the PECLA group (adjusted p = 0.056). There was no group difference in the 28-day (24 % vs. 19 %, adjusted p = 0.845) or 6-month mortality (33 % vs. 33 %).
Conclusions
In this study the use of extracorporeal carbon dioxide removal allowed avoiding intubation and invasive mechanical ventilation in the majority of patients with acute on chronic respiratory failure not responding to NIV. Compared to conventional invasive ventilation, short- and long-term survivals were similar.
Similar content being viewed by others
Introduction
Endotracheal intubation and subsequent mechanical ventilation are often a necessary and life-saving treatment for patients with severe respiratory failure. However, the side effects of the endotracheal tube, the invasive mechanical ventilation itself and the accompanying analgosedation may trigger a vicious cycle leading to prolonged weaning and may even contribute to mortality [1, 2]. The main underlying pathophysiological mechanisms are ventilator-associated pneumonia (VAP) and ventilator-induced lung injury (VILI) as well as a range of neurological disorders associated with prolonged analgosedation [3–5].
Despite the evidence that lung-protective ventilation can reduce the degree of lung injury and in turn morbidity and mortality in patients with acute respiratory distress syndrome (ARDS), avoiding invasive mechanical ventilation whenever possible is essential [6].
In selected populations, especially in patients with acute hypercapnic respiratory failure, noninvasive ventilation (NIV) is a well-established means to support the failing ventilatory pump and thus to avoid intubation and invasive mechanical ventilation [7, 8]. However, this approach often fails for a variety of reasons and is therefore followed by intubation and invasive mechanical ventilation. The prognosis of these patients depends on the severity of the chronic underlying respiratory disease. For example intubated patients with cystic fibrosis (CF) or advanced chronic obstructive pulmonary disease (COPD) have a poor prognosis [9, 10].
Extracorporeal technologies such as extracorporeal membrane oxygenation (ECMO) have been used for more than 30 years in patients with severe, life-threatening respiratory failure to improve gas exchange [11, 12]. However, its use is restricted to highly specialised centres because of invasiveness, complexity and costliness. More recently, minimally invasive extracorporeal devices for selective carbon dioxide removal have become available. So far, these have been used and studied in intubated and mechanically ventilated patients with ARDS to allow for more lung-protective ventilation [13–16].
To our knowledge, no study has yet been published that evaluates the use of a device for selective extracorporeal carbon dioxide removal in awake patients with acute hypercapnic respiratory failure in order to avoid endotracheal intubation. Therefore, the purpose of this matched case control study was to compare the feasibility, effectiveness and safety of a pumpless extracorporeal lung assist (PECLA) with conventional mechanical ventilation in patients with acute hypercapnic respiratory failure unresponsive to noninvasive ventilation.
Methods
Study design
This observational study was conducted in four tertiary-level hospitals in Germany, comprising three university hospitals [Department of Intensive Care Medicine, University Medical Center Hamburg-Eppendorf; Department of Internal Medicine, Infectious Diseases and Respiratory Medicine, Charité-Universitätsmedizin Berlin; Department of Medicine III, University of Halle (Saale)] and one, large university-affiliated hospital (Department of Cardiology and Intensive Care, Klinikum Bogenhausen, Munich). All centres have an established record of treating critically ill patients with acute respiratory insufficiency and have considerable experience with NIV and extracorporeal lung-assist devices. The data were analysed retrospectively. The institutional review boards of all participating centres approved anonymised data collection and analyses. All patients or their legal representatives had given informed consent to treatment with the PECLA at the time of intervention.
PECLA patients
All non-intubated patients who were treated with PECLA for acute hypercapnic respiratory failure between 1 January 2007 and 31 December 2010 were included. All patients initially received standard treatment including NIV, antibiotic and bronchodilator therapy, nutritional support and physiotherapy, according to international guidelines [17, 18]. Criteria for initiation of NIV were respiratory acidosis (pH < 7.35) and/or clinical signs of ventilatory pump failure in patients with chronic pulmonary disease. Criteria for failure of NIV and intubation were (1) worsening respiratory acidosis, (2) worsening oxygenation, (3) increasing respiratory rate, and (4) clinical signs suggestive of respiratory muscle fatigue and/or increased work of breathing. The decision to use a PECLA in these patients was always made by at least two senior intensivists. These procedures were applied in patients with potentially reversible respiratory failure when endotracheal intubation carried a high risk of secondary complications because of prolonged invasive mechanical ventilation.
Pumpless extracorporeal lung assist (PECLA)
The device used for extracorporeal carbon dioxide removal was a pumpless, percutaneous extracorporeal lung assist (interventional lung assist; iLA®, Novalung GmbH, Heilbronn, Germany). In brief, the cannulas are inserted under local anaesthesia without sedation in the femoral artery and vein by means of the Seldinger technique. Technical details and procedures are presented in the electronic supplementary material and have been described previously [13, 14]. For weaning of the device the sweep gas flow is reduced stepwise under close monitoring of blood gases and respiration of the patient. Finally, when a sweep gas flow of only 1 l/min is reached and respiratory parameters remain stable, the cannulas are removed manually, followed by compression of the insertion site. All patients received anticoagulation with heparin to achieve a mild prolongation of the activated partial thromboplastin time (45–55 s).
Controls
Controls were patients who had been admitted with acute hypercapnic respiratory failure and were treated with invasive mechanical ventilation after failing NIV. All controls were selected from a large database of patients who had been admitted during the study period to the Department of Intensive Care Medicine at the University Medical Center Hamburg-Eppendorf. The Department consists of ten intensive care units with 120 beds and serves all specialties of adult intensive care medicine. We compared the PECLA patients with cases matched 1:1 based on the following criteria: (1) underlying diagnosis; (2) age ± 10 years; (3) simplified acute physiology score (SAPS) II, assessed within the first 24 h after ICU admission, ± 6 points; (4) pH ± 0.05 before PECLA or intubation. If more than one match was available, a random selection was done. All control patients received analgosedation with continuous infusion of propofol and sufentanil, and underwent daily awakening and spontaneous breathing trials for ventilatory weaning according to the centre’s clinical protocols.
Data collection
All data were routine clinical data, retrospectively collected and anonymised from medical records. Aspects covered were: demographics, admission diagnoses, discharge diagnoses, length of stay in the intensive care unit (ICU) and hospital, diagnostic procedures and treatment, reason for and duration of noninvasive or invasive mechanical ventilation, ventilator settings, vital signs, laboratory values, and arterial blood gases before and after initiation of the PECLA. Survival data were obtained from records and/or telephone follow-up. Adverse events associated with the PECLA were also recorded.
Statistical Analysis
The software used for analyses of data was SigmaPlot for Windows, version 11.0 (SyStat Software, Inc., Chicago, IL, USA), SPSS 18.0 (IBM® SPSS® Statistics Version 19) or STATA 12.0 (StataCorp, College Station, TX, USA). To increase the statistical robustness with regards to the relatively small numbers of cases all quantitative findings are presented as medians (with range), irrespective of scale and distribution. Pre-post PECLA changes in the three main ventilatory physiological parameters arterial partial pressure of carbon dioxide (PaCO2), arterial pH (pH) and respiratory rate (RR) were tested for significant differences between two predefined time intervals: before and 21–24 h after implementation of PECLA. Exact Wilcoxon rank-sum tests were used for non-parametric changes with time and comparisons of matched samples. In order to adjust for residual baseline differences and to increase the power of the analyses, outcomes were additionally analysed applying mixed logistic regression models with baseline covariates as fixed and pairs as random effects or Cox regression models with baseline covariates as regressors and with pairs as shared frailties. Two-sided p < 0.05 values were considered significant.
Results
Baseline patient characteristics
Between 1 January 2007 and 31 December 2010, a total of 21 non-intubated patients received a PECLA. All patients had hypercapnic ventilatory pump failure and were started on NIV after admission to the ICU. At the time of implementation of the PECLA, all patients had failed NIV and had a clear indication for endotracheal intubation. The reasons for NIV failure were variable and in some cases multiple. In 18 cases (86 %), hypercapnia was refractory to NIV despite optimising noninvasive ventilatory settings; four patients (19 %) did not tolerate NIV and became progressively noncompliant; in 11 patients (52 %) clinical signs of respiratory muscle fatigue set in despite continuous and prolonged NIV. The median score on the Glasgow Coma Scale (GCS) on ICU admission was 14 (5–15); this fell to 11 (5–15) before initiation of the PECLA. The main cause of respiratory insufficiency refractory to NIV was acute exacerbation of severe COPD (n = 14). Figure 1 shows a flowchart of all patients admitted to the ICU at the University Medical Center Hamburg-Eppendorf with this primary diagnosis. Nine of the 21 patients (43 %) were listed for and awaiting lung transplant. Two patients (10 %) had expressed a clear desire not to be intubated under any circumstances. These 21 patients were matched with 21 controls. Complete matching was possible for all predefined criteria. The matching variables and baseline patient characteristics are shown in Table 1; a detailed list of all comorbidities is presented in the electronic supplementary material (Table 1 ESM).
Flowchart of all patients admitted to the Department of Intensive Care Medicine at the University Medical Center Hamburg-Eppendorf 2007-2010 with acute exacerbation of severe chronic obstructive pulmonary disease (AECOPD). In three (5 %) patients with NIV failure, PECLA was considered but was not possible because of peripheral arterial disease. NIV = noninvasive ventilation, PECLA = pumpless extracorporeal lung assist
Intubation rate in the PECLA group
Nineteen out of 21 patients (90 %) did not require intubation for respiratory failure after initiation of the PECLA. Of the two intubated patients (10 %), one patient with an acute exacerbation of COPD developed upper airway obstruction with subsequent hypoxaemia on the first day on PECLA. She subsequently developed multiorgan failure and died on the ventilator 21 days after ICU admission. The second patient had received a lung transplant 19 years earlier and was admitted with pneumonia under immunosuppression. He required intubation after the PECLA cannulas had to be removed because of major local bleeding on day 5. He then developed multiorgan failure and died on the ventilator 52 days after ICU admission.
Clinical course and outcomes
The median duration of PECLA support and mechanical ventilation in the PECLA group was 9 days (range 1–116); the median duration of mechanical ventilation in the control group was 21 days (range 1–47; p = 0.944). Table 2 in the electronic supplementary material gives technical details regarding the PECLA cannulas and number of membrane replacements.
Tracheostomy rates differed significantly (p = 0.004) between the PECLA group (10 %) and the control group (67 %). Patients with PECLA had a shorter median ICU (15 vs. 30 days) and hospital stay (23 vs. 42 days) than the patients with invasive mechanical ventilation. There was a trend towards a shorter length of hospital stay, but this did not reach statistical significance (adjusted p = 0.056). There was no difference in 28-day (24 % vs. 19 %) or 6-month mortality (33 % vs. 33 %) between the two groups (Table 2).
Physiological changes post PECLA
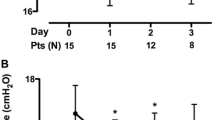
Median values for PaCO2, pH, arterial partial pressure for oxygen (PaO2), arterial O2-saturation (O2-Sat), and the PaO2/FIO2 ratio as well as the respiratory rate (RR) from the time of ICU admission until 7 days after implantation of the PECLA are displayed in the electronic supplementary material (Table 3 ESM). Changes of the three main ventilatory physiological parameters PaCO2, pH and RR from ICU admission until 24 h after PECLA start are displayed in more detail in Fig. 2. Changes in all three parameters from pre-PECLA to 21–24 h post-PECLA were significant (p < 0.001).
Complications
There were no immediate complications attributed to the implantation of the device. The haemodynamic state was not significantly altered with the institution of the PECLA. Two major and seven minor bleeding complications occurred during the course of the PECLA treatment. In one patient, a major bleed at the insertion site on day 7 required bedside surgical repair. In the second patient, bleeding led to removal of the cannulas on day 5 and to subsequent intubation and invasive mechanical ventilation. One patient developed a pseudoaneurysm of the femoral artery and another patient developed a heparin-induced thrombocytopenia type 2.
Discussion
In our study the application of a pumpless extracorporeal lung-assist (PECLA) device prevented intubation in the majority of patients with acute hypercapnic respiratory failure who were not responding to NIV. Compared to the matched control group who received invasive mechanical ventilation, there was a trend to a shorter hospital length of stay. The ICU length of stay and the overall survival rate did not differ significantly.
To our knowledge, this is the first study addressing the feasibility, effectiveness and safety of selective extracorporeal carbon dioxide removal in patients with hypercapnic respiratory failure to avoid intubation and invasive mechanical ventilation. Until now, extracorporeal carbon dioxide removal devices have been used almost exclusively to enable lung-protective ventilation in patients who are already intubated and mechanically ventilated. Only a few case reports have described their use in spontaneously breathing patients, nearly all in patients on waiting lists for lung transplantation [19, 20]. Crotti et al. reported the case of a patient with an acute hypercapnic exacerbation of COPD failing NIV who was successfully treated with a pump-driven venovenous extracorporeal system to avoid intubation [21].
Fuehner et al. recently presented a retrospective study evaluating the use of ECMO in awake patients with right heart failure or terminal respiratory failure, mostly with severe hypoxemia, as a bridge to lung transplantation [22]. Twenty of 26 patients were bridged to lung transplantation with the extracorporeal device. Despite these encouraging results, the authors stated that since ECMO is associated with serious complications, it should be used only in experienced centres. In contrast to this study, the majority of our patients had severe COPD, and only a few patients were listed for lung transplantation.
These results emphasise the potential value of using an extracorporeal lung-assist device in selected patients [23]. In our study, 14 of the 21 patients were experiencing acute hypercapnic respiratory failure because of acute exacerbations of severe COPD. It is well known that patients with severe chronic lung disease such as advanced COPD or cystic fibrosis when intubated and invasively mechanically ventilated are often difficult to wean, have a prolonged ICU stay and a high mortality [9, 10]. In particular, this is true for patients listed for lung transplantation or undergoing haematopoietic stem cell transplantation [24, 25]. Extracorporeal carbon dioxide removal can avoid the drawbacks and complications associated with intubation, sedation and prolonged mechanical ventilation, allowing patients to breath spontaneously, to communicate, to eat and drink, as well as to receive active physiotherapy (Fig. 3).
The basic concept of extracorporeal carbon dioxide removal can be seen from the original description of extracorporeal membrane oxygenation (ECMO), which appeared in the clinical setting more than 30 years ago. At that time, Hill et al. were the first to report the successful use of extracorporeal circulation to treat acute hypoxaemic respiratory failure in an adult patient [11]. In 1977, Kolobow and Gattinoni described a technique of extracorporeal removal of carbon dioxide through an extracorporeal membrane. They found that pulmonary ventilation could be supported by extracorporeal carbon dioxide removal [26, 27]. However, the high incidence of side effects, the complexity and the costs involved limited the use of this strategy. Technological improvements led to the development of minimally invasive and highly effective devices for extracorporeal carbon dioxide removal that caused relatively few side effects. Such devices include the single use, pumpless interventional lung assist used in our study (iLA®; Novalung GmbH, Heilbronn, Germany) and the decapneization system Decap® (Hemodec, Salerno, Italy) [14, 15].
Of course, the limitations and side effects of a pumpless extracorporeal lung assist need to be discussed. First, because the PECLA is a pumpless device, a cardiac index greater than 3 l/min/m2 and a mean arterial pressure above 70 mmHg are necessary to allow for the circulatory tolerance of an artificial arterio-venous shunt of up to 25 % of cardiac output. Second, the arterial cannulation can cause vascular complications. Episodes of lower limb ischaemia from arterial cannulation were frequently observed in the early period after introduction of the PECLA when large (17–19 French) cannulas were used [14]. Subsequent studies with smaller arterial cannulas (13–15 French) have had lower complication rates [16, 28]. In our series of 21 patients there were two major device-related complications, both related to local bleeding. Major complications related to lower limb ischaemia, haemolysis or thromboembolic problems were not observed. Pump-driven venovenous low-flow devices for extracorporeal CO2 removal may overcome some of the vascular and haemodynamic limitations of a pumpless system, albeit being potentially more susceptible to technical failure because of their higher technological complexity.
Our study has some methodological limitations. First, the interpretation of the results is limited by the design. Due to the retrospective nature of the study, data for short- and long-term side effects of ventilator-associated and analgosedation-associated complications are lacking. The relatively small number of patients reduces the power of the study with respect to an alpha error, not detecting a potential true difference of outcomes between the two strategies. Despite all efforts of matching, the PECLA-patients had significantly worse respiratory failure with respect to hypercapnia than the controls. Additionally, 9 of the 21 patients (43 %) in the PECLA group were listed for and awaiting a lung transplant, as opposed to none in the control group. Second, although all patients were treated as part of routine care, it needs to be emphasised that this treatment took place in expert centres. Therefore, the results may not be generalisable to other centres.
For almost 60 years now, mechanical ventilation has been used as a standard treatment procedure for patients with respiratory insufficiency [29]. We describe a novel approach in which an extracorporeal device replaces intubation and invasive mechanical ventilation in selected patients with acute hypercapnic respiratory failure. This approach would carry the modern concept of lung-protective ventilation to the next level, beyond the use of a ventilator.
Further evaluation of technological innovations and improvements in extracorporeal lung support might be key for a substantial change in the management of acute hypercapnic respiratory failure. These novel extracorporeal devices may have the potential to become a regular therapeutic option for selected patients with acute respiratory failure who fail NIV, and may avoid the excess morbidity and mortality associated with intubation and invasive mechanical ventilation [30–32].
Conclusion
The results of our preliminary study suggest that the pre-emptive application of extracorporeal carbon dioxide removal is a feasible therapeutic option to prevent intubation and invasive mechanical ventilation in selected patients with episodes of acute hypercapnic respiratory failure. Larger, prospective, randomised trials on the efficacy, effectiveness and efficiency are needed to further evaluate this new strategy.
References
Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguia C, Nightingale P, Arroliga AC, Tobin MJ (2002) Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 287:345–355
Kress JP, Pohlman AS, O’Connor MF, Hall JB (2000) Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 342:1471–1477
Melsen WG, Rovers MM, Bonten MJ (2009) Ventilator-associated pneumonia and mortality: a systematic review of observational studies. Crit Care Med 37:2709–2718
Tremblay LN, Slutsky AS (2006) Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 32:24–33
Jackson DL, Proudfoot CW, Cann KF, Walsh T (2010) A systematic review of the impact of sedation practice in the ICU on resource use, costs and patient safety. Crit Care 14:R59
Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network ((2000)) N Engl J Med 342:1301–1308
Ram FS, Picot J, Lightowler J, Wedzicha JA (2004) Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst RevCD004104
Funk GC (2012) [Non-invasive mechanical ventilation in COPD.]. Med Klin Intensivmed Notfmed
Nevins ML, Epstein SK (2001) Predictors of outcome for patients with COPD requiring invasive mechanical ventilation. Chest 119:1840–1849
Texereau J, Jamal D, Choukroun G, Burgel PR, Diehl JL, Rabbat A, Loirat P, Parrot A, Duguet A, Coste J, Dusser D, Hubert D, Mira JP (2006) Determinants of mortality for adults with cystic fibrosis admitted in Intensive Care Unit: a multicenter study. Respir Res 7:14
Hill JD, O’Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, Gerbode F (1972) Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the bramson membrane lung. N Engl J Med 286:629–634
MacLaren G, Combes A, Bartlett RH (2012) Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 38:210–220
Reng M, Philipp A, Kaiser M, Pfeifer M, Gruene S, Schoelmerich J (2000) Pumpless extracorporeal lung assist and adult respiratory distress syndrome. Lancet 356:219–220
Bein T, Weber F, Philipp A, Prasser C, Pfeifer M, Schmid FX, Butz B, Birnbaum D, Taeger K, Schlitt HJ (2006) A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med 34:1372–1377
Terragni PP, Del SL, Mascia L, Urbino R, Martin EL, Birocco A, Faggiano C, Quintel M, Gattinoni L, Ranieri VM (2009) Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 111:826–835
Nierhaus A, Frings D, Braune S, Baumann H, Schneider C, Wittenburg B, Kluge S (2011) Interventional lung assist enables lung protective mechanical ventilation in acute respiratory distress syndrome. Minerva Anestesiol 77:797–801
Global Initiative for Chronic Obstructive Lung Disease (2010) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease http://www.goldcopd.com
Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D (2004) Cystic fibrosis adult care: consensus conference report. Chest 125:1S–39S
Moscatelli A, Ottonello G, Nahum L, Lampugnani E, Puncuh F, Simonini A, Tumolo M, Tuo P (2010) Noninvasive ventilation and low-flow veno-venous extracorporeal carbon dioxide removal as a bridge to lung transplantation in a child with refractory hypercapnic respiratory failure due to bronchiolitis obliterans. Pediatr Crit Care Med 11:e8–12
Ricci D, Boffini M, Del SL, El QS, Comoglio C, Ribezzo M, Bonato R, Ranieri VM, Rinaldi M (2010) The use of CO2 removal devices in patients awaiting lung transplantation: an initial experience. Transplant Proc 42:1255–1258
Crotti S, Lissoni A, Tubiolo D, Azzari S, Tarsia P, Caspani L, Gattinoni L (2012) Artificial lung as an alternative to mechanical ventilation in COPD exacerbation. Eur Respir J 39:212–215
Fuehner T, Kuehn C, Hadem J, Wiesner O, Gottlieb J, Tudorache I, Olsson KM, Greer M, Sommer W, Welte T, Haverich A, Hoeper MM, Warnecke G (2012) Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med
Terragni P, Maiolo G, Ranieri VM (2012) Role and potentials of low-flow CO(2) removal system in mechanical ventilation. Curr Opin Crit Care 18:93–98
Hadjiliadis D, Steele MP, Govert JA, Davis RD, Palmer SM (2004) Outcome of lung transplant patients admitted to the medical ICU. Chest 125:1040–1045
Kluge S, Baumann HJ, Nierhaus A, Kroger N, Meyer A, Kreymann G (2008) Safety of percutaneous dilational tracheostomy in hematopoietic stem cell transplantation recipients requiring long-term mechanical ventilation. J Crit Care 23:394–398
Kolobow T, Gattinoni L, Tomlinson TA, Pierce JE (1977) Control of breathing using an extracorporeal membrane lung. Anesthesiology 46:138–141
Kolobow T, Gattinoni L, Tomlinson T, Pierce JE (1978) An alternative to breathing. J Thorac Cardiovasc Surg 75:261–266
Zimmermann M, Bein T, Arlt M, Philipp A, Rupprecht L, Mueller T, Lubnow M, Graf BM, Schlitt HJ (2009) Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: a prospective pilot study. Crit Care 13:R10
Lassen HC (1953) A preliminary report on the 1952 epidemic of poliomyelitis in copenhagen with special reference to the treatment of acute respiratory insufficiency. Lancet 1:37–41
Pesenti A, Patroniti N, Fumagalli R (2010) Carbon dioxide dialysis will save the lung. Crit Care Med 38:S549–S554
Del SL, Ranieri VM (2010) We do not need mechanical ventilation any more. Crit Care Med 38:S555–S558
Stewart NI, Jagelman TA, Webster NR (2011) Emerging modes of ventilation in the intensive care unit. Br J Anaesth
Conflicts of interest
AN, ME and SR have received lecture honoraria from Novalung GmbH, Heilbronn, Germany. SK is a member of the advisory board of Novalung GmbH and therefore has received advisor honoraria. All other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Kluge and S. A. Braune contributed equally to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kluge, S., Braune, S.A., Engel, M. et al. Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med 38, 1632–1639 (2012). https://doi.org/10.1007/s00134-012-2649-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2649-2