-
PDF
- Split View
-
Views
-
Cite
Cite
Deborah Tomlinson, Leonard A. Mermel, Marie-Chantal Ethier, Anne Matlow, Biljana Gillmeister, Lillian Sung, Defining Bloodstream Infections Related to Central Venous Catheters in Patients With Cancer: A Systematic Review, Clinical Infectious Diseases, Volume 53, Issue 7, 1 October 2011, Pages 697–710, https://doi.org/10.1093/cid/cir523
Close - Share Icon Share
Abstract
The objective of this review was to determine whether consistent definitions were used in published studies of bloodstream infections due to central venous catheters in patients with cancer (ie, catheter-related or catheter-associated bloodstream infections). Review of 191 studies reporting catheter-related or catheter-associated bloodstream infections in patients with cancer revealed a lack of uniformity in these definitions. We grouped definitions by type, with 39 articles failing to cite or report a definition. Definitions included those of the Centers for Disease Control and Prevention (n = 39) and the Infectious Diseases Society of America (n = 18). The criteria included in the definitions in studies were also tabulated. Clinical manifestations were frequently included.
Definitions used have been highly variable; comparability of risk factors, incidence, management, and outcomes of such infections is difficult to achieve across studies. Future research should focus on development of a common definition of catheter-related and catheter-associated bloodstream infections for both adults and children with cancer.
Central venous catheters (CVCs) are frequently used in patients receiving treatment for cancer. They provide more easily accessible, long-term venous access for blood testing and the necessary delivery of treatment, including chemotherapy, blood products, and occasionally, parenteral nutrition [1]. However, use of CVCs can lead to bloodstream infection, frequently referred to as catheter-related bloodstream infection (CRBSI) or catheter-associated bloodstream infection (CABSI). Such infections are associated with serious morbidity and mortality and with increased health care costs [2–6]. Patients with cancer with CVCs are at particular risk of CRBSI and/or CABSI [4, 7, 8]. Signs and symptoms of these infections may be altered in patients with cancer due to neutropenia or steroid administration [7]. For example, CRBSI in neutropenic patients may be unaccompanied by inflammation or purulence at the catheter site [9].
The incidence of catheter-related infections reported in the literature varies from 9% to 80%, depending on catheter type and patient risk factors and on the definition of CRBSI or CABSI that is used [10]. Also, 70%–85% of patients with suspected catheter-related infections are proven not to have such infections after assessment of the results of catheter tip and blood cultures [11]. An accurate diagnosis of CRBSI and/or CABSI is important for several reasons: effective and timely treatment may reduce further complications; the diagnosis may influence subsequent treatment and potential catheter removal; and this outcome is an important end point for supportive care clinical trials.
The gold standard for diagnosis of CRBSI is the isolation of the same organism from a peripheral blood culture as that isolated from the tip of the removed CVC [12]. However, this definition is problematic, because the vast majority of patients suspected of having a bloodstream infection associated with a long-term CVC will not have their catheter removed. Consequently, many definitions have been proposed for CRBSI in the absence of catheter removal. A recently published guideline for the prevention of intravascular catheter infection describes the confusion regarding the terminology used to describe such infections because of the interchangeability of the definitions used for CRBSI and CABSI throughout the medical literature [13].
Definitions are used by infection control professionals for surveillance purposes to compare the incidence density of infections in similar contexts (ie, CABSI), by clinicians caring for patients on a day to day basis, and in clinical research studies, such as those comparing 2 intravascular devices in which a rigorous, unambiguous definition is required (eg, for CRBSI). The latter definition requires percutaneously obtained blood samples for culture, either catheter tip cultures or catheter-obtained blood cultures measured quantitatively, or both compared with regard to the differential growth rate of microbes in the percutaneously obtained and catheter-obtained blood samples for culture. Quantitative blood cultures are not universally done in clinical, nonstudy settings, and differential growth rates of blood culture bottles may not be reported by the microbiology laboratory. Thus, surveillance definitions (ie, for CABSI) are used to compare the incidence of bloodstream infections associated with CVCs in different units or institutions. Surveillance definitions overestimate the true incidence of CRBSI [13]. Despite the number of proposed definitions of CRBSI and CABSI [13–18], many published studies fail to make this distinction apparent. Because many definitions are applied for CRBSI or CABSI, comparisons between studies and interpretation of meta-analyses are difficult [19]. Optimally, investigators should use a common definition of CRBSI and CABSI, and a standard definition has been recommended for hematological patients [20].
The objectives of this study were to (1) describe the definitions used in studies that investigate CRBSI or CABSI in patients with cancer and (2) compare and contrast these definitions to assist in determining an optimal definition of CRBSI and CABSI in an oncological population.
METHODS
Search Strategy for Identification of Studies
We conducted literature searches with use of the OVID search platform MEDLINE, EMBASE, and Cochrane Controlled Trial Register from the database inception until 13 October 2010. The search strategy is attached as Appendix 1. We retrieved a total of 3010 references from all 3 databases. The search strategy included the available vocabulary terms and text words for bacterial infections and catheters and study designs. Studies focusing on renal dialysis catheters were excluded from the review with use of the Not Boolean operator. References were limited to English language. A total of 1033 duplicates were excluded.
Strategy for Selection of Articles for Review
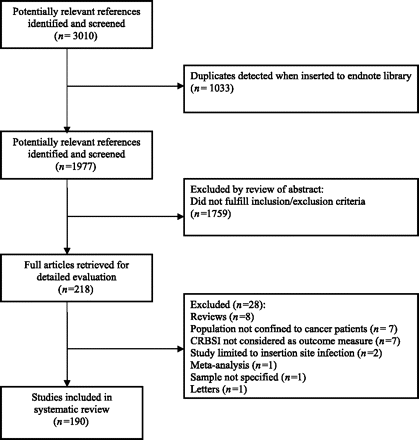
Articles were included if they were clinical research studies that reported on CRBSI or CABSI as an outcome measure in patients with cancer. Because of the imperceptible irregularities in the use of the 2 definitions, we considered it appropriate to include articles that reported on either CRBSI or CABSI. All CVC types were considered in our review, including totally implanted and partially implanted catheters and tunneled and nontunneled catheters. Studies were excluded if (1) they were reviews, guidelines, commentaries, or published abstracts, or if (2) the population was not specified to be oncological or stem cell transplant recipients. The term “central line–associated bloodstream infection” was also considered. There was no restriction by age. Figure 1 shows the flow of studies. One author (DT) reviewed the abstracts of the remaining 1977 unique references, and with use of the aforementioned inclusion and exclusion criteria, a total of 218 articles were retrieved for full text review. Of the retrieved full articles, 28 were excluded. Thus, a total of 190 studies were included for review.
Flow diagram of study identification and selection from literature search.
Where articles cited definite, probable, and possible definitions of CRBSI, only the definite definitions were reviewed. We also examined whether there was a distinction between CRBSI and CASBI definitions. Definitions were extracted, sorted, and tabulated according to similarity of definition. Criteria associated with the definitions were examined and listed for each definition, to determine frequency of cited criteria.
RESULTS
As noted above, 190 articles were included in our review. Table 1 lists the definitions used, demonstrating a large amount of variability. Only 14 of these articles predated the development of the Centers for Disease Control and Prevention (CDC) CRBSI definition (ie, articles were published before or during 1990). There were 16 unique CRBSI and/or CABSI definitions. A total of 39 articles that reported CRBSI and/or CABSI in patients with cancer did not cite or reference any definition of CRBSI and/or CABSI [174–212]. Despite several recent articles referring to central line–associated bloodstream infection [213–215], we did not find this term in the studies included in our review.
Catheter-Related Bloodstream Infection (CRBSI) and Catheter-Associated Bloodstream Infection (CABSI) Definitions Used in 190 Studies Involving Patients With Cancer
| Definition/reference used or cited in study | Number of studies | Study reference | |
| 1. | Centers for Disease Control and Prevention (CDC)/National Nosocomial Infections Surveillance (NNIS)/Healthcare Infection Control Practices Advisory Committee (HICPAC) [12, 13, 21, 22]: Catheter Associated Bloodstream Infection (CABSI) defined as: a) Bacteremia/fungemia in a patient with an intravascular catheter with at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infection (ie, fever, chills, and/or hypotension), and no apparent source for the bloodstream infection except the catheter[21] (may be referred to as CRBSI in some studies); OR b) Bloodstream infections are considered to be associated with a central line if the line was in use during the 48-hour period before the development of the bloodstream infection. (O'Grady, 2011 #13) Catheter Related Bloodstream Infection (CRBSI) defined as: c) Clinical manifestations and at least one positive blood culture from a peripheral vein and no other apparent source, with either positive semiquantitative (>15 CFU/catheter segment) or quantitative (>103 CFU/catheter segment) culture, whereby the same organism (species and antibiogram) is isolated from the catheter segment and a peripheral blood sample; simultaneous quantitative cultures of blood samples with a ratio of ≥3:1 (CVC vs. peripheral); differential period of CVC culture versus peripheral blood culture positivity of 2 h [11, 13]; OR d) Isolation of the same organism from semiquantitative or quantitative culture segment and from blood (preferably from a peripheral vein) of a patient with accompanying symptoms of bloodstream infection and no other apparent source of infection [12, 22] e) CDC definition cited but not confirmed in text Note: CDC definition may not have been cited in all cases, but definition was comparable. | =39* | a) [8, 23–31] b) [32–43] c) [44–48] d) [43, 49–54] e) [55–60] |
| 2. | Requires positive catheter tip/segment culture and positive peripheral blood culture (ie, requires catheter removal). (Ref #72, 73 Alternative of purulence from insertion site) (Ref #61 Definition also includes positive cultures from CVC) | 19 | [61–79] |
| 3. | Infectious Disease Society of America [11] Bacteremia or fungemia in a patient who has an intravascular device and ≥1 positive result of culture of blood samples obtained from the peripheral vein, clinical manifestations of infection (eg fever, chills, and/or hypotension), and no apparent source for bloodstream infection (with the exception of the catheter). One of the following should be present: a positive result of semiquantitative (≥15 CFU per catheter segment) or quantitative (≥1000 CFU per catheter segment) culture, whereby the same organism (species and antibiogram) is isolated from a catheter segment and a peripheral blood sample; simultaneous quantitative cultures of blood samples with a ratio of ≥3:1 (CVC vs. peripheral); differential time to positivity (ie, a positive result of culture from a CVC is obtained at least 2 h earlier than is a positive result of culture from peripheral blood). Note: IDSA definition may not have been cited in all cases, but definition was comparable | 18* | [41, 44, 48, 55, 80–93] |
| 4. | Requires positive culture from CVC blood only. | 12 | [94–105] |
| 5. | Requires positive blood cultures from both CVC and peripheral blood. | 11 | [106–116] |
| 6. | Requires positive CVC blood culture, with either a negative peripheral blood culture or a lower number of CFU in peripheral blood compared with CVC blood culture. | 11 | [117–127] |
| 7. | Any positive blood culture (with CVC in situ). | 9 | [128–136] |
| 8. | Clinical manifestations of infection that improve following removal of CVC (may or may not include positive blood cultures). | 8 | [137–144] |
| 9. | Includes positive culture swab from CVC site (may include other positive cultures from blood or catheter tip). | 6 | [145–150] |
| 10. | a) Greater than 10-fold increase in CFUs of organism/ml of blood obtained through catheter in comparison with simultaneously obtained peripheral blood cultures; b) In the absence of peripheral blood cultures, >1000 CFUs of organism/ml of blood obtained through the catheter; OR c) Positive catheter-tip culture when removed in clinical setting. | 5 | [151–155] |
| 11. | a) ≥10 CFU/ml through device compared with peripheral; b) >103 CFU/ml through device with negative peripheral cultures; c) Same organism from CVC sample and from swab of site; OR d) Relationship between CVC manipulation and onset of fever and rigors. | 1 | [156] |
| 12. | a) Temperature >38 with chills and rigors within 1 h of flushing or manipulation; b) Isolation of pathogen from blood culture drawn through catheter but not from another blood culture drawn from peripheral vein at the same time; c) Isolation of same pathogen from catheter tip and blood; OR d) Isolation of same pathogen from blood and purulent material draining from catheter exit site or subcutaneous tunnel. | 1 | [157] |
| 13. | Temperature ≥ 38C with positive blood cultures derived from the catheter and at least one of the following: a) Negative peripheral blood cultures; b) If simultaneously taken peripheral blood cultures were also positive, cultures from the catheter became positive at least 2 h earlier (differential time to positivity, DTP); c) Culture of the removed catheter tip grew ≥15 CFUs of the organism (semi-quantitative catheter segment culture); OR d) If peripheral blood cultures were not taken and the tip not removed or not sent for culture, there was no other obvious clinical, radiological or microbiological focus of infection. | 1 | [158] |
| 14. | Clinical syndrome compatible with sepsis in the absence of any clinically apparent source other than a central venous access device. | 1 | [159] |
| 15. | Clinical manifestations and positive venipuncture blood cultures. | 1 | [160] |
| 16. | Clinical manifestation of infection in absence of any other source of bloodstream infection except the catheter, in addition to one of following: a) Catheter colonization with at least 15 CFUs by roll plate or at least 1000 CFUs by sonication [161] with the same organism isolated from the bloodstream; OR b) Positive quantitative culture of blood drawn through the catheter, yielding five-fold or greater colony count than a quantitative culture of concurrently drawn peripheral venous blood growing the same organism. | 2 | [162, 163] |
| 17. | a) Clinical signs of infection, but no other identifiable focus of infection; AND b) Isolation of the same microorganism from blood and exudates from catheter exit site in presence of signs and inflammation, or isolation of same microorganism from blood and catheter tip. | 1 | [164] |
| 18. | High clinical suspicion with fever, requiring catheter removal. | 1 | [165] |
| 19. | a) Recognized pathogen cultured from one or more blood cultures; b) Common skin contaminant cultured from two or more blood cultures, both drawn at separate occasions; OR c) Skin contaminant identified from at least one blood culture in association with clinical signs | 1 | [166] |
| 20. | a) Culture of removed catheter tip with same organism isolated from the catheter tip and peripheral blood; OR b) Indicative differential time to positivity (ie, blood culture from Hickman became positive at least 2 h earlier than positive simultaneously-drawn peripheral blood culture) | 1 | [167] |
| 21. | No other primary source of infection identified with a) At least two sets of blood cultures positive for the same organism; OR b) One positive set accompanied by a positive drainage or catheter tip culture. | 1 | [168] |
| 22. | a) Clinical manifestations and positive culture; AND b) Catheter colonized with same organism; OR CVC blood culture ≥10-fold CFUs than peripheral blood culture. | 1 | [169] |
| 23. | Increase in temperature (>38.5 C), associated with chills or rigors which settled spontaneously or with antipyretic measures, in an otherwise well child, following flushing of the Broviac catheter; subsequent culture of Broviac intraluminal catheter fluid was undertaken to confirm infection. | 1 | [170] |
| 24. | Clinical features with quantitative blood culture ratio of >5:1 (CVC vs peripheral) or isolation of >100 CFU/ml from CVC-drawn blood culture. | 1 | [171] |
| 25. | a) Fever or clinical signs/symptoms of infection, blood cultures (at least one from CVC and one peripheral) are positive; AND b) Insertion site swab, CVC tip culture or positive CVC intraluminal (lock) culture yields growth identical to blood cultures. | 1 | [172] |
| 26. | a) Fever >38C with chills and rigors within 1 h after catheter flushing or manipulation; b) Isolation of a pathogen from CVC-drawn blood culture, but not from a simultaneously-obtained peripheral blood culture; c) Isolation of same pathogen from catheter tip and blood; OR d) Isolation of same organism from blood and from purulent material at exit site or subcutaneous tunnel. | 1 | [173] |
| 27. | Not defined | 39 | [174–212] |
| Definition/reference used or cited in study | Number of studies | Study reference | |
| 1. | Centers for Disease Control and Prevention (CDC)/National Nosocomial Infections Surveillance (NNIS)/Healthcare Infection Control Practices Advisory Committee (HICPAC) [12, 13, 21, 22]: Catheter Associated Bloodstream Infection (CABSI) defined as: a) Bacteremia/fungemia in a patient with an intravascular catheter with at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infection (ie, fever, chills, and/or hypotension), and no apparent source for the bloodstream infection except the catheter[21] (may be referred to as CRBSI in some studies); OR b) Bloodstream infections are considered to be associated with a central line if the line was in use during the 48-hour period before the development of the bloodstream infection. (O'Grady, 2011 #13) Catheter Related Bloodstream Infection (CRBSI) defined as: c) Clinical manifestations and at least one positive blood culture from a peripheral vein and no other apparent source, with either positive semiquantitative (>15 CFU/catheter segment) or quantitative (>103 CFU/catheter segment) culture, whereby the same organism (species and antibiogram) is isolated from the catheter segment and a peripheral blood sample; simultaneous quantitative cultures of blood samples with a ratio of ≥3:1 (CVC vs. peripheral); differential period of CVC culture versus peripheral blood culture positivity of 2 h [11, 13]; OR d) Isolation of the same organism from semiquantitative or quantitative culture segment and from blood (preferably from a peripheral vein) of a patient with accompanying symptoms of bloodstream infection and no other apparent source of infection [12, 22] e) CDC definition cited but not confirmed in text Note: CDC definition may not have been cited in all cases, but definition was comparable. | =39* | a) [8, 23–31] b) [32–43] c) [44–48] d) [43, 49–54] e) [55–60] |
| 2. | Requires positive catheter tip/segment culture and positive peripheral blood culture (ie, requires catheter removal). (Ref #72, 73 Alternative of purulence from insertion site) (Ref #61 Definition also includes positive cultures from CVC) | 19 | [61–79] |
| 3. | Infectious Disease Society of America [11] Bacteremia or fungemia in a patient who has an intravascular device and ≥1 positive result of culture of blood samples obtained from the peripheral vein, clinical manifestations of infection (eg fever, chills, and/or hypotension), and no apparent source for bloodstream infection (with the exception of the catheter). One of the following should be present: a positive result of semiquantitative (≥15 CFU per catheter segment) or quantitative (≥1000 CFU per catheter segment) culture, whereby the same organism (species and antibiogram) is isolated from a catheter segment and a peripheral blood sample; simultaneous quantitative cultures of blood samples with a ratio of ≥3:1 (CVC vs. peripheral); differential time to positivity (ie, a positive result of culture from a CVC is obtained at least 2 h earlier than is a positive result of culture from peripheral blood). Note: IDSA definition may not have been cited in all cases, but definition was comparable | 18* | [41, 44, 48, 55, 80–93] |
| 4. | Requires positive culture from CVC blood only. | 12 | [94–105] |
| 5. | Requires positive blood cultures from both CVC and peripheral blood. | 11 | [106–116] |
| 6. | Requires positive CVC blood culture, with either a negative peripheral blood culture or a lower number of CFU in peripheral blood compared with CVC blood culture. | 11 | [117–127] |
| 7. | Any positive blood culture (with CVC in situ). | 9 | [128–136] |
| 8. | Clinical manifestations of infection that improve following removal of CVC (may or may not include positive blood cultures). | 8 | [137–144] |
| 9. | Includes positive culture swab from CVC site (may include other positive cultures from blood or catheter tip). | 6 | [145–150] |
| 10. | a) Greater than 10-fold increase in CFUs of organism/ml of blood obtained through catheter in comparison with simultaneously obtained peripheral blood cultures; b) In the absence of peripheral blood cultures, >1000 CFUs of organism/ml of blood obtained through the catheter; OR c) Positive catheter-tip culture when removed in clinical setting. | 5 | [151–155] |
| 11. | a) ≥10 CFU/ml through device compared with peripheral; b) >103 CFU/ml through device with negative peripheral cultures; c) Same organism from CVC sample and from swab of site; OR d) Relationship between CVC manipulation and onset of fever and rigors. | 1 | [156] |
| 12. | a) Temperature >38 with chills and rigors within 1 h of flushing or manipulation; b) Isolation of pathogen from blood culture drawn through catheter but not from another blood culture drawn from peripheral vein at the same time; c) Isolation of same pathogen from catheter tip and blood; OR d) Isolation of same pathogen from blood and purulent material draining from catheter exit site or subcutaneous tunnel. | 1 | [157] |
| 13. | Temperature ≥ 38C with positive blood cultures derived from the catheter and at least one of the following: a) Negative peripheral blood cultures; b) If simultaneously taken peripheral blood cultures were also positive, cultures from the catheter became positive at least 2 h earlier (differential time to positivity, DTP); c) Culture of the removed catheter tip grew ≥15 CFUs of the organism (semi-quantitative catheter segment culture); OR d) If peripheral blood cultures were not taken and the tip not removed or not sent for culture, there was no other obvious clinical, radiological or microbiological focus of infection. | 1 | [158] |
| 14. | Clinical syndrome compatible with sepsis in the absence of any clinically apparent source other than a central venous access device. | 1 | [159] |
| 15. | Clinical manifestations and positive venipuncture blood cultures. | 1 | [160] |
| 16. | Clinical manifestation of infection in absence of any other source of bloodstream infection except the catheter, in addition to one of following: a) Catheter colonization with at least 15 CFUs by roll plate or at least 1000 CFUs by sonication [161] with the same organism isolated from the bloodstream; OR b) Positive quantitative culture of blood drawn through the catheter, yielding five-fold or greater colony count than a quantitative culture of concurrently drawn peripheral venous blood growing the same organism. | 2 | [162, 163] |
| 17. | a) Clinical signs of infection, but no other identifiable focus of infection; AND b) Isolation of the same microorganism from blood and exudates from catheter exit site in presence of signs and inflammation, or isolation of same microorganism from blood and catheter tip. | 1 | [164] |
| 18. | High clinical suspicion with fever, requiring catheter removal. | 1 | [165] |
| 19. | a) Recognized pathogen cultured from one or more blood cultures; b) Common skin contaminant cultured from two or more blood cultures, both drawn at separate occasions; OR c) Skin contaminant identified from at least one blood culture in association with clinical signs | 1 | [166] |
| 20. | a) Culture of removed catheter tip with same organism isolated from the catheter tip and peripheral blood; OR b) Indicative differential time to positivity (ie, blood culture from Hickman became positive at least 2 h earlier than positive simultaneously-drawn peripheral blood culture) | 1 | [167] |
| 21. | No other primary source of infection identified with a) At least two sets of blood cultures positive for the same organism; OR b) One positive set accompanied by a positive drainage or catheter tip culture. | 1 | [168] |
| 22. | a) Clinical manifestations and positive culture; AND b) Catheter colonized with same organism; OR CVC blood culture ≥10-fold CFUs than peripheral blood culture. | 1 | [169] |
| 23. | Increase in temperature (>38.5 C), associated with chills or rigors which settled spontaneously or with antipyretic measures, in an otherwise well child, following flushing of the Broviac catheter; subsequent culture of Broviac intraluminal catheter fluid was undertaken to confirm infection. | 1 | [170] |
| 24. | Clinical features with quantitative blood culture ratio of >5:1 (CVC vs peripheral) or isolation of >100 CFU/ml from CVC-drawn blood culture. | 1 | [171] |
| 25. | a) Fever or clinical signs/symptoms of infection, blood cultures (at least one from CVC and one peripheral) are positive; AND b) Insertion site swab, CVC tip culture or positive CVC intraluminal (lock) culture yields growth identical to blood cultures. | 1 | [172] |
| 26. | a) Fever >38C with chills and rigors within 1 h after catheter flushing or manipulation; b) Isolation of a pathogen from CVC-drawn blood culture, but not from a simultaneously-obtained peripheral blood culture; c) Isolation of same pathogen from catheter tip and blood; OR d) Isolation of same organism from blood and from purulent material at exit site or subcutaneous tunnel. | 1 | [173] |
| 27. | Not defined | 39 | [174–212] |
Abbreviations: CFU, colony forming unit; CVC, central venous catheter.
a Four studies used two definitions.
Catheter-Related Bloodstream Infection (CRBSI) and Catheter-Associated Bloodstream Infection (CABSI) Definitions Used in 190 Studies Involving Patients With Cancer
| Definition/reference used or cited in study | Number of studies | Study reference | |
| 1. | Centers for Disease Control and Prevention (CDC)/National Nosocomial Infections Surveillance (NNIS)/Healthcare Infection Control Practices Advisory Committee (HICPAC) [12, 13, 21, 22]: Catheter Associated Bloodstream Infection (CABSI) defined as: a) Bacteremia/fungemia in a patient with an intravascular catheter with at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infection (ie, fever, chills, and/or hypotension), and no apparent source for the bloodstream infection except the catheter[21] (may be referred to as CRBSI in some studies); OR b) Bloodstream infections are considered to be associated with a central line if the line was in use during the 48-hour period before the development of the bloodstream infection. (O'Grady, 2011 #13) Catheter Related Bloodstream Infection (CRBSI) defined as: c) Clinical manifestations and at least one positive blood culture from a peripheral vein and no other apparent source, with either positive semiquantitative (>15 CFU/catheter segment) or quantitative (>103 CFU/catheter segment) culture, whereby the same organism (species and antibiogram) is isolated from the catheter segment and a peripheral blood sample; simultaneous quantitative cultures of blood samples with a ratio of ≥3:1 (CVC vs. peripheral); differential period of CVC culture versus peripheral blood culture positivity of 2 h [11, 13]; OR d) Isolation of the same organism from semiquantitative or quantitative culture segment and from blood (preferably from a peripheral vein) of a patient with accompanying symptoms of bloodstream infection and no other apparent source of infection [12, 22] e) CDC definition cited but not confirmed in text Note: CDC definition may not have been cited in all cases, but definition was comparable. | =39* | a) [8, 23–31] b) [32–43] c) [44–48] d) [43, 49–54] e) [55–60] |
| 2. | Requires positive catheter tip/segment culture and positive peripheral blood culture (ie, requires catheter removal). (Ref #72, 73 Alternative of purulence from insertion site) (Ref #61 Definition also includes positive cultures from CVC) | 19 | [61–79] |
| 3. | Infectious Disease Society of America [11] Bacteremia or fungemia in a patient who has an intravascular device and ≥1 positive result of culture of blood samples obtained from the peripheral vein, clinical manifestations of infection (eg fever, chills, and/or hypotension), and no apparent source for bloodstream infection (with the exception of the catheter). One of the following should be present: a positive result of semiquantitative (≥15 CFU per catheter segment) or quantitative (≥1000 CFU per catheter segment) culture, whereby the same organism (species and antibiogram) is isolated from a catheter segment and a peripheral blood sample; simultaneous quantitative cultures of blood samples with a ratio of ≥3:1 (CVC vs. peripheral); differential time to positivity (ie, a positive result of culture from a CVC is obtained at least 2 h earlier than is a positive result of culture from peripheral blood). Note: IDSA definition may not have been cited in all cases, but definition was comparable | 18* | [41, 44, 48, 55, 80–93] |
| 4. | Requires positive culture from CVC blood only. | 12 | [94–105] |
| 5. | Requires positive blood cultures from both CVC and peripheral blood. | 11 | [106–116] |
| 6. | Requires positive CVC blood culture, with either a negative peripheral blood culture or a lower number of CFU in peripheral blood compared with CVC blood culture. | 11 | [117–127] |
| 7. | Any positive blood culture (with CVC in situ). | 9 | [128–136] |
| 8. | Clinical manifestations of infection that improve following removal of CVC (may or may not include positive blood cultures). | 8 | [137–144] |
| 9. | Includes positive culture swab from CVC site (may include other positive cultures from blood or catheter tip). | 6 | [145–150] |
| 10. | a) Greater than 10-fold increase in CFUs of organism/ml of blood obtained through catheter in comparison with simultaneously obtained peripheral blood cultures; b) In the absence of peripheral blood cultures, >1000 CFUs of organism/ml of blood obtained through the catheter; OR c) Positive catheter-tip culture when removed in clinical setting. | 5 | [151–155] |
| 11. | a) ≥10 CFU/ml through device compared with peripheral; b) >103 CFU/ml through device with negative peripheral cultures; c) Same organism from CVC sample and from swab of site; OR d) Relationship between CVC manipulation and onset of fever and rigors. | 1 | [156] |
| 12. | a) Temperature >38 with chills and rigors within 1 h of flushing or manipulation; b) Isolation of pathogen from blood culture drawn through catheter but not from another blood culture drawn from peripheral vein at the same time; c) Isolation of same pathogen from catheter tip and blood; OR d) Isolation of same pathogen from blood and purulent material draining from catheter exit site or subcutaneous tunnel. | 1 | [157] |
| 13. | Temperature ≥ 38C with positive blood cultures derived from the catheter and at least one of the following: a) Negative peripheral blood cultures; b) If simultaneously taken peripheral blood cultures were also positive, cultures from the catheter became positive at least 2 h earlier (differential time to positivity, DTP); c) Culture of the removed catheter tip grew ≥15 CFUs of the organism (semi-quantitative catheter segment culture); OR d) If peripheral blood cultures were not taken and the tip not removed or not sent for culture, there was no other obvious clinical, radiological or microbiological focus of infection. | 1 | [158] |
| 14. | Clinical syndrome compatible with sepsis in the absence of any clinically apparent source other than a central venous access device. | 1 | [159] |
| 15. | Clinical manifestations and positive venipuncture blood cultures. | 1 | [160] |
| 16. | Clinical manifestation of infection in absence of any other source of bloodstream infection except the catheter, in addition to one of following: a) Catheter colonization with at least 15 CFUs by roll plate or at least 1000 CFUs by sonication [161] with the same organism isolated from the bloodstream; OR b) Positive quantitative culture of blood drawn through the catheter, yielding five-fold or greater colony count than a quantitative culture of concurrently drawn peripheral venous blood growing the same organism. | 2 | [162, 163] |
| 17. | a) Clinical signs of infection, but no other identifiable focus of infection; AND b) Isolation of the same microorganism from blood and exudates from catheter exit site in presence of signs and inflammation, or isolation of same microorganism from blood and catheter tip. | 1 | [164] |
| 18. | High clinical suspicion with fever, requiring catheter removal. | 1 | [165] |
| 19. | a) Recognized pathogen cultured from one or more blood cultures; b) Common skin contaminant cultured from two or more blood cultures, both drawn at separate occasions; OR c) Skin contaminant identified from at least one blood culture in association with clinical signs | 1 | [166] |
| 20. | a) Culture of removed catheter tip with same organism isolated from the catheter tip and peripheral blood; OR b) Indicative differential time to positivity (ie, blood culture from Hickman became positive at least 2 h earlier than positive simultaneously-drawn peripheral blood culture) | 1 | [167] |
| 21. | No other primary source of infection identified with a) At least two sets of blood cultures positive for the same organism; OR b) One positive set accompanied by a positive drainage or catheter tip culture. | 1 | [168] |
| 22. | a) Clinical manifestations and positive culture; AND b) Catheter colonized with same organism; OR CVC blood culture ≥10-fold CFUs than peripheral blood culture. | 1 | [169] |
| 23. | Increase in temperature (>38.5 C), associated with chills or rigors which settled spontaneously or with antipyretic measures, in an otherwise well child, following flushing of the Broviac catheter; subsequent culture of Broviac intraluminal catheter fluid was undertaken to confirm infection. | 1 | [170] |
| 24. | Clinical features with quantitative blood culture ratio of >5:1 (CVC vs peripheral) or isolation of >100 CFU/ml from CVC-drawn blood culture. | 1 | [171] |
| 25. | a) Fever or clinical signs/symptoms of infection, blood cultures (at least one from CVC and one peripheral) are positive; AND b) Insertion site swab, CVC tip culture or positive CVC intraluminal (lock) culture yields growth identical to blood cultures. | 1 | [172] |
| 26. | a) Fever >38C with chills and rigors within 1 h after catheter flushing or manipulation; b) Isolation of a pathogen from CVC-drawn blood culture, but not from a simultaneously-obtained peripheral blood culture; c) Isolation of same pathogen from catheter tip and blood; OR d) Isolation of same organism from blood and from purulent material at exit site or subcutaneous tunnel. | 1 | [173] |
| 27. | Not defined | 39 | [174–212] |
| Definition/reference used or cited in study | Number of studies | Study reference | |
| 1. | Centers for Disease Control and Prevention (CDC)/National Nosocomial Infections Surveillance (NNIS)/Healthcare Infection Control Practices Advisory Committee (HICPAC) [12, 13, 21, 22]: Catheter Associated Bloodstream Infection (CABSI) defined as: a) Bacteremia/fungemia in a patient with an intravascular catheter with at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infection (ie, fever, chills, and/or hypotension), and no apparent source for the bloodstream infection except the catheter[21] (may be referred to as CRBSI in some studies); OR b) Bloodstream infections are considered to be associated with a central line if the line was in use during the 48-hour period before the development of the bloodstream infection. (O'Grady, 2011 #13) Catheter Related Bloodstream Infection (CRBSI) defined as: c) Clinical manifestations and at least one positive blood culture from a peripheral vein and no other apparent source, with either positive semiquantitative (>15 CFU/catheter segment) or quantitative (>103 CFU/catheter segment) culture, whereby the same organism (species and antibiogram) is isolated from the catheter segment and a peripheral blood sample; simultaneous quantitative cultures of blood samples with a ratio of ≥3:1 (CVC vs. peripheral); differential period of CVC culture versus peripheral blood culture positivity of 2 h [11, 13]; OR d) Isolation of the same organism from semiquantitative or quantitative culture segment and from blood (preferably from a peripheral vein) of a patient with accompanying symptoms of bloodstream infection and no other apparent source of infection [12, 22] e) CDC definition cited but not confirmed in text Note: CDC definition may not have been cited in all cases, but definition was comparable. | =39* | a) [8, 23–31] b) [32–43] c) [44–48] d) [43, 49–54] e) [55–60] |
| 2. | Requires positive catheter tip/segment culture and positive peripheral blood culture (ie, requires catheter removal). (Ref #72, 73 Alternative of purulence from insertion site) (Ref #61 Definition also includes positive cultures from CVC) | 19 | [61–79] |
| 3. | Infectious Disease Society of America [11] Bacteremia or fungemia in a patient who has an intravascular device and ≥1 positive result of culture of blood samples obtained from the peripheral vein, clinical manifestations of infection (eg fever, chills, and/or hypotension), and no apparent source for bloodstream infection (with the exception of the catheter). One of the following should be present: a positive result of semiquantitative (≥15 CFU per catheter segment) or quantitative (≥1000 CFU per catheter segment) culture, whereby the same organism (species and antibiogram) is isolated from a catheter segment and a peripheral blood sample; simultaneous quantitative cultures of blood samples with a ratio of ≥3:1 (CVC vs. peripheral); differential time to positivity (ie, a positive result of culture from a CVC is obtained at least 2 h earlier than is a positive result of culture from peripheral blood). Note: IDSA definition may not have been cited in all cases, but definition was comparable | 18* | [41, 44, 48, 55, 80–93] |
| 4. | Requires positive culture from CVC blood only. | 12 | [94–105] |
| 5. | Requires positive blood cultures from both CVC and peripheral blood. | 11 | [106–116] |
| 6. | Requires positive CVC blood culture, with either a negative peripheral blood culture or a lower number of CFU in peripheral blood compared with CVC blood culture. | 11 | [117–127] |
| 7. | Any positive blood culture (with CVC in situ). | 9 | [128–136] |
| 8. | Clinical manifestations of infection that improve following removal of CVC (may or may not include positive blood cultures). | 8 | [137–144] |
| 9. | Includes positive culture swab from CVC site (may include other positive cultures from blood or catheter tip). | 6 | [145–150] |
| 10. | a) Greater than 10-fold increase in CFUs of organism/ml of blood obtained through catheter in comparison with simultaneously obtained peripheral blood cultures; b) In the absence of peripheral blood cultures, >1000 CFUs of organism/ml of blood obtained through the catheter; OR c) Positive catheter-tip culture when removed in clinical setting. | 5 | [151–155] |
| 11. | a) ≥10 CFU/ml through device compared with peripheral; b) >103 CFU/ml through device with negative peripheral cultures; c) Same organism from CVC sample and from swab of site; OR d) Relationship between CVC manipulation and onset of fever and rigors. | 1 | [156] |
| 12. | a) Temperature >38 with chills and rigors within 1 h of flushing or manipulation; b) Isolation of pathogen from blood culture drawn through catheter but not from another blood culture drawn from peripheral vein at the same time; c) Isolation of same pathogen from catheter tip and blood; OR d) Isolation of same pathogen from blood and purulent material draining from catheter exit site or subcutaneous tunnel. | 1 | [157] |
| 13. | Temperature ≥ 38C with positive blood cultures derived from the catheter and at least one of the following: a) Negative peripheral blood cultures; b) If simultaneously taken peripheral blood cultures were also positive, cultures from the catheter became positive at least 2 h earlier (differential time to positivity, DTP); c) Culture of the removed catheter tip grew ≥15 CFUs of the organism (semi-quantitative catheter segment culture); OR d) If peripheral blood cultures were not taken and the tip not removed or not sent for culture, there was no other obvious clinical, radiological or microbiological focus of infection. | 1 | [158] |
| 14. | Clinical syndrome compatible with sepsis in the absence of any clinically apparent source other than a central venous access device. | 1 | [159] |
| 15. | Clinical manifestations and positive venipuncture blood cultures. | 1 | [160] |
| 16. | Clinical manifestation of infection in absence of any other source of bloodstream infection except the catheter, in addition to one of following: a) Catheter colonization with at least 15 CFUs by roll plate or at least 1000 CFUs by sonication [161] with the same organism isolated from the bloodstream; OR b) Positive quantitative culture of blood drawn through the catheter, yielding five-fold or greater colony count than a quantitative culture of concurrently drawn peripheral venous blood growing the same organism. | 2 | [162, 163] |
| 17. | a) Clinical signs of infection, but no other identifiable focus of infection; AND b) Isolation of the same microorganism from blood and exudates from catheter exit site in presence of signs and inflammation, or isolation of same microorganism from blood and catheter tip. | 1 | [164] |
| 18. | High clinical suspicion with fever, requiring catheter removal. | 1 | [165] |
| 19. | a) Recognized pathogen cultured from one or more blood cultures; b) Common skin contaminant cultured from two or more blood cultures, both drawn at separate occasions; OR c) Skin contaminant identified from at least one blood culture in association with clinical signs | 1 | [166] |
| 20. | a) Culture of removed catheter tip with same organism isolated from the catheter tip and peripheral blood; OR b) Indicative differential time to positivity (ie, blood culture from Hickman became positive at least 2 h earlier than positive simultaneously-drawn peripheral blood culture) | 1 | [167] |
| 21. | No other primary source of infection identified with a) At least two sets of blood cultures positive for the same organism; OR b) One positive set accompanied by a positive drainage or catheter tip culture. | 1 | [168] |
| 22. | a) Clinical manifestations and positive culture; AND b) Catheter colonized with same organism; OR CVC blood culture ≥10-fold CFUs than peripheral blood culture. | 1 | [169] |
| 23. | Increase in temperature (>38.5 C), associated with chills or rigors which settled spontaneously or with antipyretic measures, in an otherwise well child, following flushing of the Broviac catheter; subsequent culture of Broviac intraluminal catheter fluid was undertaken to confirm infection. | 1 | [170] |
| 24. | Clinical features with quantitative blood culture ratio of >5:1 (CVC vs peripheral) or isolation of >100 CFU/ml from CVC-drawn blood culture. | 1 | [171] |
| 25. | a) Fever or clinical signs/symptoms of infection, blood cultures (at least one from CVC and one peripheral) are positive; AND b) Insertion site swab, CVC tip culture or positive CVC intraluminal (lock) culture yields growth identical to blood cultures. | 1 | [172] |
| 26. | a) Fever >38C with chills and rigors within 1 h after catheter flushing or manipulation; b) Isolation of a pathogen from CVC-drawn blood culture, but not from a simultaneously-obtained peripheral blood culture; c) Isolation of same pathogen from catheter tip and blood; OR d) Isolation of same organism from blood and from purulent material at exit site or subcutaneous tunnel. | 1 | [173] |
| 27. | Not defined | 39 | [174–212] |
Abbreviations: CFU, colony forming unit; CVC, central venous catheter.
a Four studies used two definitions.
Of the remaining 151 studies, the most common definition cited was that stated by the CDC (n = 39) [8, 23–60]. This definition [13] is applied often, but its use is not limited to surveillance studies. The CDC definition is the only apparent definition that specifies a difference between CABSI and CRBSI. However, despite referencing the CDC definitions, the actual definition used varied from the CDC definitions (eg, some articles referred to CDC definitions but defined CRBSI with positive results of culture of percutaneously and CVC-obtained blood samples without any differential considerations). In addition, the term CABSI was used often interchangeably with CRBSI. A few studies cited CABSI as the outcome measure, which does not include catheter tip cultures [32–36].
The next most frequently used definitions included aspects of the CDC definition; one definition included a positive catheter tip culture result (ie, requiring catheter removal; n = 19) [61–79], and the other by the Infectious Diseases Society of America (IDSA) included a positive catheter tip culture result or a differential growth rate or quantitative results between percutaneously and CVC-obtained blood cultures (n = 18) [41, 44, 48, 55, 80–93].
Other definitions that do not require the presence of clinical manifestations were requirement of a positive result of culture of CVC blood sample only (n = 12) [94–105], positive result of blood culture from both CVC and peripheral blood (n = 11) [106–116], differential between CVC and peripheral blood cultures (n = 11) [117–127], any positive blood culture result (n = 9) [128–136], and positive result of culture of a CVC exit site sample (n = 6) [145–150]. Some studies defined CRBSI as improvement of clinical manifestations after removal of the CVC (n = 8) [137–144].
Table 2 shows the various components included for each definition. Fifty-four definitions of CRBSI and/or CABSI were included in 42 articles published since 2006. Among all included articles, clinical manifestations were most frequently cited (n = 135). Three studies citing clinical manifestations of infection were concerned only with symptoms if they were related to flushing or manipulation of the CVC, as shown in Table 2 [156, 157, 173]. Positive peripheral blood culture result was the next most frequently cited criterion (n = 67), with a similar number of definitions requiring a positive catheter tip culture result (n = 50) or a positive CVC blood culture result (n = 50). Table 2 also shows the proportion of studies with a combination of criteria that were published more recently (ie, during or after 2006). Although obvious trends were not apparent, 65% of 17 studies requiring clinical manifestations and peripheral blood cultures were published during or after 2006.
Criteria Associated With Definitions of Catheter-Related Bloodstream Infection (CRBSI) in Patients With Cancer
| Author | Number of papers | Criteria | Defined as catheter- associated or catheter- related | Number of papers published from 2006 onwards (n = 54) | |||||||
| Clinical manifestation | CVC-drawn blood culture positive | CVC-tip culture positive | Peripheral blood culture positive | EITHER CVC OR peripheral blood culture positive | Differential between CVC and peripheral culturesa | CVC insertion site culture positive | |||||
| Present | Resolve after CVC removal | ||||||||||
| [137–144] | 8 | ✓ | ✓ | ✓ | 3 CRBSI; 1 CABSI 2 CR septicemia 2 unspecified | 0/8, 0% | |||||
| [77, 172] | 2 | ✓ | ✓ | ✓ | ✓ | 2 CRBSI | 1/2, 50% | ||||
| [158, 162, 163] | 3 | ✓ | ✓ | ✓ | 3 CRBSI | 1/3, 33% | |||||
| [150, 172] | 2 | ✓ | ✓ | ✓ | ✓ | 1 CR-infection; 1 CRBSI | 1/2, 50% | ||||
| [23, 51, 53, 58–60, 106, 147, 148] | 9 | ✓ | ✓ | ✓ | 6 CRBSI; 2 unspecified; 1 CABSI | 2/9, 22% | |||||
| [141, 147] | 2 | ✓ | ✓ | ✓ | 1 CRBSI; 1 unspecified | 0/2, 0% | |||||
| [28–30, 95, 96, 98–101, 104, 105, 146, 170] | 13 | ✓ | ✓ | 6 CRBSI; 1 CABSI; 1 CA-septicemia 3 CR-septicemia; 1 intra-catheter infection 1 unspecified | 4/13, 31% | ||||||
| [52, 63, 65–68, 70, 71, 73, 78, 80, 81, 127, 164] | 14 | ✓ | ✓ | ✓ | 12 CRBSI 1 CR-septicemia 1 CABSI | 3/14, 21% | |||||
| [38, 43, 48–50, 54, 79, 93, 142, 146, 149, 169] | 12 | ✓ | ✓ | ✓ | 10 CRBSI; 1 CR-septicemia 1 unspecified | 3/12, 25% | |||||
| [31, 102, 104] | 3 | ✓ | ✓ | 3 CRBSI | 2/3, 67% | ||||||
| [44, 127] | 2 | ✓ | ✓ | ✓ | 1 CRBSI; 1 CR-septicemia | 1/2, 50% | |||||
| [24–26, 31, 35–37, 39–42, 45, 55–57, 103, 160] | 17 | ✓ | ✓ | 7 CABSI; 4 unspecified; 6 CRBSI | 11/17, 65% | ||||||
| [38, 50, 142, 146, 149, 164] | 6 | ✓ | ✓ | ✓ | 4 CRBSI; 1 CR-septicemia 1unspecified | 1/6, 17% | |||||
| [8, 27, 32–34, 43, 128–136, 166] | 16 | ✓ | ✓ | 8 CABSI; 3 CRBSI; 3 CR-sepsis; 2 unspecified | 8/16, 50% | ||||||
| [41, 46–48, 80, 81, 85–93, 117, 118, 120, 125, 127, 142, 146, 147, 158, 162, 163, 169, 171] | 28 | ✓ | ✓ | 20 CRBSI; 5 CR-septicemia; 1 CA-bacteremia; 2 unspecified | 8/28, 29% | ||||||
| [159, 165, 173] | 3 | ✓ | 1 CRBSI; 1 CR sepsis; 1 unspecified | 0/3, 0% | |||||||
| [64, 69, 107–112, 114–116] | 11 | ✓ | ✓ | 5 CRBSI; 3 CR septicemia; 3 unspecified | 1/11, 9% | ||||||
| [94, 97, 103] | 3 | ✓ | 1 CRBSI; 2 unspecified | 0/3, 0% | |||||||
| [151–155] | 5 | >1000 CFUs | 5 CRBSI | 0/5, 0% | |||||||
| [61, 62, 72, 74–76, 126, 167] | 8 | ✓ | ✓ | 6 CRBSI; 2 CR-septicemia | 1/8, 13% | ||||||
| [92, 157, 168, 173] | 4 | ✓ | ✓ | 4 CRBSI | 2/4, 50% | ||||||
| [151–155] | 5 | ✓ | 5 CRBSI | 0/5, 0% | |||||||
| [145, 157] | 2 | ✓ | ✓ | 2 CRBSI | 1/2, 50% | ||||||
| [168,173] | 2 | ✓ | ✓ | CRBSI | 0/2; 0% | ||||||
| [82–84, 113, 119, 121–124, 126, 151–157, 167, 173] | 19 | ✓ | 2 unspecified, 2 CR-septicemia 14 CRBSI; 1 CR infection | 3/19, 16% | |||||||
| Total number of papers with cited criteria | 135 | 8 | 50 | 50 | 67 | 46 | 45 | 17 | 54 | ||
| Author | Number of papers | Criteria | Defined as catheter- associated or catheter- related | Number of papers published from 2006 onwards (n = 54) | |||||||
| Clinical manifestation | CVC-drawn blood culture positive | CVC-tip culture positive | Peripheral blood culture positive | EITHER CVC OR peripheral blood culture positive | Differential between CVC and peripheral culturesa | CVC insertion site culture positive | |||||
| Present | Resolve after CVC removal | ||||||||||
| [137–144] | 8 | ✓ | ✓ | ✓ | 3 CRBSI; 1 CABSI 2 CR septicemia 2 unspecified | 0/8, 0% | |||||
| [77, 172] | 2 | ✓ | ✓ | ✓ | ✓ | 2 CRBSI | 1/2, 50% | ||||
| [158, 162, 163] | 3 | ✓ | ✓ | ✓ | 3 CRBSI | 1/3, 33% | |||||
| [150, 172] | 2 | ✓ | ✓ | ✓ | ✓ | 1 CR-infection; 1 CRBSI | 1/2, 50% | ||||
| [23, 51, 53, 58–60, 106, 147, 148] | 9 | ✓ | ✓ | ✓ | 6 CRBSI; 2 unspecified; 1 CABSI | 2/9, 22% | |||||
| [141, 147] | 2 | ✓ | ✓ | ✓ | 1 CRBSI; 1 unspecified | 0/2, 0% | |||||
| [28–30, 95, 96, 98–101, 104, 105, 146, 170] | 13 | ✓ | ✓ | 6 CRBSI; 1 CABSI; 1 CA-septicemia 3 CR-septicemia; 1 intra-catheter infection 1 unspecified | 4/13, 31% | ||||||
| [52, 63, 65–68, 70, 71, 73, 78, 80, 81, 127, 164] | 14 | ✓ | ✓ | ✓ | 12 CRBSI 1 CR-septicemia 1 CABSI | 3/14, 21% | |||||
| [38, 43, 48–50, 54, 79, 93, 142, 146, 149, 169] | 12 | ✓ | ✓ | ✓ | 10 CRBSI; 1 CR-septicemia 1 unspecified | 3/12, 25% | |||||
| [31, 102, 104] | 3 | ✓ | ✓ | 3 CRBSI | 2/3, 67% | ||||||
| [44, 127] | 2 | ✓ | ✓ | ✓ | 1 CRBSI; 1 CR-septicemia | 1/2, 50% | |||||
| [24–26, 31, 35–37, 39–42, 45, 55–57, 103, 160] | 17 | ✓ | ✓ | 7 CABSI; 4 unspecified; 6 CRBSI | 11/17, 65% | ||||||
| [38, 50, 142, 146, 149, 164] | 6 | ✓ | ✓ | ✓ | 4 CRBSI; 1 CR-septicemia 1unspecified | 1/6, 17% | |||||
| [8, 27, 32–34, 43, 128–136, 166] | 16 | ✓ | ✓ | 8 CABSI; 3 CRBSI; 3 CR-sepsis; 2 unspecified | 8/16, 50% | ||||||
| [41, 46–48, 80, 81, 85–93, 117, 118, 120, 125, 127, 142, 146, 147, 158, 162, 163, 169, 171] | 28 | ✓ | ✓ | 20 CRBSI; 5 CR-septicemia; 1 CA-bacteremia; 2 unspecified | 8/28, 29% | ||||||
| [159, 165, 173] | 3 | ✓ | 1 CRBSI; 1 CR sepsis; 1 unspecified | 0/3, 0% | |||||||
| [64, 69, 107–112, 114–116] | 11 | ✓ | ✓ | 5 CRBSI; 3 CR septicemia; 3 unspecified | 1/11, 9% | ||||||
| [94, 97, 103] | 3 | ✓ | 1 CRBSI; 2 unspecified | 0/3, 0% | |||||||
| [151–155] | 5 | >1000 CFUs | 5 CRBSI | 0/5, 0% | |||||||
| [61, 62, 72, 74–76, 126, 167] | 8 | ✓ | ✓ | 6 CRBSI; 2 CR-septicemia | 1/8, 13% | ||||||
| [92, 157, 168, 173] | 4 | ✓ | ✓ | 4 CRBSI | 2/4, 50% | ||||||
| [151–155] | 5 | ✓ | 5 CRBSI | 0/5, 0% | |||||||
| [145, 157] | 2 | ✓ | ✓ | 2 CRBSI | 1/2, 50% | ||||||
| [168,173] | 2 | ✓ | ✓ | CRBSI | 0/2; 0% | ||||||
| [82–84, 113, 119, 121–124, 126, 151–157, 167, 173] | 19 | ✓ | 2 unspecified, 2 CR-septicemia 14 CRBSI; 1 CR infection | 3/19, 16% | |||||||
| Total number of papers with cited criteria | 135 | 8 | 50 | 50 | 67 | 46 | 45 | 17 | 54 | ||
Alternative definitions given within one study have been included separately within table.Abbreviations: CABSI, catheter-associated bloodstream infection; CR, catheter-related; CVC, central venous catheter.
a Differential is defined as (1) quantitative difference in colony forming unit load (including 1 positive and 1 negative culture result) or (2) differential time to positive detection of growth in blood cultures.
Criteria Associated With Definitions of Catheter-Related Bloodstream Infection (CRBSI) in Patients With Cancer
| Author | Number of papers | Criteria | Defined as catheter- associated or catheter- related | Number of papers published from 2006 onwards (n = 54) | |||||||
| Clinical manifestation | CVC-drawn blood culture positive | CVC-tip culture positive | Peripheral blood culture positive | EITHER CVC OR peripheral blood culture positive | Differential between CVC and peripheral culturesa | CVC insertion site culture positive | |||||
| Present | Resolve after CVC removal | ||||||||||
| [137–144] | 8 | ✓ | ✓ | ✓ | 3 CRBSI; 1 CABSI 2 CR septicemia 2 unspecified | 0/8, 0% | |||||
| [77, 172] | 2 | ✓ | ✓ | ✓ | ✓ | 2 CRBSI | 1/2, 50% | ||||
| [158, 162, 163] | 3 | ✓ | ✓ | ✓ | 3 CRBSI | 1/3, 33% | |||||
| [150, 172] | 2 | ✓ | ✓ | ✓ | ✓ | 1 CR-infection; 1 CRBSI | 1/2, 50% | ||||
| [23, 51, 53, 58–60, 106, 147, 148] | 9 | ✓ | ✓ | ✓ | 6 CRBSI; 2 unspecified; 1 CABSI | 2/9, 22% | |||||
| [141, 147] | 2 | ✓ | ✓ | ✓ | 1 CRBSI; 1 unspecified | 0/2, 0% | |||||
| [28–30, 95, 96, 98–101, 104, 105, 146, 170] | 13 | ✓ | ✓ | 6 CRBSI; 1 CABSI; 1 CA-septicemia 3 CR-septicemia; 1 intra-catheter infection 1 unspecified | 4/13, 31% | ||||||
| [52, 63, 65–68, 70, 71, 73, 78, 80, 81, 127, 164] | 14 | ✓ | ✓ | ✓ | 12 CRBSI 1 CR-septicemia 1 CABSI | 3/14, 21% | |||||
| [38, 43, 48–50, 54, 79, 93, 142, 146, 149, 169] | 12 | ✓ | ✓ | ✓ | 10 CRBSI; 1 CR-septicemia 1 unspecified | 3/12, 25% | |||||
| [31, 102, 104] | 3 | ✓ | ✓ | 3 CRBSI | 2/3, 67% | ||||||
| [44, 127] | 2 | ✓ | ✓ | ✓ | 1 CRBSI; 1 CR-septicemia | 1/2, 50% | |||||
| [24–26, 31, 35–37, 39–42, 45, 55–57, 103, 160] | 17 | ✓ | ✓ | 7 CABSI; 4 unspecified; 6 CRBSI | 11/17, 65% | ||||||
| [38, 50, 142, 146, 149, 164] | 6 | ✓ | ✓ | ✓ | 4 CRBSI; 1 CR-septicemia 1unspecified | 1/6, 17% | |||||
| [8, 27, 32–34, 43, 128–136, 166] | 16 | ✓ | ✓ | 8 CABSI; 3 CRBSI; 3 CR-sepsis; 2 unspecified | 8/16, 50% | ||||||
| [41, 46–48, 80, 81, 85–93, 117, 118, 120, 125, 127, 142, 146, 147, 158, 162, 163, 169, 171] | 28 | ✓ | ✓ | 20 CRBSI; 5 CR-septicemia; 1 CA-bacteremia; 2 unspecified | 8/28, 29% | ||||||
| [159, 165, 173] | 3 | ✓ | 1 CRBSI; 1 CR sepsis; 1 unspecified | 0/3, 0% | |||||||
| [64, 69, 107–112, 114–116] | 11 | ✓ | ✓ | 5 CRBSI; 3 CR septicemia; 3 unspecified | 1/11, 9% | ||||||
| [94, 97, 103] | 3 | ✓ | 1 CRBSI; 2 unspecified | 0/3, 0% | |||||||
| [151–155] | 5 | >1000 CFUs | 5 CRBSI | 0/5, 0% | |||||||
| [61, 62, 72, 74–76, 126, 167] | 8 | ✓ | ✓ | 6 CRBSI; 2 CR-septicemia | 1/8, 13% | ||||||
| [92, 157, 168, 173] | 4 | ✓ | ✓ | 4 CRBSI | 2/4, 50% | ||||||
| [151–155] | 5 | ✓ | 5 CRBSI | 0/5, 0% | |||||||
| [145, 157] | 2 | ✓ | ✓ | 2 CRBSI | 1/2, 50% | ||||||
| [168,173] | 2 | ✓ | ✓ | CRBSI | 0/2; 0% | ||||||
| [82–84, 113, 119, 121–124, 126, 151–157, 167, 173] | 19 | ✓ | 2 unspecified, 2 CR-septicemia 14 CRBSI; 1 CR infection | 3/19, 16% | |||||||
| Total number of papers with cited criteria | 135 | 8 | 50 | 50 | 67 | 46 | 45 | 17 | 54 | ||
| Author | Number of papers | Criteria | Defined as catheter- associated or catheter- related | Number of papers published from 2006 onwards (n = 54) | |||||||
| Clinical manifestation | CVC-drawn blood culture positive | CVC-tip culture positive | Peripheral blood culture positive | EITHER CVC OR peripheral blood culture positive | Differential between CVC and peripheral culturesa | CVC insertion site culture positive | |||||
| Present | Resolve after CVC removal | ||||||||||
| [137–144] | 8 | ✓ | ✓ | ✓ | 3 CRBSI; 1 CABSI 2 CR septicemia 2 unspecified | 0/8, 0% | |||||
| [77, 172] | 2 | ✓ | ✓ | ✓ | ✓ | 2 CRBSI | 1/2, 50% | ||||
| [158, 162, 163] | 3 | ✓ | ✓ | ✓ | 3 CRBSI | 1/3, 33% | |||||
| [150, 172] | 2 | ✓ | ✓ | ✓ | ✓ | 1 CR-infection; 1 CRBSI | 1/2, 50% | ||||
| [23, 51, 53, 58–60, 106, 147, 148] | 9 | ✓ | ✓ | ✓ | 6 CRBSI; 2 unspecified; 1 CABSI | 2/9, 22% | |||||
| [141, 147] | 2 | ✓ | ✓ | ✓ | 1 CRBSI; 1 unspecified | 0/2, 0% | |||||
| [28–30, 95, 96, 98–101, 104, 105, 146, 170] | 13 | ✓ | ✓ | 6 CRBSI; 1 CABSI; 1 CA-septicemia 3 CR-septicemia; 1 intra-catheter infection 1 unspecified | 4/13, 31% | ||||||
| [52, 63, 65–68, 70, 71, 73, 78, 80, 81, 127, 164] | 14 | ✓ | ✓ | ✓ | 12 CRBSI 1 CR-septicemia 1 CABSI | 3/14, 21% | |||||
| [38, 43, 48–50, 54, 79, 93, 142, 146, 149, 169] | 12 | ✓ | ✓ | ✓ | 10 CRBSI; 1 CR-septicemia 1 unspecified | 3/12, 25% | |||||
| [31, 102, 104] | 3 | ✓ | ✓ | 3 CRBSI | 2/3, 67% | ||||||
| [44, 127] | 2 | ✓ | ✓ | ✓ | 1 CRBSI; 1 CR-septicemia | 1/2, 50% | |||||
| [24–26, 31, 35–37, 39–42, 45, 55–57, 103, 160] | 17 | ✓ | ✓ | 7 CABSI; 4 unspecified; 6 CRBSI | 11/17, 65% | ||||||
| [38, 50, 142, 146, 149, 164] | 6 | ✓ | ✓ | ✓ | 4 CRBSI; 1 CR-septicemia 1unspecified | 1/6, 17% | |||||
| [8, 27, 32–34, 43, 128–136, 166] | 16 | ✓ | ✓ | 8 CABSI; 3 CRBSI; 3 CR-sepsis; 2 unspecified | 8/16, 50% | ||||||
| [41, 46–48, 80, 81, 85–93, 117, 118, 120, 125, 127, 142, 146, 147, 158, 162, 163, 169, 171] | 28 | ✓ | ✓ | 20 CRBSI; 5 CR-septicemia; 1 CA-bacteremia; 2 unspecified | 8/28, 29% | ||||||
| [159, 165, 173] | 3 | ✓ | 1 CRBSI; 1 CR sepsis; 1 unspecified | 0/3, 0% | |||||||
| [64, 69, 107–112, 114–116] | 11 | ✓ | ✓ | 5 CRBSI; 3 CR septicemia; 3 unspecified | 1/11, 9% | ||||||
| [94, 97, 103] | 3 | ✓ | 1 CRBSI; 2 unspecified | 0/3, 0% | |||||||
| [151–155] | 5 | >1000 CFUs | 5 CRBSI | 0/5, 0% | |||||||
| [61, 62, 72, 74–76, 126, 167] | 8 | ✓ | ✓ | 6 CRBSI; 2 CR-septicemia | 1/8, 13% | ||||||
| [92, 157, 168, 173] | 4 | ✓ | ✓ | 4 CRBSI | 2/4, 50% | ||||||
| [151–155] | 5 | ✓ | 5 CRBSI | 0/5, 0% | |||||||
| [145, 157] | 2 | ✓ | ✓ | 2 CRBSI | 1/2, 50% | ||||||
| [168,173] | 2 | ✓ | ✓ | CRBSI | 0/2; 0% | ||||||
| [82–84, 113, 119, 121–124, 126, 151–157, 167, 173] | 19 | ✓ | 2 unspecified, 2 CR-septicemia 14 CRBSI; 1 CR infection | 3/19, 16% | |||||||
| Total number of papers with cited criteria | 135 | 8 | 50 | 50 | 67 | 46 | 45 | 17 | 54 | ||
Alternative definitions given within one study have been included separately within table.Abbreviations: CABSI, catheter-associated bloodstream infection; CR, catheter-related; CVC, central venous catheter.
a Differential is defined as (1) quantitative difference in colony forming unit load (including 1 positive and 1 negative culture result) or (2) differential time to positive detection of growth in blood cultures.
For the diagnosis of CRBSI without CVC removal, experts have recommended use of quantitative blood cultures or measurement of the differential time to positivity of blood cultures [19]. Differential microbial load or time to positivity between peripheral and CVC blood cultures were included as criteria for CRBSI in 45 studies in our review, with only 11 of these studies published within the past 5 years. One study included a definition of CRBSI that compared the results of quantitative blood cultures of the lumens of double-lumen CVCs [82].
DISCUSSION
Despite past efforts to attain a standard definition for CRBSI or CABSI [15, 19], a consistent definition for such infections in patients with cancer has not yet been used in the literature. A considerable number of articles (39 [21%] of 190) did not report the definition or cite a reference for a definition, but these were included in our review to emphasize the lack of uniformity in the study of CRBSI or CABSI. Although the CDC definition was most frequently cited, this accounted for only 39 (26%) of the 151 studies that provided a definition for CRBSI or CABSI as an outcome. After 1990, CDC definitions accounted for 34 (25%) of the 137 definitions used. Surprisingly, we found 26 definitions used in studies of CRBSI and/or CABSI in patients with cancer.
Criteria including clinical manifestations alone are not conclusive for defining a CRBSI or CABSI. A diagnosis of CRBSI requires removal of the catheter for quantitative or semiquantitative catheter tip culture, with concordant growth on culture of a percutaneously obtained blood sample, at those institutions not using differential time to positivity or quantitative blood cultures to diagnose CRBSI [11]. However, only 15%–25% of CVCs removed because of suspected infection actually have significant microbial growth, which implies that clinical manifestations of such infections are not sensitive or specific [216]. Definitions of CABSI do not require catheter removal and catheter tip culture, but this term needs to be applied with greater homogeneity with the realization that its original intent is for surveillance purposes and comparisons of rates of infection in different patient care units or different institutions; lastly, it should be used with a clear understanding that CABSI has reduced specificity and an increased number of false-positive results [13]. To avoid the removal of a catheter and the risk associated with placement of a new catheter, other diagnostic tests, such as differential quantitative blood cultures of samples taken simultaneously from the catheter and a peripheral vein, have been proposed [216]. The IDSA has adopted this as one of their measures in the diagnosis of CRBSI, and we found 15 studies that used this microbiologic method. However, despite its high specificity, this culture method is labor intensive and costly [216].
The measurement of differential time to positivity between cultures of CVC-obtained and percutaneously obtained blood samples has also been used to diagnose CRBSI. The CDC and IDSA have incorporated this criterion into their definitions. This is defined as the time to positive detection of growth in peripheral blood cultures minus that of the CVC-obtained blood cultures. Blot et al [216] used a cutoff value of +120 minutes, the differential time to positivity of the paired blood samples, and reported 91% specificity and 94% sensitivity for the diagnosis of CRBSI. However, because the accuracy of this method depends on inoculum size, it is important that the same volume of blood per bottle be submitted for culture. This may be a problem when it is difficult to obtain percutaneous blood samples (eg, in infants and children) [19]. Nevertheless, this is a preferred method for the diagnosis of CRBSI in institutions that do not perform quantitative blood cultures [19, 93]. Of note, only 45 (30%) of 190 studies in our review used differential time to positivity of blood cultures.
Of note, 67 (44%) of 151 CRBSI definitions provided in studies required performance of peripheral blood cultures. Some investigators have suggested that peripheral cultures are not necessary in the assessment of febrile patients with cancer [95]. However, omission of percutaneously obtained blood cultures would reduce specificity and may lead to excessive antimicrobial use, and it would lessen the rigor of epidemiological studies and the interpretation of trials designed to measure the impact of interventions aimed at reducing CRBSIs.
Because of the reluctance to obtain peripheral blood samples from children with a CVC in place, there has been interest in obtaining samples from different CVC lumens and using quantitative blood cultures or assessing differential time to positivity to diagnose CRBSI. A 5-fold difference in colony forming units or a differential growth time of ≥180 minutes between samples obtained from different catheter lumens has been suggested as a way to define CRBSI [19, 82, 84]. However, only 2 studies in our review that cited use of the IDSA definition used this technique [82, 84]. Of interest, a recent study that investigated CRBSI in double- and triple-lumen catheters in a population that included patients with cancer concluded that blood samples should be obtained from all lumens [217]. Capdevilla et al [16] applied a definition of isolation of ≥100 colony-forming units/mL in a quantitative blood culture from a CVC as highly suggestive of a CRBSI. Gaur et al [84] found that this definition had a positive predictive value of 79%–92%, depending on the approach used to analyze the data.
An important factor to consider in patients with cancer is the requirement that signs and symptoms of infection be present to meet criteria for CRBSI [218]. However, corticosteroid treatment reduces signs of inflammation, and neutropenic patients have a limited ability to produce purulent exudates. Consequently, signs and symptoms of CVC-related infections may differ in this population, although it is not yet known whether a definition specific to patients with cancer is necessary.
Our review has shown that, because of the many definitions used, it is difficult to make comparisons across studies. The incidences of CRBSI or CABSI vary considerably depending on the definition adopted. Future research should determine whether a different definition of CRBSI and CABSI in adult and pediatric patients with cancer is needed.
We thank Elizabeth Uleryk, for her valuable assistance with the search strategies necessary for this review, and Rhonda Adams for retrieving many of the articles that we reviewed.
Financial support. This work was supported by the Canadian Institutes of Health Research (New Investigator Grant to L. S.) and the employees of Kraft Canada Inc.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments