Abstract
Purpose
Pump-driven veno-venous extracorporeal CO2-removal (ECCO2-R) increasingly takes root in hypercapnic lung failure to minimize ventilation invasiveness or to avoid intubation. A recently developed device (iLA activve®, Novalung, Germany) allows effective decarboxylation via a 22 French double lumen cannula. To assess determinants of gas exchange, we prospectively evaluated the performance of ECCO2-R in ten patients receiving iLA activve® due to hypercapnic respiratory failure.
Methods
Sweep gas flow was increased in steps from 1 to 14 L/min at constant blood flow (phase 1). Similarly, blood flow was gradually increased at constant sweep gas flow (phase 2). At each step gas transfer via the membrane as well as arterial blood gas samples were analyzed.
Results
During phase 1, we observed a significant increase in CO2 transfer together with a decrease in PaCO2 levels from a median of 66 mmHg (range 46–85) to 49 (31–65) mmHg from 1 to 14 L/min sweep gas flow (p < 0.0001), while arterial oxygenation deteriorated with high sweep gas flow rates. During phase 2, oxygen transfer significantly increased leading to an increase in PaO2 from 67 (49–87) at 0.5 L/min to 117 (66–305) mmHg at 2.0 L/min (p < 0.0001). Higher blood flows also significantly enhanced decarboxylation (p < 0.0001).
Conclusions
Increasing sweep gas flow results in effective CO2-removal, which can be further reinforced by raising blood flow. The clinically relevant oxygenation effect in this setting could broaden the range of indications of the system and help to set up an individually tailored configuration.
Similar content being viewed by others
Introduction
Extracorporeal gas exchange as supportive therapy in severe lung failure has become increasingly popular during the last years since technological progress has resulted in more biocompatible and effective systems. Replacement of failing lungs usually is achieved by veno-venous extracorporeal membrane oxygenation (ECMO) systems providing oxygenation as well as decarboxylation. The amount of CO2-removal is mainly dependent on sweep gas flow and can be effectively achieved with blood flows through a gas exchange membrane from as low as 0.3–0.4 L/min to a maximum of about 1.0–1.5 L/min [1]. Oxygenation in contrast mainly depends on blood flow and usually requires flows of more than 60 % of cardiac output to achieve adequate oxygen delivery by concomitantly reducing invasiveness of mechanical ventilation [2].
Extracorporeal CO2-removal (ECCO2-R) is used to enable tidal volume reduction even beyond standard of care lung-protective ventilation from the currently recommended 6 mL/kg IBW (ideal body weight) towards the range of 3–4 mL/kg. While this concept called “ultra-protective ventilation” has been shown to be feasible [3, 4], no definitive proof of beneficial effects on outcome in patients with severe respiratory failure exists to date. In isolated hypercapnic lung failure like in acute exacerbated COPD or in terminal chronic lung failure during bridging to lung transplantation, ECCO2-R may effectively support non-invasive ventilation with the aim to avoid intubation or facilitate weaning from the ventilator [5–8].
Systems for ECCO2-R usually are designed to provide low blood flows as well as low invasivity and simple handling. The first device developed exclusively for ECCO2-R comprises the iLA® system (Interventional Lung Assist, Novalung, Germany), a pumpless arterio–venous system with blood flowing from the femoral artery through a gas exchange membrane to a femoral vein passively driven by the pressure gradient. Due to the arteriovenous configuration and the low blood flows achievable, no clinically relevant effect on oxygenation occurs. As arterial cannulation is associated with risk of bleeding and limb ischemia [3], pump-driven veno-venous systems to provide CO2-removal have been developed. All of these systems share the concept of low blood flows, miniaturized pumps, low volume tubing systems as well as special cannulas to guarantee low invasivity. A summary on role and potentials of low-flow CO2 removal systems is given in a review by Terragni et al. [9]. Gas transfer in low-flow ECCO2-R systems as well as in high-flow ECMO systems has been well described [2, 9], whereas data on gas exchange in the so called “mid-flow range” of blood flow from 1.0 up to 2.5 L/min are however lacking. The aim of our investigation was to gain insights into gas exchange with different settings of pump speed and sweep gas flow using the novel iLA activve® modular system in a setting of intermediate invasivity comprising a miniaturized centrifugal pump together with low volume tubing, a gas exchange membrane optimized for low to midrange blood flows as well as a jugular unicaval 22 F double lumen cannula.
Patients and methods
The study was conducted on a medical intensive care unit of a tertiary care university hospital. The protocol was approved by the local ethical review board. According to Austrian law, informed consent of unresponsive patients was obtained from themselves after regaining responsiveness.
Patients
We included ten critically ill patients undergoing extracorporeal gas exchange with the iLA activve® system in an ECCO2-R setting matching the following criteria: pressure controlled mechanical ventilation, established sedation without spontaneous breathing, stable hemodynamic conditions and gas exchange at the time of study entry defined by FiO2 ≤0.80, breathing rate ≤20/min, SaO2 >92 %, pH-value between 7.35 and 7.45 and hemoglobin ≥8 g/dl. Exclusion criteria were age <18 or >89 years, pregnancy and participation in another experimental protocol, respectively. Patients´ characteristics are shown in Table 1.
ECCO2-R
iLA activve® is a compact extracorporeal pump-driven gas exchange system consisting of a miniaturized centrifugal pump and a polymethylpenthene gas exchange membrane (iLA membrane ventilator®, Novalung, Germany). In all patients a 22 F in diameter and 17 cm in length unicaval double lumen cannula (Novaport® twin, Novalung, Germany) was used to establish jugular venous access. The details of the system and its ability to effectively remove CO2 are described elsewhere [10]. As tubing and cannula are heparin coated, no pre-heparinization was performed. Patients received a heparin bolus of 5000 units immediately after insertion of the cannula followed by continuous heparin infusion for systemic anticoagulation titrated to keep the activated partial thromboplastin time (aPTT) between 50 and 60 s.
Study protocol
The study was performed in two independent stages: During phase 1, sweep gas flow was increased stepwise from 1 L/min to a maximum of 14 L/min at a constant blood flow of 1.0 L/min. Fifteen minutes after setting each step, blood gas analyses were performed from the patient’s arterial line, as well as from iLA tubing at the inlet and the outlet of the gas exchange membrane. At all steps, iLA settings (pump speed, blood flow) as well as pressures (venous suction pressure, intra-membraneous as well as arterial re-perfusion pressure) were recorded.
During phase 2, which was performed 60 min after phase 1, sweep gas flow was set to a constant level providing a pH within the normal range. Blood flow was increased stepwise from 0.5 to a maximum of 2.0 L/min by increasing pump speed. Fifteen minutes after each increase, the same measurements as during phase 1 were performed. Additionally, to assess hemolysis associated with higher pump speed and more negative suction pressures, free hemoglobin was measured 15 min after setting each step.
Blood gases and hemoglobin values were measured by a standard blood gas analyzer (Radiometer ABL 800 Flex), baseline cardiac output was estimated by pulse curve analysis (Vigileo®, Edwards Lifesciences, Irvine, USA). Ventilator settings were kept constant throughout the study period. In case of spontaneous breathing during the study period, sedation was increased to suppress breathing efforts. Oxygen and crude CO2 transfer were calculated as difference between oxygen and CO2 content prior to and after the gas exchange membrane according to standard equations [11]. Since variations in blood PCO2 values would result in variable CO2 removal rates, normalized CO2 exchange rate was calculated based on a moving average of venous PCO2 according to Wearden et al. [12]. The equations used to calculate gas transfer are given in the “Appendix”.
During the study period all patients were monitored by an indwelling arterial line, ECG and pulse oximetry. The study was stopped if oxygen saturation declined to values below 88 %, if hemodynamic instability occurred, or if arterial pH was lower than 7.25 or higher than 7.55.
Statistical analysis
Non-parametric tests were chosen because of the small population studied. Continuous data are given as median and range, categorical variables as percentages. To compare the changes at different iLA settings, a non-parametric one-way ANOVA for repeated measures (Kruskal–Wallis Test) was used. Dunn’s multiple comparison post test was used to compare pairs of time points. Calculations were performed by a statistic software package (GraphPad Prism®, GraphPad Software, San Diego, USA). Differences with a p-level less than 0.05 were considered as statistically significant.
Results
Ten patients on ECCO2-R on the iLA activve® system were included into the study. Six patients were female, median age was 47 (range 25–67) years. Median hemoglobin level was 10.3 (8.3–12.4) g/dL, median cardiac output was 5.3 (4.2–6.9) L/min. Patients’ characteristics and baseline data prior to study entry are given in Table 1. Ventilatory settings prior to start of ECCO2-R as well as during ECCO2-R immediately prior to study entry are shown in Table 2. During ECCO2-R, PaCO2 decreased significantly with concomitant reduction of ventilatory driving pressure and tidal volume. Two of the patients (20 %) had to be switched to full veno-venous ECMO during their further course, seven patients survived to hospital discharge (70 %). Seven patients remained without device associated complications, in two patients clotting of the gas exchange membrane occurred during ECCO2-R, in one patient thrombosis of the right jugular vein after cannula removal was diagnosed.
Phase 1
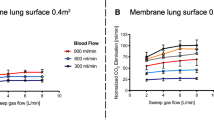
Crude CO2 transfer rate increased significantly with increasing sweep gas flow reaching a plateau from 8 to 14 L/min (p < 0.0001, supplemental figure S1a). This plateau was not observed when calculating normalized CO2 transfer rate, which increased continuously with increasing sweep gas flow (p < 0.0001, Fig. 1a). This was paralleled by continuously decreasing PaCO2 from a median of 66 (range 46–85) mmHg at 1 L/min to 49 (31–65) mmHg at 14 L/min (p < 0.0001, Fig. 1b).
Oxygen transfer tended to increase at 10 and 14 L/min (p = 0.06, Fig. 1c). PaO2 however, remained stable until increasing sweep gas flow to 10 and 14 L/min which led to a significant decrease during these two steps from 80 (58–103) mmHg at 1 L/min to 65 (36–95) mmHg at 14 L/min (p < 0.0001, Fig. 1d).
Phase 2
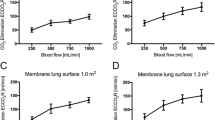
Sweep gas flow was set to a median of 4 L/min (range 2–12 L/min). Together with an increasing crude as well as normalized CO2 transfer rate (p < 0.0001, Fig. 2a; supplemental figure S1b), PaCO2 decreased continuously from a median of 60 (39–72) mmHg at 0.5 L/min to 48 (38–62) mmHg at 2.0 L/min with increasing blood flow (p < 0.0001, Fig. 2b).
Oxygen transfer increased significantly with higher blood flow (p < 0.0001, Fig. 2c). Concomitantly, PaO2 increased significantly from 67 (49–87) mmHg at 0.5 L/min to 117 (66–305) mmHg at 2.0 L/min (p < 0.0001, Fig. 2d), resembling a significant and clinically relevant increase in PaO2/FiO2 from 107 (76–218) to 206 (89–508), p < 0.0001.
To achieve the desired steps of blood flow, pump speed had to be increased from 2500 (2100–2800) rpm for a blood flow of 0.5 L/min to 6600 (5600–7300) rpm for 2.0 L/min. Venous suction pressure decreased from −6 (−2 to −14) mmHg at 0.5 L/min to −122 (−88 to −185) mmHg at 2.0 L/min (Fig. 3a). Intramembraneous and arterial pressures increased significantly with higher blood flows (p < 0.0001, data not shown), while transmembraneous pressure increased only slightly from 4 (1–24) mmHg at 0.5 L/min to 12 (3–22) mmHg at 2.0 L/min (p = 0.007). At a blood flow of 2.0 L/min free hemoglobin measured 15 min after adjustment of pump speed rose significantly (p = 0.03, Fig. 3b).
Discussion
In the particular setting of extracorporeal gas exchange described herein, effective removal of CO2 with increasing sweep gas flow could be demonstrated. By using the same gas exchange membrane as in our study (iLA, Novalung, Germany) in a pumpless arterio–venous configuration, comparable results have been generated in a sheep model [13] as well as in patients with acute lung failure [3]. Zhou and co-workers demonstrated increasing CO2 removal with increasing sweep gas flow up to 10 L/min, while a further increase was associated with only minimally higher rate of CO2 removal [13]. The same was true in our study with respect to crude CO2 transfer, which reached a maximum at 8 L/min. PaCO2 however, decreased continuously up to a sweep gas flow of 14 L/min. Patients were sedated to suppress spontaneous breathing for the study period, thus ventilation was kept unchanged throughout the study. As variations in CO2 production within the short study period under unchanged conditions seems unlikely, this phenomenon most likely reflects the influence of blood CO2 values on elimination rate. Indeed, when calculating normalized CO2 elimination rate, a continuous increase in CO2 transfer could be observed. Moreover, a time dependent effect leading to further decrease of arterial CO2 could account for this finding. CO2 elimination seems to undergo a biphasic elimination during ECCO2-R with rapid removal of physically dissolved CO2 during the first 2 h followed by a smaller reduction in PaCO2 afterwards due to the delayed liberation of bicarbonate from slow compartments [14]. The intervals of 15 min between each step were chosen arbitrarily based on clinical experience. The study period for titrating sweep gas flow thus resulted in 105 min, which could be too short a time for carbon dioxide levels to reach a stable equilibrium. This limitation has to be taken into account when interpreting our findings.
In our study, increasing blood flow at constant sweep gas flow led to a less pronounced, but significant increase in CO2 transfer together with a decrease in PaCO2. These findings are in line with the iLA sheep models of Jayroe et al. and Zhou et al. [1, 13], who both demonstrated increasing CO2 removal by raising blood flow up to 1.4 and 1.6 L/min, respectively. Our data suggest an increase in CO2 elimination up to even 2.0 mL/min of blood flow. Recently, a study in patients on veno-venous ECMO did not reveal any effect on CO2 by raising blood flow from more than 2 L/min to higher levels [2]. Summing up these data, an increase in blood flow up to about 2 L/min might add to CO2 elimination while at higher levels CO2 removal seems to be dependent on sweep gas flow only. In clinical practice however, arterio–venous iLA as well as the setting described in our study using the pump driven iLA system, have been shown to effectively eliminate CO2 at blood flow rates between 1.0 and 1.5 mL/min [10, 15].
In veno-venous extracorporeal gas exchange, oxygenation depends mainly on blood flow. In a study on patients suffering from severe ARDS undergoing veno-venous ECMO therapy, increasing blood flow to 60 % of cardiac output was necessary to adequately supply oxygen and concomitantly enable reduction of invasiveness of ventilation [2]. In our study, increasing blood flow to more than 1.0 L/min led to a clinically significant increase in oxygen transfer and PaO2. A concomitant beneficial effect on oxygenation could be due to reduction of alveolar pCO2 and a concomitant increase in alveolar pO2, according to Dalton’s Law. This effect however could not be observed during increasing sweep gas flow with even more pronounced reduction in PCO2. Although a blood flow of 1.5 and 2.0 L/min resembled only 28 and 38 % of the cohort’s median cardiac output, respectively, the increase in PaO2/FiO2 would have enabled a less invasive ventilatory support in most patients. It has to be taken into account, however, that suction pressures at a blood flow of 2 L/min in many patients approached negative values associated with possible shear stress to blood cells and ensuing hemolysis [16]. Rising levels of free haemoglobin underline this hypothesis and it may be speculated that keeping patients for longer than 15 min at these negative suction pressures would have led to pronounced damage of blood cells. Suction pressures at 1.5 L/min could be kept below −100 mmHg and can be regarded as safe [16]. The interpretation of the relation of extracorporeal blood flow to cardiac output has to be regarded with caution. It has to be emphasized that in our study cardiac output was measured by pulse wave analysis via a Vigileo® monitor (Edwards Lifesciences, Irvine, USA). Referring to Slagt et al. an inherent shortcoming of this technique using uncalibrated arterial pressure waveform analysis is a reduced ability of exact measurement of cardiac output [17]. Additionally, transient vasopressor therapy could have led to further errors in cardiac output estimation [18]. A lack of reliability should therefore be considered regarding the accuracy of values.
Surprisingly, despite a tendency for increasing oxygen transfer with increasing sweep gas flow, systemic oxygenation worsened. Taken into account that iLA blood flow, ventilation settings as well as hemodynamics including cardiac output also remained unchanged throughout the titration of sweep gas flow, an effect on pulmonary gas exchanged must be assumed. Schmidt et al. demonstrated a decrease in arterial pulmonary pressures with increasing sweep gas flow, indicating pulmonary vasodilatation [2]. Better oxygenation in the pulmonary artery due to higher oxygen transfer could have led to abolition of physiologic hypoxic vasoconstriction and consequently to an increase of pulmonary shunt fraction, thus resembling administration of a systemic vasodilator. It has been shown that unselective vasodilation leads to increase of shunt fraction as well as deterioration of systemic oxygenation [19]. However, in phase 2, while oxygen transfer did increase with higher blood flows, arterial oxygenation improved significantly. We hypothesize that the more pronounced effect on oxygen transfer observed during phase 2 might have outweighed the possible negative effect on pulmonary shunt fraction. Our data, however, are not sufficient to prove this hypothesis.
In summary, our results prove that decarboxylation with iLA activve® efficiently takes place with increasing sweep gas flow up to 10 L/min. Higher sweep gas flow rates yield impaired oxygenation and should therefore be avoided. Increasing blood flow to more than 1 L/min seems to amplify the decarboxylation effect and should be considered in severe hypercapnic conditions. Increasing blood flow to ≥1.5 L/min leads to a relevant oxygenation effect. The role of extracorporeal gas exchange systems able to provide blood flows in the so called “mid range” has not yet been defined. Although these systems appear to offer lower invasivity by single venous access, smaller then bicaval ECMO single vessel cannulas as well as smaller extracorporeal blood volumes, it remains to be elucidated if they may be advantageous with respect to complications and outcome. Our data show however, that modern systems of extracorporeal gas exchange offer high flexibility and a wide range of respiratory support, which should enable intensivists to tailor the setting to the individual need of a patient. This may offer the possibility of broaden indications from isolated hypercapnia towards additional oxygen supply in patients with mild to moderate hypoxic respiratory failure. Individualizing extracorporeal gas exchange to a specific clinical scenario may well help to enable avoidance of invasive mechanical ventilation in selected groups of high risk patients like those suffering from acute exacerbated COPD, severe asthma or immunosuppression. The findings presented in here may contribute to prepare clinical trials focusing on outcome of acute respiratory failure in these particular groups of patients, if extracorporeal gas exchange with the goal of maintaining spontaneous ventilation is established early.
References
Jayroe JB, Wang D, Deyo DJ, Alpard SK, Bidani A, Zwischenberger JB (2003) The effect of augmented hemodynamics on blood flow during arteriovenous carbon dioxide removal. ASAIO J 49:30–34
Schmidt M, Tachon G, Devilliers C, Muller G, Hekimian G, Brechot N, Merceron S, Luyt CE, Trouillet JL, Chastre J, Leprince P, Combes A (2013) Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensiv Care Med 39:838–846
Zimmermann M, Bein T, Arlt M, Philipp A, Rupprecht L, Mueller T, Lubnow M, Graf BM, Schlitt HJ (2009) Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: a prospective pilot study. Crit Care 13:R10
Bein T, Weber-Carstens S, Goldmann A, Muller T, Staudinger T, Brederlau J, Muellenbach R, Dembinski R, Graf BM, Wewalka M, Philipp A, Wernecke KD, Lubnow M, Slutsky AS (2013) Lower tidal volume strategy (approximately 3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS : the prospective randomized Xtravent-study. Intensiv Care Med 39:847–856
Fischer S, Simon AR, Welte T, Hoeper MM, Meyer A, Tessmann R, Gohrbandt B, Gottlieb J, Haverich A, Strueber M (2006) Bridge to lung transplantation with the novel pumpless interventional lung assist device NovaLung. J Thorac Cardiovasc Surg 131:719–723
Fischer S, Hoeper MM, Bein T, Simon AR, Gottlieb J, Wisser W, Frey L, Van Raemdonck D, Welte T, Haverich A, Strueber M (2008) Interventional lung assist: a new concept of protective ventilation in bridge to lung transplantation. ASAIO J 54:3–10
Kluge S, Braune SA, Engel M, Nierhaus A, Frings D, Ebelt H, Uhrig A, Metschke M, Wegscheider K, Suttorp N, Rousseau S (2012) Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensiv Care Med 38:1632–1639
Abrams DC, Brenner K, Burkart KM, Agerstrand CL, Thomashow BM, Bacchetta M, Brodie D (2013) Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc 10:307–314
Terragni P, Maiolo G, Ranieri VM (2012) Role and potentials of low-flow CO(2) removal system in mechanical ventilation. Curr Opin Crit Care 18:93–98
Hermann A, Staudinger T, Bojic A, Riss K, Wohlfarth P, Robak O, Sperr WR, Schellongowski P (2014) First experience with a new miniaturized pump-driven venovenous extracorporeal CO2 removal system (iLA Activve): a retrospective data analysis. ASAIO J 60:342–347
Lehle K, Philipp A, Hiller KA, Zeman F, Buchwald D, Schmid C, Dornia C, Lunz D, Muller T, Lubnow M (2014) Efficiency of gas transfer in venovenous extracorporeal membrane oxygenation: analysis of 317 cases with four different ECMO systems. Intensiv Care Med 40:1870–1877
Wearden PD, Federspiel WJ, Morley SW, Rosenberg M, Bieniek PD, Lund LW, Ochs BD (2012) Respiratory dialysis with an active-mixing extracorporeal carbon dioxide removal system in a chronic sheep study. Intensiv Care Med 38:1705–1711
Zhou X, Loran DB, Wang D, Hyde BR, Lick SD, Zwischenberger JB (2005) Seventy-two hour gas exchange performance and hemodynamic properties of NOVALUNG iLA as a gas exchanger for arteriovenous carbon dioxide removal. Perfusion 20:303–308
Muller T, Lubnow M, Philipp A, Bein T, Jeron A, Luchner A, Rupprecht L, Reng M, Langgartner J, Wrede CE, Zimmermann M, Birnbaum D, Schmid C, Riegger GA, Pfeifer M (2009) Extracorporeal pumpless interventional lung assist in clinical practice: determinants of efficacy. Eur Respir J 33:551–558
Schellongowski P, Riss K, Staudinger T, Ullrich R, Krenn CG, Sitzwohl C, Bojic A, Wohlfarth P, Sperr WR, Rabitsch W, Aigner C, Taghavi S, Jaksch P, Klepetko W, Lang G (2014) Extracorporeal CO removal as bridge to lung transplantation in life-threatening hypercapnia. Transpl Int (Epub ahead of print)
Mulholland JW, Massey W, Shelton JC (2000) Investigation and quantification of the blood trauma caused by the combined dynamic forces experienced during cardiopulmonary bypass. Perfusion 15:485–494
Slagt C, Helmi M, Malagon I, Groeneveld AB (2015) Calibrated versus uncalibrated arterial pressure waveform analysis in monitoring cardiac output with transpulmonary thermodilution in patients with severe sepsis and septic shock: an observational study. Eur J Anaesthesiol 32:5–12
Monnet X, Vaquer S, Anguel N, Jozwiak M, Cipriani F, Richard C, Teboul JL (2015) Comparison of pulse contour analysis by Pulsioflex and Vigileo to measure and track changes of cardiac output in critically ill patients. Br J Anaesth 114:235–243
Radermacher P, Santak B, Becker H, Falke KJ (1989) Prostaglandin E1 and nitroglycerin reduce pulmonary capillary pressure but worsen ventilation-perfusion distributions in patients with adult respiratory distress syndrome. Anesthesiology 70:601–606
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Two of the authors (Thomas Staudinger, Peter Schellongowski) received speaker fees from Novalung.
Additional information
Take-home message ECCO2-R using iLA activve® in a setting of intermediate invasivity and mid-range blood flow can effectively remove CO2. A clinically relevant oxygenation effect could be observed even at relatively low blood flow, which may help in designing an extracorporeal circuit tailored to a patient’s individual needs.
Electronic supplementary material
Below is the link to the electronic supplementary material.

134_2015_3957_MOESM1_ESM.jpg
Supplementary material 1: Panel a: Crude CO2 transfer during stepwise increase of sweep gas flow (study phase 1), panel b: Crude CO2 transfer during stepwise increase of blood flow (study phase 2). Asterisks denote statistically significant changes compared to baseline (p < 0.0001). (JPEG 428 kb)
Appendix
Appendix
Calculation of O2 content, CO2 content, O2 transfer and CO2 transfer.
-
O2 content (mL O2/dL blood) = 1.34 × (Hb) × SaO2 + 0.0031 × PO2. The factor 1.34 is specified in mL × g−1 and the factor 0.0031 is specified in mL × dL−1 × mmHg−1.
-
CO2 content (mL CO2/dL blood) = 22.4 × (HCO3 −) + 0.030 × PCO2. The factor 22.4 (molecular volume of a gas) is specified in L × Mol−1, and the factor 0.030 is specified in mMol × mmHg−1.
-
O2 transfer (mL/min) = [(O2 content)out − (O2 content)in] × blood flow × 10;
-
CO2 transfer (mL/min) = [(CO2 content)in − (CO2 content)out] × blood flow × 10.
Normalized CO2 transfer rate.
-
VCO2(norm) = VCO2(actual) x (45 mmHg/PCO2in).
Rights and permissions
About this article
Cite this article
Hermann, A., Riss, K., Schellongowski, P. et al. A novel pump-driven veno-venous gas exchange system during extracorporeal CO2-removal. Intensive Care Med 41, 1773–1780 (2015). https://doi.org/10.1007/s00134-015-3957-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-3957-0