Abstract
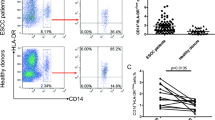
Accumulating evidence has demonstrated that myeloid-derived suppressor cells (MDSCs), a heterogeneous population of cells, play an important role in the subversion, inhibition, and downregulation of the immune response to cancer. However, the characteristics of these cells, particularly clinical relevance, in malignant tumors remain unclear due to a lack of specific markers. In this study, we characterized peripheral CD14+HLA-DR-/low cells, a new human MDSC subpopulation, in 89 patients with non-small cell lung cancer (NSCLC). As expected, both frequency and absolute number of CD14+HLA-DR-/low cells were significantly increased in the peripheral blood of NSCLC patients compared with that of the healthy controls and indicated an association with metastasis, response to chemotherapy, and progression-free survival. These cells showed decreased expression of CD16 and CD86 compared with HLA-DR+ monocytes. Unlike classical monocytes, these populations showed significantly decreased allostimulatory activity and showed the ability to inhibit autologous T cell proliferation and IFN-γ production in a cell-contact-dependent manner. Furthermore, we demonstrated that CD14+HLA-DR-/low cells expressed the NADPH oxidase component gp91phox and generated high level of reactive oxygen species (ROS). Moreover, inactivation of ROS reversed their immunosuppressive capacity on T cell response. These results prove, for the first time, the existence of ROS-producing CD14+HLA-DR-/low myeloid-derived suppressor cells in NSCLC patients, which mediate tumor immunosuppression and might thus represent a potential target for therapeutic intervention.





Similar content being viewed by others
Abbreviations
- MDSCs:
-
Myeloid-derived suppressor cells
- AS:
-
Adenocarcinoma
- SCC:
-
Squamous cell carcinoma
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- PFS:
-
Progression-free survival
- L-NMMA:
-
NG-Methyl-l-arginine acetate salt
- nor-NOHA:
-
Nω-Hydroxy-nor-l-arginine, diacetate salt
- CD14depl :
-
CD14-depleted
- Arg1:
-
Arginase-1
- iNOS:
-
Inducible nitric oxide synthase
- Cox2:
-
Cyclooxygenase 2
- IDO:
-
Indoleamine 2, 3-dioxygenase
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- DCF:
-
2′,7′-dichlorofluorescein
- ROS:
-
Reactive oxygen species
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
Molina JR, Yang PG, Cassivi SD, Schild SE, Adjei AA (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83(5):584–594
Shepherd FA, Douillard JY, Blumenschein GR (2011) Immunotherapy for non-small cell lung cancer novel approaches to improve patient outcome. J Thorac Oncol 6(10):1763–1773. doi:10.1097/JTO.0b013e31822e28fc
Alberts WM (2007) Diagnosis and management of lung cancer executive summary: ACCP evidence-based clinical practice guidelines (2nd Edition). Chest 132(3 Suppl):1S–19S
Srivastava MK, Bosch JJ, Wilson AL, Edelman MJ, Ostrand-Rosenberg S (2010) MHC II lung cancer vaccines prime and boost tumor-specific CD4+ T cells that cross-react with multiple histologic subtypes of nonsmall cell lung cancer cells. Int J Cancer 127(11):2612–2621. doi:10.1002/ijc.25462
Nagaraj S, Gabrilovich DI (2008) Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res 68(8):2561–2563. doi:10.1158/0008-5472.CAN-07-6229
Srivastava MK, Zhu L, Harris-White M, Kar U, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S, Sharma S (2012) Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One 7(7):e40677. doi:10.1371/journal.pone.0040677
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9(3):162–174. doi:10.1038/nri2506
Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V (2006) Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 116(10):2777–2790. doi:10.1172/JCI28828
Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI (2001) Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 166(1):678–689
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 58(1):49–59. doi:10.1007/s00262-008-0523-4
Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S (2008) Lung cancer patients’ CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother 57(10):1493–1504. doi:10.1007/s00262-008-0490-9
Feng PH, Lee KY, Chang YL, Chan YF, Kuo LW, Lin TY, Chung FT, Kuo CS, Yu CT, Lin SM, Wang CH, Chou CL, Huang CD, Kuo HP (2012) CD14+ S100A9+ monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer. Am J Respir Crit Care Med 186(10):1025–1036. doi:10.1164/rccm.201204-0636O
Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, Lee KY, Lin SM, Lin HC, Wang CH, Yu CT, Kuo HP (2010) Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(−)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol 136(1):35–45. doi:10.1007/s00432-009-0634-0
Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB (2011) Immunosuppressive CD14+ HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood 117(3):872–881. doi:10.1182/blood-2010-05-283820
Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L (2006) Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res 66(18):9290–9298
Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L (2007) Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor based antitumor vaccine. J Clin Oncol 25(18):2546–2553. doi:10.1200/jco.2006.08.5829
Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R (2010) Immature immunosuppressive CD14(+)HLA-DR-/low cells in melanoma patients are Stat3(hi) and overexpress CD80, CD83, and DC-sign. Cancer Res 70(11):4335–4345. doi:10.1158/0008-5472
Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F (2008) A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135(1):234–243. doi:10.1053/i.gastro.2008.03.020
Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao XH, Dietz AB (2010) Immunosuppressive CD14(+)HLA-DRlow/- monocytes in prostate cancer. Prostate 70(4):443–455. doi:10.1002/pros.21078
Gustafson MP, Lin Y, New KC, Bulur PA, O’Neill BP, Gastineau DA, Dietz AB (2010) Systemic immune suppression in glioblastoma: the interplay between CD14+ HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol 12(7):631–644. doi:10.1093/neuonc/noq001
Chikamatsu K, Sakakura K, Toyoda M, Takahashi K, Yamamoto T, Masuyama K (2012) Immunosuppressive activity of CD14(+) HLA-DR- cells in squamous cell carcinoma of the head and neck. Cancer Sci 103(6):976–983. doi:10.1111/j.1349-7006.2012.02248.x
Yuan XK, Zhao XK, Xia YC, Zhu X, Xiao P (2011) Increased circulating immunosuppressive CD14(+)HLA-DR-/low cells correlate with clinical cancer stage and pathological grade in patients with bladder carcinoma. J Int Med Res 39(4):1381–1391
Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, Svane IM (2010) Increased level of both CD4+ FOXP3+ regulatory T cells and CD14+ HLA-DR(-)/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol 72(6):540–547. doi:10.1111/j.1365-3083.2010.02463.x
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Beasley MB, Brambilla E, Travis WD (2005) The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 40(2):90–97
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Rebelato E, Mares-Guia TR, Graciano MF, Labriola L, Britto LR, Garay-Malpartida HM, Curi R, Sogayar MC, Carpinelli AR (2012) Expression of NADPH oxidase in human pancreatic islets. Life Sci 91(7–8):244–249. doi:10.1016/j.lfs.2012.07.004
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer Immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565–1570. doi:10.1126/science.1203486
Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R (2012) Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother 61(6):827–838. doi:10.1007/s00262-011-1143-y
Yu L, Quinn MT, Cross AR, Dinauer MC (1998) Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci USA 95(14):7993–7998
Choi J, Suh B, Ahn YO, Kim TM, Lee JO, Lee SH, Heo DS (2012) CD15(+)/CD16(low) human granulocytes from terminal cancer patients: granulocytic myeloid-derived suppressor cells that have suppressive function. Tumour Biol 33(1):121–129. doi:10.1007/s13277-011-0254-6
Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW (2011) Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 60(10):1419–1430. doi:10.1007/s00262-011-1028-0
Ziegler-Heitbrock L (2007) The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 81(3):584–592
Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F (2010) Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33(3):375–386. doi:10.1016/j.immuni.2010.08.012
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2(8):706–714
Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H (2012) Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 18(8):1254–1261. doi:10.1038/nm.2883
Vollbrecht T, Stirner R, Tufman A, Roider J, Huber RM, Bogner JR, Lechner A, Bourquin C, Draenert R (2012) Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. Aids 26(12):F31–F37. doi:10.1097/QAD.0b013e328354b43f
Zhao F, Hoechst B, Duffy A, Gamrekelashvili J, Fioravanti S, Manns MP, Greten TF, Korangy F (2012) S100A9 a new marker for monocytic human myeloid-derived suppressor cells. Immunology 136(2):176–183. doi:10.1111/j.1365-2567.2012.03566.x
Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, Zanovello P (2009) IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol 182(10):6562–6568. doi:10.4049/jimmunol.0803831
Sinha P, Parker KH, Horn L, Ostrand-Rosenberg S (2012) Tumor-induced myeloid-derived suppressor cell function is independent of IFN-gamma and IL-4Ralpha. Eur J Immunol 42(8):2052–2059. doi:10.1002/eji.201142230
Filipazzi P, Huber V, Rivoltini L (2012) Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother 61(2):255–263. doi:10.1007/s00262-011-1161-9
Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS (2011) Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology 55(2):343–353. doi:10.1002/hep.24700
Benedyk M, Sopalla C, Nacken W, Bode G, Melkonyan H, Banfi B, Kerkhoff C (2007) HaCaT keratinocytes overexpressing the S100 proteins S100A8 and S100A9 show increased NADPH oxidase and NF-kappaB activities. J Invest Dermatol 127(8):2001–2011
Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 205(10):2235–2249. doi:10.1084/jem.20080132
Aurelius J, Thoren FB, Akhiani AA, Brune M, Palmqvist L, Hansson M, Hellstrand K, Martner A (2012) Monocytic AML cells inactivate antileukemic lymphocytes: role of NADPH oxidase/gp91(phox) expression and the PARP-1/PAR pathway of apoptosis. Blood 119(24):5832–5837. doi:10.1182/blood-2011-11-391722
Acknowledgments
This study was supported by grants from the Ministry of Science and Technology of China (“973” projects) (No. 2012CB917104) and the National Natural Science Foundation of China (No. 81172853 and No. 81201605).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ang Huang and Bo Zhang contributed equally to this work as first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, A., Zhang, B., Wang, B. et al. Increased CD14+HLA-DR-/low myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother 62, 1439–1451 (2013). https://doi.org/10.1007/s00262-013-1450-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1450-6