Key Points
-
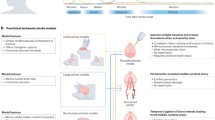
Biomaterials are having an increasingly important role in brain protection, repair and regeneration.
-
Micro and nano-technologies have provided tools that allow cell or tissue transplants to be effectively delivered into the brain.
-
Biomaterial scaffolds placed into the damaged area or cavity potentially provide support for the surrounding brain tissue, function as a substrate for cell growth, axon regeneration and neurite formation, and allow cell infiltration.
-
Biomaterials are being used to promote regeneration and repair of damaged neuronal pathways with stem cell therapies.
-
Current technologies allow greater control over material–cell interactions that mimic specific developmental processes and cellular responses including differentiation, migration and outgrowth.
Abstract
Biomaterials are likely to have an increasingly important role in the treatment of nervous system disorders. Recently developed biomaterials can enable and augment the targeted delivery of drugs or therapeutic proteins to the brain, allow cell or tissue transplants to be effectively delivered to the brain and help to rebuild damaged circuits. Similarly, biomaterials are being used to promote regeneration and to repair damaged neuronal pathways in combination with stem cell therapies. Many of these approaches are gaining momentum because nanotechnology allows greater control over material–cell interactions that induce specific developmental processes and cellular responses including differentiation, migration and outgrowth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pardridge, W. M. Drug targeting to the brain. Pharm. Res. 24, 1733–1744 (2007).
Pardridge W. M. Molecular biology of the blood-brain barrier. Mol. Biotechnol. 30, 57–70 (2005). Excellent introduction to the structural and molecular aspects of the blood–brain barrier that limit drug entry into the brain.
Popovic, N. & Brundin, P. Therapeutic potential of controlled drug delivery systems in neurodegenerative disorders. Int. J. Pharm. 314, 120–126 (2006).
Silva, G. A. Nanotechnology approaches for the regeneration and neuroprotection of the central nervous system. Surg. Neurol. 63, 301–306 (2005).
Halberstadt C and Emerich, D. F. (Eds) Cellular Transplants: From Lab to Clinic. (Academic Press, 2007).
Zhong, Z. & Bellamkonda, R. Biomaterials for the central nervous system. J. R. Soc. Interface 5, 957–975 (2008).
Pangalos, M. N., Schechter, L. E. & Hurko, O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nature Rev. Drug Discov. 6, 521–532 (2007).
Pardridge, W. M. The blood-brain barrier: bottleneck in brain development. NeuroRx 2, 3–14 (2005).
Neuwelt, E. et al. Strategies to advance translational research into brain barriers. Lancet Oncol. 7, 84–96 (2008). Expert meeting report on essential and high-priority recommendations to propel brain barrier research in different topical areas.
Weinstein, J. N., Blumenthal, R., Sharrow, S. O. & Henkart, P. A. Antibody mediated targeting of liposomes. Binding to lymphocytes does not ensure incorporation of vesicle contents into the cells. Biochem. Biophys. Acta 509, 272–288 (1978).
Béduneau, A., Saulnier, P. & Benoit, J. P. Active targeting of brain tumors using nanocarriers. Biomaterials 28, 4947–4967 (2007). Introductory review on the use of targeted colloidal systems used for drug delivery to the brain.
Siwak, D. R., Tari, A. M. & Lopez-Berestein, G. The potential of drug-carrying immunoliposomes as anticancer agents. Clin. Cancer Res. 8, 955–956 (2002).
Begley, D. J. Delivery of therapeutic agents to the central nervous system: the problems and the difficulties. Pharmacol. Ther. 104, 29–45 (2004).
Denora, N., Trapani, A., Laquintana, V., Lopedota, A. & Trapani, G. Recent advances in medicinal chemistry and pharmaceutical technology strategies for drug delivery to the brain. Curr. Topics Med. Chem. 9, 182–196 (2009).
Sahoo, S. K. & Labhasetwar, V. Nanotech approaches to drug delivery and imaging. Drug Discov. Today 8, 1112–1120 (2003).
Schnyder, A. & Huwyler, J. Drug transport to brain with targeted liposomes. NeuroRx 2, 99–107 (2005).
Lian, T. & Ho, R. J. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 90, 667–680 (2001).
Misra, A., Ganesh, S., Shahiwala, A. & Shah, S. P. Drug delivery to the central nervous system: a review. J. Pharm. Pharmacol. Sci. 6, 252–273 (2003).
Allen, T. M. Long-circulating (sterically stabilized) liposomes for targeted drug delivery. Trends Pharmacol. Sci. 15, 215–220 (1994).
Pardridge, W. M., Boado, R. J., Black, K. L. & Cancilla, P. A. Blood-brain barrier and new approaches to brain drug delivery. West J. Med. 156, 281–286 (1992).
Zhang, Y., Calon, F., Zhu, C., Boado, R. J. & Pardridge, W. M. Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism. Hum. Gene Ther. 14, 1–12 (2003).
Lockman, P. R., Mumper, R. J., Khan, M. A. & Allen, D. D. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev. Ind. Pharm. 28, 1–13 (2002).
Kreuter, J. et al. Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm. Res. 20, 409–416 (2003).
Menei, P. et al. Intracerebral implantation of NGF-releasing biodegradable microspheres protects striatum against excitotoxic damage. Exper. Neurol. 161, 259–272 (2000).
Mc Rae, A. & Dahlstrom, A. Transmitter-loaded polymeric microspheres induce regrowth of dopaminergic nerve terminals in striata of rats with 6-OH-DA induced parkinsonism. Neurochem. Int. 25, 27–33 (1994).
Schubert, D., Dargusch, R., Raitano, J. & Chan S. W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. 342, 86–91 (2006).
Blasi, P., Giovagnoli, S., Schoubben, A., Ricci, M. & Rossi, C. Solid lipid nanoparticles for targeted brain drug delivery. Adv. Drug Del. Rev. 59, 454–477 (2007). Interesting introductory review on the potential use of solid-lipid nanoparticles for targeted brain drug delivery.
Kaur, I. P., Bhandari, R., Bhandari, S. & Kakkar, V. Potential of solid lipid nanoparticles in brain drug targeting. J. Control Rel. 127, 97–109 (2008).
Brioschi, A. et al. Solid lipid nanoparticles: could they help to improve the efficacy of pharmacologic treatments for brain tumors? Neurol. Res. 29, 324–330 (2007).
Chattopadhyay, N. et al. Solid lipid nanoparticles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharm. Res. 25, 2262–2271 (2008).
Liu, L. et al. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG-TAT for drug delivery across the blood brain barrier. Biomaterials 29, 1509–1517 (2008).
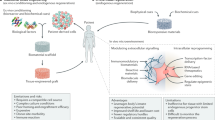
Orive, G. et al. Cell encapsulation: promise and progress. Nature Med. 9, 104–107 (2003). A critical view on cell microencapsulation technology proposed by a panel of experts in the field that summarizes recent progress in the field and outlines what is needed to bring this technology closer to clinical application.
De Vos P. et al. Multiscale requirements for bioencapsulation in medicine and biotechnology. Biomaterials 30, 2559–2570 (2009).
Czech, K. A. & Sagen, J. Update on cellular transplantation into the rat CNS as a novel therapy for chronic pain, Prog. Neurobiol., 46, 507–515 (1995).
Lindner, M. D. et al., Somatic delivery of catecholamines in the striatum attenuate parkinsonian symptoms and widen the therapeutic window or oral Sinemet in rats. Exper. Neurol. 14, 130–140 (1997).
Yasuhara, T. et al. Early transplantation of an encapsulated glial cell line-derived neurotrophic factor-producing cell demonstrating strong neuroprotective effects in a rat model of Parkinson disease. J. Neurosurg. 102, 80–89 (2005).
Sajadi A., Bensadoun J. C., Schneider B. L., Lo Bianco C. & Aebischer P. Transient striatal delivery of GDNF via encapsulated cells leads to sustained behavioral improvement in a bilateral model of Parkinson disease. Neurobiol. Dis. 22, 119–129 (2006).
Emerich, D. F. et al. Protective effects of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington's disease. Nature 386, 395–399 (1997).
Winn, S. R. et al. Polymer-encapsulated cells genetically modified to secrete human nerve growth factor promote the survival of axotomized septal cholinergic neurons, Proc. Natl. Acad. Sci. USA, 91, 23–28 (1994).
Tan, S. A. et al. Rescue of motoneurons from axotomy-induced cell death by polymer encapsulated cells genetically engineered to release CNTF. Cell Transpl. 5, 577–587 (1996).
Visted, T. & Lund-Johansen, M., Progress and challenges for cell encapsulation in brain tumor therapy. Expert Opin. Biol. Ther. 3, 551–561 (2003).
Emerich, D. F. et al. Extensive neuroprotection by choroid plexus transplants in excitotoxin lesioned monkeys. Neurobiol. Dis. 23, 471–480 (2006).
Bloch, J. et al. Neuroprotective gene therapy for Huntington's disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum. Gene Ther. 15, 968–975 (2004).
Aebischer, P. et al. Intrathecal delivery of CNTF using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nature Med. 2, 696–699 (1996). Initial clinical experience using xenogeneic, genetically modified cells to deliver a potentially therapeutic protein.
Zurn, A. D. et al. Evaluation of an intrathecal immune response in amyotrophic lateral sclerosis patients implanted with encapsulated genetically-engineered xenogeneic cells. Cell Transpl. 9, 471–484 (2000).
Sieving, A. et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants Proc. Natl Acad. Sci. USA 103, 3896–3901 (2006). Important demonstration of stable, long-term delivery of CNTF using genetically modified cells transplanted into the vitreous humor.
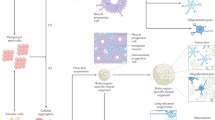
Fitch, M. T., Doller, C., Combs, C. K., Landreth, G. E. & Silver, J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 19, 8182–8198 (1999).
Nomura, H., Tator, C. H. & Shoichet, M. S. Bioengineered strategies for spinal cord repair. J. Neurotrauma 23, 496–507 (2006).
Geller, H. M. & Fawcett, J. W. Building a bridge: engineering spinal cord repair. Exp. Neurol. 174, 125–136 (2002).
Tsai, E. C., Dalton, P. D., Shoichet, M. S. & Tator, C. H. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. J. Neurotrauma 21, 789–804 (2004).
Piantino, J., Burdick, J. A., Goldberg, D., Langer, R. & Benowitz, L. I. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp. Neurol. 201, 359–367 (2006).
Park, K. I., Teng, Y. D. & Snyder, E. Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nature Biotechnol. 20, 1091–1093 (2002). Convincing demonstration that polymer scaffolds can reduce secondary brain tissue loss and support new and target appropriate tissue regeneration.
Tate, M. C. et al. Fibronectin promotes survival and migration of primary neural stem cells transplanted into the traumatically injured mouse brain. Cell Transpl. 11, 283–295 (2002).
Tate, C. C., et al. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. J. Tiss. Engin. Regen. Med. Feb. 19 Epub (2009).
Lee, Y. L. & Mooney, D. J. Cell-Interactive Polymers for Tissue Engineering. Fibers and Polymers 2, 51–57 (2001).
Misra, A., Ganesh, S., Shahiwala, A. & Shah, S. P. Drug delivery to the central nervous system: a review. J. Pharm. Pharmacol. Sci. 6, 252–273 (2003).
Wong, D. Y., Krebsbach, P. H., & Hollister, S. J. Brain cortex regeneration affected by scaffold architectures. J. Neurosurg. 109, 715–722, (2008).
Wong, D. Y., Hollister, S. J., Krebsbach, P. H. & Nosrat, C. Poly(ɛ-caprolactone) and poly (L-lactic-co-glycolic acid) degradable polymer sponges attenuate astrocyte response and lesion growth in acute traumatic brain injury. Tissue Engineer. 13, 2515–2523 (2007).
Zhang, H. et al. Gelatin-siloxane hybrid scaffolds with vascular endothelial growth factor induces brain tissue regeneration. Curr. Neurovasc. Res. 5, 112–117 (2008).
Potter, W., Kalil, R. E. & Kao, W. J. Biomimetic material systems for neural progenitor cell-based therapy. Front. Biosci. 13, 806–821, (2008).
Teixeira, A., Duckworth, J. K. & Hermanson, O. Getting the right stuff: controlling neural stem cell state and fate in vivo and in vitro with biomaterials. Cell Res. 17, 56–61 (2007).
Leach, J. K. Multifunctional cell-instructive materials for tissue regeneration; Reg. Med. 1, 447–455 (2006).
Norman, J. J. & Desai, T. A. Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann. Biomed. Eng. 34, 89–101 (2006).
Yamada, K. M. & Olden, K. Fibronectins-adhesive glycoproteins of cell surface and blood. Nature 275, 179–184, (1978).
Ruoslahti, E. & Pierschbacher, M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell 44, 517–518 (1986).
Woerly, S., Pinet, E., de Robertis, L., Van Diep, D. & Bousmina M. Spinal cord repair with PHPMA hydrogel containing RGD peptides (NeurogelTM). Biomaterials 20, 2213–2221 (2001).
Aota, S., Nomizu, M. & Yamada, K. M. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 269, 24756–24761 (1994).
Potter, W., Kalil, R. E. & Kao, W. J. Biomimetic material systems for neural progenitor cell-based therapy. Front. Biosci. 13, 806–821, (2008).
Smyth, N. et al. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J. Cell Biol. 144, 151–160 (1999).
Graf, J. et al. A pentapeptide from the laminin B1 chainmediates cell adhesion and binds the 67, 000 laminin receptor. Biochemistry 26, 6896–6900 (1987).
Jucker, M., Kleinman, H. K. & Ingram, D. K. Fetal rat septal cells adhere to and extend processes on basement membrane, laminin, and a synthetic peptide from the laminin A chain sequence. J. Neurosci. Res. 28, 507–517 (1991).
Yu, T. T. ; &. Shoichet, M. S. Guided cell adhesion and outgrowth in peptide-modified channels for neural tissue engineering. Biomaterials 26, 1507–1514 (2005).
Itoh, S. et al. Development of a nerve scaffold using a tendon chitosan tube. Artif. Organs 27, 1079–1088 (2003).
Ellis-Behnke, R. G. et al. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc. Natl Acad. Sci. USA 103, 5054–5059 (2006). Important demonstration that nanoscaffolds foster regeneration. In this study, the optic tract in hamsters regenerated following implantation of a self-assembled nanofibre scaffold resulting in histological and visual recovery.
Nisbet, D. R. et al. Interaction of embryonic cortical neurons on nanofibrous scaffolds for neural tissue engineering. J. Neural Engineer. 4, 35–41 (2007).
Silva, G. A. et al. Selective differentiation of neural progenitor cells by high epitope density nanofibers. Science 303, 1352–1355 (2004). Important demonstration of the capacity of nanofibres to rapidly and predictably control stem cell differentiation.
Willerth, S. M., Arendas, K. J., Gottlieb, D. I. & Sakiyama Elbert, S. E. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials 27, 5990–6003 (2006).
Brannvall, K. et al. Enhanced neuronal differentiation in a three-dimensional collagen–hyaluronan matrix. J. Neurosci. Res. 85, 2138–2146 (2007).
Bhang, S. H., Lim, J. S., Choi, C. Y., Kwon, Y. K. & Kim, B. S. The behavior of neural stem cells on biodegradable synthetic polymers. J. Biomater. Sci. Polym. Edin. 18, 223–239 (2007).
Hung, C. H., Lin, Y. L. & Young, T. H. The effect of chitosan and PVDF substrates on the behavior of embryonic rat cerebral cortical stem cells. Biomaterials 27, 4461–4469 (2006).
Young, T. H. & Hung, C. H. Behavior of embryonic rat cerebral cortical stem cells on the PVA and EVAL substrates. Biomaterials 26, 4291–4299 (2005).
Kulbatski, I., Mothe, A. J., Nomura, H. & Tator, C. H. Endogenous and exogenous CNS derived stem/progenitor cell approaches for neurotrauma. Curr. Drug Targets 6, 111–126 (2005).
Levenberg, S. et al. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc. Natl Acad. Sci. USA 100, 12741–12746 (2003).
Bible, E., Chau, D. Y. S., Alexander, M. R., Price, J., Shakesheff, K. M. & Modo M. The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials 30, 2985–2994 (2009).
Mahoney, M. J. & Saltzman, W. M. Transplantation of brain cells assembled around a programmable synthetic microenvironment. Nature Biotechnol. 19, 934–939 (2001).
Lu, D., Mahmood, A., Qu, C., Hong, X., Kaplan, D. & Chopp, M. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery 61, 596–602, (2007).
Nomura, H., Tator, C. H. & Shoichet, M. S. Bioengineered strategies for spinal cord repair. J. Neurotrauma 23, 496–507 (2006).
Jain, A., Kim, Y. T., McKeon, R. J. & Bellamkonda, R. V. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials 27, 497–504 (2001).
Hou, S. et al. The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. J. Neurosci. Methods 148, 60–70 (2005).
Novikov, L. N. et al. A novel biodegradable implant for neuronal rescue and regeneration after spinal cord injury. Biomaterials 23, 3369–3376 (2002).
Kamada T. et al. Transplantation of bone marrow stromal cell-derived Schwann cells promotes axonal regeneration and functional recovery after complete transection of adult rat spinal cord. J. Neuropathol. Exp. Neurol. 64, 37–45 (2005).
Rhodes, K. E. & Fawcett, J. W. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J. Anat. 204, 33–48 (2004).
Friedman, J. A. et al. Biodegradable polymer grafts for surgical repair of the injured spinal cord. Neurosurgery 51, 751–752 (2002).
Farokhzad, O. C. & Langer, R. Impact of nanotechnology on drug delivery. ACS Nano. 3, 16–20 (2009).
Gu, H., Long, D., Song, C. & Li X. Recombinant human NGF-loaded microspheres promote survival of basal forebrain cholinergic neurons and improve memory impairments of spatial learning in the rat model of Alzheimer's disease with fimbria-fornix lesion. Neurosci Lett 453, 204–209 (2009).
Péan, J. M., Menei, P., Morel, O., Montero-Menei, C. N. & Benoit, J. P. Intraseptal implantation of NGF-releasing microspheres promote the survival of axotomized cholinergic neurons. Biomaterials. 20, 2097–2101 (2000).
Garbayo, E. et al. Effective GDNF brain delivery using microspheres—a promising strategy for Parkinson's disease. J. Control Release 135, 119–126 (2009).
Buchser, E. et al. Immunoisolated xenogeneic chromaffin cell therapy for chronic pain. Initial clinical experience. Anesthesiology 5, 1005–1112 (1996).
Bachoud-Levi, A. C. et al. Neuroprotective gene therapy for Huntington's disease using a polymer encapsulated BHK cell line engineered to secrete human CNTF. Hum. Gene. Ther. 11, 1723–1729 (2000).
Leone, P. et al. Aspartoacylase gene transfer to the mammalian central nervous system with therapeutic implications for Canavan disease. Ann. Neurol. 48, 27–38 (2000).
Acknowledgements
We thank L. Sánchez for her assistance in preparing the original figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.O. and E.A are research scientists at the Biotechnology Institute (BTI), Vitoria, Spain. D.F.E. works for InCytu Inc., Lincoln, Rhode Island, USA.
Related links
Glossary
- Proteomics
-
The large-scale study of proteins, particularly their structures and functions.
- Oligonucleotide
-
A short nucleic-acid polymer, typically with 20 or fewer bases.
- Extracellular matrix
-
(ECM). Connective tissue produced largely by fibroblasts and astrocytes that provides diverse inhibitory and growth promoting signals to neurons and their extensions.
- Active targeting
-
A process whereby the surface of a particle is modified with a ligand so that when injected into the body it can bind to, or target, a specific cell receptor.
- Bioavailability
-
The fraction of an administered dose of drug that reaches the systemic circulation. By definition, when a drug is administered intravenously, its bioavailability is 100%.
- Reticuloendothelial system
-
(RES). Part of the immune system that consists of the phagocytic cells located in reticular connective tissue, primarily monocytes and macrophages.
- Immunoliposome
-
A spherical vesicle consisting of one or more concentric lipid bilayers enclosing one or more aqueous compartments that is coupled to an antibody.
- Biodegradable
-
The chemical breakdown of materials by a physiological environment.
- Entrapment
-
The process of incorporating a drug into a particle, scaffold or device. It is also a measurement to determine and compare the efficiency of drug incorporation.
- Covalent link
-
A bond characterized by the sharing of pairs of electrons between atoms, or between other covalent bonds. In short, attraction-to-repulsion stability that forms between atoms when they share electrons.
- Reactive oxygen species
-
(ROS). Ions or very small molecules that include oxygen ions, free radicals and peroxides, both inorganic and organic. They are highly reactive due to the presence of unpaired valence shell electrons.
- Surfactants
-
Wetting agents that lower the surface tension of a liquid, allowing easier spreading, and that lower the interfacial tension between two liquids.
- Amphiphilic block copolymers
-
Two or more homopolymer subunits linked by covalent bonds with an intermediate non-repeating subunit, known as a junction block. The amphiphilic copolymer has both lipophilic and hydrophilic parts.
- Encapsulation
-
The enclosure of a drug, peptide, cell or any other material within selectively permeable thermoplastics or three-dimensional particles surrounded or not by additional layers.
- Thermoplastic
-
A polymer that turns to a liquid when heated and freezes to a very glassy state when cooled sufficiently.
- Glial scar formation (gliosis)
-
A reactive cellular process involving astrogliosis that occurs after injury to the CNS. The glial scar is the body's mechanism to protect and begin the healing process in the nervous system.
- Nanogel
-
A nanomaterial based on hydrogels of crosslinked polymer network that often combine ionic and non-ionic chains and which enhances the transport of incorporated molecules into the brain.
- Cross linking
-
The joining of adjacent chains of a polymer or protein by creating covalent bonds.
- Self assembly
-
Processes in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. An example is the automatic arrangement of phospholipids into a cell wall.
Rights and permissions
About this article
Cite this article
Orive, G., Anitua, E., Pedraz, J. et al. Biomaterials for promoting brain protection, repair and regeneration. Nat Rev Neurosci 10, 682–692 (2009). https://doi.org/10.1038/nrn2685
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn2685
This article is cited by
-
In vivo chemical reprogramming of astrocytes into neurons
Cell Discovery (2021)
-
Systemic dendrimer-drug nanomedicines for long-term treatment of mild-moderate cerebral palsy in a rabbit model
Journal of Neuroinflammation (2020)
-
Foreign body responses in mouse central nervous system mimic natural wound responses and alter biomaterial functions
Nature Communications (2020)
-
Protective Mechanism and Treatment of Neurogenesis in Cerebral Ischemia
Neurochemical Research (2020)