Abstract
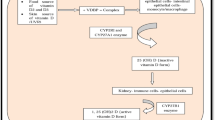
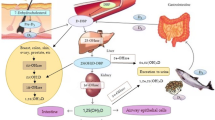
Bronchiectasis is a chronic infective and inflammatory respiratory disease that causes significant morbidity and mortality. Patients with non-cystic-fibrosis bronchiectasis are frequently vitamin D deficient, and vitamin D levels correlate with disease severity. Infection-specific actions of vitamin D include the enhancement of innate immunity and the moderation of inflammation caused by the adaptive immune response. Potentially, vitamin D could influence the processes that lead to bronchiectasis and the frequency and severity of acute exacerbations. Randomized trials of vitamin D supplementation have shown effects that are likely to be protective against the development of bronchiectasis. Several issues need to be clarified before the development of clinical trials to investigate the role of vitamin D in bronchiectasis. These include an optimal vitamin D supplementation dose and appropriate and sensitive outcome measures that include assessment of exacerbation frequency and severity, lung function, and health-related quality of life.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf). 2012;76:315–25. An excellent recent review of the role of vitamin D in human immunity.
Chishimba L, Thickett DR, Stockley RA, et al. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax. 2010;65:456–62.
•• Chalmers J, McHugh B, Docherty C, et al. Vitamin-D deficiency is associated with chronic bacterial colonisation and disease severity in bronchiectasis. Thorax 2013;68:39–47. Recent case series documenting the association of low 25(OH)D levels with bronchiectasis and bronchiectasis severity.
• Camargo Jr CA, Ganmaa D, Frazier A, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130:e561–7. A well-designed RCT showing a halving in incidence of upper respiratory tract infection in vitamin D deficient children given daily vitamin D supplementation.
Urashima M, Segawa T, Okazaki M, et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60.
Manaseki-Holland S, Qader G, Isaq Masher M, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health. 2010;15:1148–55.
•• Coussens A, Wilkinson R, Hanifa Y, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci USA. 2012;109:15449–54. An important clinical study documenting the anti-inflammatory actions of vitamin D in a clinical situation.
Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2010.
Bradley JM, Moran F, Greenstone M. Physical training for bronchiectasis. Cochrane Database Syst Rev. 2002;3:CD002166.
Kapur N, Bell S, Kolbe J, et al. Inhaled steroids for bronchiectasis. Cochrane Database Syst Rev. 2009;1:CD000996.
Lasserson T, Holt K, Greenstone M. Oral steroids for bronchiectasis (stable and acute exacerbations). Cochrane Database Syst Rev. 2001;4:CD002162.
Pizzutto SJ, Upham JW, Yerkovich ST, et al. Inhaled non-steroid anti-inflammatories for children and adults with bronchiectasis. Cochrane Database Syst Rev. 2010;4:CD007525.
• Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380:660–7. Azithromycin appears an important antibiotic in bronchiectasis management.
Pincikova T, Nilsson K, Moen IE, et al. Inverse relation between vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur J Clin Nutr. 2011;65:102–9.
King P. The pathophysiology of bronchiectasis. Int J Chron Obstruct Pulmon Dis. 2009;4:411–9.
Holick MF. Vitamin D, deficiency. N Eng J Med. 2007;357:266–81.
Carlberg C, Molnar F. Current status of vitamin D signaling and its therapeutic applications. Curr Top Med Chem. 2012;12:528–47.
Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–95.
Kim ST, Cha HE, Kim DY, et al. Antimicrobial peptide LL-37 is upregulated in chronic nasal inflammatory disease. Acta Otolaryngol. 2003;123:81–5.
Bals R, Wang X, Zasloff M, et al. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A. 1998;95:9541–6.
Gudmundsson GH, Agerberth B. Neutrophil antibacterial peptides, multifunctional effector molecules in the mammalian immune system. J Immunol Methods. 1999;232:45–54.
Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–50.
Rockett KA, Brookes R, Udalova I, et al. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998;66:5314–21.
Desjardins M, Huber LA, Parton RG, et al. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol. 1994;124:677–88.
Russell DG. Mycobacterium and Leishmania: stowaways in the endosomal network. Trends Cell Biol. 1995;5:125–8.
Pieters J. Evasion of host cell defense mechanisms by pathogenic bacteria. Curr Opin Immunol. 2001;13:37–44.
Hmama Z, Sendide K, Talal A, et al. Quantitative analysis of phagolysosome fusion in intact cells: inhibition by mycobacterial lipoarabinomannan and rescue by an 1alpha,25-dihydroxyvitamin D3-phosphoinositide 3-kinase pathway. J Cell Sci. 2004;15:2131–40.
Coussens A, Timms PM, Boucher BJ, et al. 1alpha,25-dihydroxyvitamin D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. 2009;127:539–48.
Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70:345–52.
Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26.
Mahnke K, Johnson TS, Ring S, et al. Tolerogenic dendritic cells and regulatory T cells: a two-way relationship. J Dermatol Sci. 2007;46:159–67.
Medzhitov R, Janeway Jr C. Innate immunity. N Engl J Med. 2000;343:338–44.
Margulies DH. TCR avidity: it’s not how strong you make it, it’s how you make it strong. Nat Immunol. 2001;2:669–70.
Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–7.
Lemire JM, Archer DC, Beck L, et al. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125(6 Suppl):1704S–8.
Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep. 2011;11:29–36.
Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Review Immunol. 2008;8:523–32.
Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–67.
Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47.
Dimeloe S, Nanzer A, Ryanna K, et al. Regulatory T cells, inflammation and the allergic response - The role of glucocorticoids and Vitamin D. J Steroid Biochem Mol Biol. 2010;120:86–95.
Jirapongsananuruk O, Melamed I, Leung DY. Additive immunosuppressive effects of 1,25(OH)2D3 and corticosteroids on Th1, but not Th2, responses. J Allergy Clin Immunol. 2000;106:981–5.
Pichler J, Gerstmayr M, Szepfalusi Z, et al. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52:12–8.
• Rothers J, Wright A, Halonen M, et al. Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J Allergy Clin Immunol. 2011;128:1093–9. This article has shown that both high and low 25-hydroxyvitamin D levels are associated with aeroallergen sensitization.
Dunne Jr WM. Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev. 2002;15:155–66.
Otto M. Bacterial evasion of antimicrobial peptides by biofilm formation. Curr Top Microbiol Immunol. 2006;306:251–8.
Schmidtchen A, Frick I-M, Andersson E, et al. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol Microbiol. 2002;46:157–68.
Rosenfeld Y, Shai Y. Lipopolysaccharide (Endotoxin)-host defense antibacterial peptides interactions: role in bacterial resistance and prevention of sepsis. Biochim Biophys Acta. 2006;1758:1513–22.
Overhage J, Campisano A, Bains M, et al. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Imm. 2008;76:4176–82.
Chennupati SK, Chiu AG, Tamashiro E, et al. Effects of an LL-37-derived antimicrobial peptide in an animal model of biofilm Pseudomonas sinusitis. Am J Rhinol Allergy. 2009;23:46–51.
Matheson EM, Mainous 3rd AG, Hueston WJ, et al. Vitamin D and methicillin-resistant Staphylococcus aureus nasal carriage. Scand J Infect Dis. 2010;42:455–60.
Olsen K, Falch BM, Danielsen K, et al. Staphylococcus aureus nasal carriage is associated with serum 25-hydroxyvitamin D levels. Eur J Clin Microbiol Infect Dis. 2012;31:465–73.
Eastham KM, Freeman R, Kearns AM, et al. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax. 2004;59:522–5.
Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Resp Crit Care Med. 2000;162:1277–84.
Kolbe J, Wells A. Bronchiectasis: a neglected cause of respiratory morbidity and mortality. Respirology. 1996;1:221–5.
Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respir Med. 2010;104:981–5.
Seitz AE, Olivier KN, Steiner CA, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993–2006. Chest. 2010;138:944–9.
Field CE. Bronchiectasis. A long-term follow-up of medical and surgical cases from childhood. Arch Dis Child. 1961;36:587–603.
Twiss J, Metcalfe R, Edwards E, et al. New Zealand national incidence of bronchiectasis “too high” for a developed country. Arch Dis Child. 2005;90:737–40.
Haidopoulou K, Calder A, Jones A, et al. Bronchiectasis secondary to primary immunodeficiency in children: longitudinal changes in structure and function. Pediatr Pulmonol. 2009;44:669–75.
Eastham KM, Fall AJ, Mitchell L, et al. The need to redefine non-cystic fibrosis bronchiectasis in childhood. Thorax. 2004;59:324–7.
Courtney J, Kelly M, Watt A, et al. Quality of life and inflammation in exacerbations of bronchiectasis. Chron Respir Dis. 2008;5:161–8.
Dupont M, Gacouin A, Lena H, et al. Survival of patients with bronchiectasis after the first ICU stay for respiratory failure. Chest. 2004;125:1815–20.
Singleton R, Morris A, Redding G, et al. Bronchiectasis in Alaska Native children: causes and clinical courses. Pediatr Pulmonol. 2000;29:182–7.
Roberts ME, Lowndes L, Milne DG, et al. Socioeconomic deprivation, readmissions, mortality and acute exacerbations of bronchiectasis. Intern Med J. 2012;42:e129–36.
Roberts H, Wells A, Milne D, et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax. 2000;55:198–204.
Gaga M, Bentley AM, Humbert M, et al. Increases in CD4+ T lymphocytes, macrophages, neutrophils and interleukin 8 positive cells in the airways of patients with bronchiectasis. Thorax. 1998;53:685–91.
Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383–93.
Zheng L, Tipoe G, Lam W, et al. Endothelin-1 in stable bronchiectasis. Eur Respir J. 2000;16:146–9.
Tsang KW, Chan K, Ho P, et al. Sputum elastase in steady-state bronchiectasis. Chest. 2000;117:420–6.
Richman-Eisenstat JB, Jorens PG, Hebert CA, et al. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol. 1993;264:L413–8.
Shum DK, Chan SC, Ip MS. Neutrophil-mediated degradation of lung proteoglycans: stimulation by tumor necrosis factor-alpha in sputum of patients with bronchiectasis. Am J Resp Crit Care Med. 2000;162:1925–31.
Wilson R, Cole PJ. The effect of bacterial products on ciliary function. Am Rev Resp Dis. 1988;138:S49–53.
• Beck JM, Young VB, Huffnagle GB. The microbiome of the lung. Transl Res. 2012;160:258–66. An important review article about a rapidly evolving area of microbiology.
Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Resp Crit Care Med. 2011;184:957–63.
Hare KM, Grimwood K, Leach AJ, et al. Respiratory bacterial pathogens in the nasopharynx and lower airways of Australian indigenous children with bronchiectasis. J Pediatr. 2010;157:1001–5.
Godoy JM, Godoy AN, Ribalta G, et al. Bacterial pattern in chronic sinusitis and cystic fibrosis. Otolaryngol Head Neck Surg. 2011;145:673–6.
Guilemany JM, Angrill J, Alobid I, et al. United airways: the impact of chronic rhinosinusitis and nasal polyps in bronchiectasic patient’s quality of life. Allergy. 2009;64:1524–9.
Bardin P, Van Heerden B, Joubert J. Absence of pulmonary aspiration of sinus contents in patients with asthma and sinusitis. J Allergy Clin Immunol. 1990;86:82–3.
Brehm J, Schuemann B, Fuhlbrigge A, et al. Serum vitamin D levels and severe asthma exacerbations in the childhood asthma management program study. J Allergy Clin Immunol. 2010;126:52–8.
Brehm JM, Acosta-Perez E, Klei L, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Resp Crit Care Med. 2012;186:140–6.
Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156:105–14.
•• Bartley J, Camargo CA Jr. Vitamin D and infection. In: Vitamin D: oxidation, immunity & aging. Edited by Gombart A. Taylor & Francis Group, CRC Press; 2012:323–48. A recent review of clinical studies investigating the role of vitamin D in infectious disease.
Majak P, Olszowiec-Chlebna M, Smejda K, et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294–6.
Manaseki-Holland S, Maroof Z, Bruce J, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet. 2012;379:1419–27.
Martineau AR, Timms PM, Bothamley GH, et al. High-dose vitamin D3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377:242–50.
• Murdoch D, Slow S, Chambers S, et al. Effect of vitamin D3 supplementation on upper respiratory infections in healthy adults: A randomised, double blind, placebo-controlled trial. JAMA. 2012;308:1333–9. A recent RCT indicating that vitamin D levels above 75nmol/L may not provide protection against upper respiratory infection.
Nielsen NO, Skifte T, Andersson M, et al. Both high and low serum vitamin D concentrations are associated with tuberculosis: a case–control study in Greenland. Br J Nutr. 2010;104:1487–91.
Martineau AR. Bolus-dose vitamin D and prevention of childhood pneumonia. Lancet. 2012;379:1373–5.
Stephenson A, Brotherwood M, Robert R, et al. Cholecalciferol significantly increases 25-hydroxyvitamin D concentrations in adults with cystic fibrosis. Am J Clin Nutr. 2007;85:1307–11.
Grossmann RE, Zughaier SM, Kumari M, et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: A randomized, controlled trial. Dermatoendocrinol. 2012;4:191–7.
Grossmann RE, Zughaier SM, Liu S, et al. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr. 2012;66:1072–4.
Reid D, Toole B, Knox S, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011;93:1006–11.
Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28.
Calverley P, Pauwels Dagger R, Lofdahl C, et al. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. Eur Respir J. 2005;26:406–13.
Murray MP, Turnbull K, MacQuarrie S, et al. Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Eur Resp J. 2009;34:125–31.
Eaton T, Young P, Fergusson W, et al. The Dartmouth COOP Charts: a simple, reliable, valid and responsive quality of life tool for chronic obstructive pulmonary disease. Qual Life Res. 2005;14:575–85.
Djukanovic R, Sterk P, Fahy J, et al. Standardised methodology of sputum induction and processing. Eur Resp J. 2002;37:1s–2.
Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–6. author reply 7–8.
Avenell A, Cook JA, Maclennan GS, et al. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing. 2007;36:574–7.
Li-Ng M, Aloia JF, Pollack S, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137:1396–404.
Laaksi I, Ruohola JP, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. J Inf Dis. 2010;202:809–14.
Acknowledgments
Dr. Bartley, Dr. Garrett, and Professor Camargo Jr. have received a Health Research Council of New Zealand grant with which they are investigating the role of Vitamin D supplementation in adult bronchiectasis.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartley, J., Garrett, J., Grant, C.C. et al. Could Vitamin D Have a Potential Anti-Inflammatory and Anti-Infective Role in Bronchiectasis?. Curr Infect Dis Rep 15, 148–157 (2013). https://doi.org/10.1007/s11908-013-0321-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-013-0321-9