Summary
Abstract
Specifically formulated for nebulisation using the PARI LC PLUS™ reusable nebuliser, tobramycin solution for inhalation (TSI) [TOBI®] provides a high dose of tobramycin (an aminoglycoside antibacterial with good activity against Pseudomonas aeruginosa) to the lungs of patients with cystic fibrosis, while maintaining low serum concentrations of the drug, thus reducing the risk of systemic toxicity.
Intermittent (28-day on/28-day off) treatment with TSI 300mg twice daily significantly (p < 0.001) improved lung function and sputum P. aeruginosa density compared with placebo (randomised double-blind trials), and was significantly (p = 0.008) more effective than colistin for improvement in forced expiratory volume in 1 second (small nonblind trial) in patients aged ≥6 years with cystic fibrosis and chronic P. aeruginosa infection. Improvements in lung function were most marked in adolescent patients (aged 13–17 years) in placebo-controlled trials. Improvements were maintained for up to 96 weeks in patients in an open-label extension study. Fewer TSI than placebo recipients required parenteral antipseudomonal agents or hospitalisation. In addition, TSI 300mg twice daily for 28 days reduced P. aeruginosa density in the lower airways of patients aged <6 years with early colonisation and cystic fibrosis, although TSI is not currently indicated in this patient group.
A decrease in tobramycin susceptibility of P. aeruginosa isolates and an increase in fungal organisms (Candida albicans and Aspergillus species) during prolonged intermittent treatment with TSI 300mg twice daily was not associated with adverse clinical outcome. There was no evidence of selection for the most resistant isolates.
TSI is generally well tolerated, with no renal toxicity or hearing loss in clinical trials, although transient mild or moderate tinnitus occurred more frequently in TSI than placebo recipients. Bronchospasm after administration of TSI was transient and occurred with a similar incidence to that with placebo; TSI is preservative free and specifically formulated for the lung in terms of osmolality and pH.
In conclusion, TSI provides an effective means of delivering tobramycin to the lungs of patients with cystic fibrosis with chronic P. aeruginosa infection, improving lung function and sputum P. aeruginosa density in these patients without the nephrotoxicity or ototoxicity of parenteral aminoglycosides. Further data on the potential for and clinical significance of increased tobramycin resistance and fungal colonisation during TSI treatment would be beneficial, as would longer-term data. In the meantime, TSI represents a valuable option for suppressive antipseudomonal therapy in patients with cystic fibrosis.
Antibacterial Activity
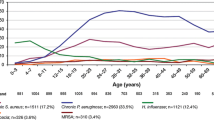
Tobramycin, an aminoglycoside antibacterial, had good in vitro activity against Pseudomonas aeruginosa in sputum isolates from patients with cystic fibrosis; the minimum concentrations required to inhibit the growth of 90% (MIC90) of strains was 8 μg/mL in the largest study (involving 1240 isolates) [MIC90 = 8–64 μg/mL in smaller studies]. According to breakpoint values for systemic antibacterials issued by the US National Committee for Clinical Laboratory Standards (NCCLS) [MIC ≤4 μg/mL susceptible, MIC 8 μg/mL intermediate, MIC ≥16 μg/mL resistant], 89% of 1240 P. aeruginosa isolates from 508 patients with cystic fibrosis were susceptible to tobramycin and 5.4% were resistant; the range of resistance for other antibacterial agents was 11–21%. Increased resistance of non-mucoid compared with mucoid strains was evident with tobramycin in one study (although statistical analysis was not performed) but not in another study. Tobramycin was active against most (65–84%) nonaminoglycoside single- or multiple-drug resistant isolates. About one- to two-thirds of isolates resistant to tobramycin were susceptible to other agents. Resistance to tobramycin results mostly from impermeability of isolates.
In phase III placebo-controlled trials in patients aged ≥6 years with cystic fibrosis, there was a significant decrease in the tobramycin susceptibility of P. aeruginosa isolates (characterised by an increase in MIC90 from 8 [baseline] to 16 μg/mL [weeks 20 and 24]) and an increase in fungal organisms (Candida albicans and Aspergillus species) during intermittent treatment with tobramycin solution for inhalation (TSI) 300mg twice daily. However, clinical outcome was not adversely affected. Furthermore there was no evidence of selection for the most resistant isolates.
Similarly, improvement in lung function was not affected by tobramycin MIC after 96 weeks of alternating treatment (i.e. 28 days on/28 days off) with TSI 300mg twice daily in patients with cystic fibrosis, despite reduced susceptibility of P. aeruginosa to tobramycin. In a subgroup of adolescent patients, increases were observed in the percentage of resistant isolates (5% [baseline] to 19% [study end]) and MIC90 (from 8 to 32 μg/mL).
Pharmacokinetic Properties
TSI achieved lung concentrations of tobramycin sufficient for an antibacterial effect against P. aeruginosa in most patients aged ≥6 with moderate-to-severe cystic fibrosis, while maintaining minimal systemic exposure. Ten minutes after single-dose TSI 300mg, sputum concentrations of tobramycin were 35–7417 μg/g (mean 1237 μg/g; median 959 μg/g), demonstrating wide interindividual variability. At 60 minutes after TSI, serum tobramycin concentrations remained low (≤3.62 μg/mL, mean 0.95 μg/mL, median 0.91 μg/mL). There was no accumulation of tobramycin in sputum or serum after multiple-dose administration (measured at week 20). The median ratio of serum-to-sputum tobramycin concentrations was 0.01 after TSI administration, demonstrating an improved therapeutic ratio compared with parenteral aminoglycosides.
The estimated bioavailability of tobramycin following administration of TSI 300mg was 11.7% in a population pharmacokinetic analysis using systemic clearance of 5.79 L/h and an apparent clearance of 49.6 L/h.
In children aged <6 years with cystic fibrosis, peak serum drug concentrations (0.6 μg/mL [range <0.2–1.2 μg/mL]) were reached 1 hour after administration of TSI 300mg, and were lower than the maximum accepted trough concentrations (2 μg/mL) for parenteral administration of tobramycin. Lower respiratory tract drug concentrations were within the bactericidal range for P. aeruginosa, with lung epithelial lining fluid concetrations being 16–204 μg/mL (mean 90 μg/mL) and above the target of 20 μg/mL (i.e. 10 times the MIC90 for P. aeruginosa) in 11 of 12 patients.
After inhalation, systemically absorbed tobramycin is assumed to be eliminated primarily by glomerular filtration, and unabsorbed tobramycin residing in the endobronchial space is probably eliminated primarily in expectorated sputum.
Therapeutic Efficacy
In Patients aged ≥6 years with cystic fibrosis and chronic P. aeruginosa respiratory infection, TSI 300mg twice daily significantly improved lung function and sputum P. aeruginosa density versus placebo or baseline in well designed trials. In addition, TSI 300mg twice daily was significantly more effective than colistin for improvement in forced expiratory volume in 1 second (FEV1) in a nonblind trial of short duration (involving only the antibacterial phase of one 28-day on/28-day off cycle of TSI) in 100 patients with cystic fibrosis and chronic P. aeruginosa infection.
In two identically designed, randomised, double-blind, placebo-controlled trials (pooled analysis, n = 520), marked improvements in lung function (≈12% increase from baseline in FEV1) occurred within 2 weeks of treatment initiation and were maintained throughout the treatment period (three 28-day on/28-day off cycles). At treatment end, the total treatment effect (absolute difference between TSI and placebo) was ≈12%. Mean FEV1 was maintained above pretreatment (baseline) for up to 96 weeks in an open-label extension phase. Patients aged 13–17 years were more responsive to treatment than other patient age-groups. There were no observed differences according to gender, disease severity or dornase alfa use. These results can be contextualised when one considers the progressive decline in lung function of ≈2% per year generally observed in patients with cystic fibrosis.
Mean sputum P. aeruginosa density showed marked reductions from baseline during the first two active TSI-treatment periods and tended back towards baseline during the non-treatment phases in these placebo-controlled trials. The effect of TSI on sputum P. aeruginosa density was significantly (p = 0.01) reduced with increasing age.
Fewer TSI than placebo recipients required hospitalisation or intravenous antibacterial treatment, and the mean duration of both was shorter with TSI than placebo, during the controlled phase of clinical trials. Similar trends for hospitalisation rate and duration of stay were recorded during the 96-week open-label extension phase. Reductions in hospitalisation rates and the use of intravenous antibacterials produced cost savings that partially off-set the acquisition cost of TSI in a small UK observational economic evaluation in patients with cystic fibrosis and P. aeruginosa infection. Global ratings for health-related quality of life were significantly (p ≤ 0.03) better for TSI than placebo recipients during each treatment cycle in a retrospective analysis using a nonvalidated single-item questionnaire.
In 21 patients aged <6 years with cystic fibrosis and early P. aeruginosa colonisation in a randomised, double-blind, placebo-controlled trial, TSI 300mg twice daily was more effective than placebo for change in bronchoalveolar lavage (BAL) P. aeruginosa density (primary endpoint; between group difference 5.36 log10 colony forming units/mL, p < 0.0001) and eradication rate at 28 days (p < 0.0001), with all eight patients in the TSI group having a negative BAL culture.
Tolerability
Children and adults generally tolerated TSI treatment well, with recipients in clinical trials showing few of the adverse events associated with parenteral aminoglycosides as a class (ototoxicity, nephrotoxicity, neuromuscular interference and fetal harm in pregnant women). Inhaled aminoglycoside treatment is expected to result in fewer systemic adverse events because of poor absorption through the respiratory epithelium. This was the case in trials with TSI 300mg twice daily where no nephrotoxicity or neuromuscular interference was experienced. The only adverse events that occurred significantly more frequently in TSI than placebo recipients in the largest trials were transient mild or moderate tinnitus (3% vs 0%, p = 0.003) and voice changes (12.8% vs 6.5%, p = 0.02), neither of which increased with subsequent cycles of treatment or were cause for TSI discontinuation. No reproductive toxicology studies have been conducted with TSI.
Objectively measured hearing loss did not occur in TSI-treated patients in clinical trials even if they experienced tinnitus, nor did patients complain of hearing loss. However, in postmarketing experience, some TSI recipients reported hearing loss and this was frequently associated with tinnitus. Some of these patients had had previous or concurrent systemic aminoglycoside treatment.
Bronchospasm observed in patients shortly after administration of TSI was transient and similar to that observed after inhalation of placebo. The TSI formulation does not contain phenol, EDTA (edetic acid) or sodium metabisulphite which may cause airway reactivity.
Dosage and Administration
TSI 300mg twice daily (taken as close to 12 hours apart as possible, but not less than 6 hours apart) for alternating periods (28 days on/28 days off) is indicated for the management of P. aeruginosa infection in patients with cystic fibrosis. Subsequent to publication of the current US label, which states that safety have not been demonstrated in patients <6 years of age, a clinical trial has been conducted in this younger age group. TSI is inhaled over a 15-minute period using the PARI LC PLUS™ reusable nebuliser with a compressor.
TSI should be used with caution in patients with known or suspected renal, auditory, vestibular or neuromuscular dysfunction, and in consultation with a physician in pregnant or breast-feeding women. It should not be used concomitantly with ethacrynic acid, furosemide, urea or mannitol, and/or concurrently or sequentially with other drugs with neurotoxic or ototoxic potential.






Similar content being viewed by others
Notes
Use of tradename is for product identification purposes only and does not imply endorsement.
References
Pai VB, Nahata MC. Efficacy and safety of aerosolized tobramycin in cystic fibrosis. Pediatr Pulmonol 2001 Oct; 32(4): 314–27
Lamb HM, Goa KL. Management of patients with cystic fibrosis: defining the role of inhaled tobramycin. Dis Manag Health Outcomes 1999; 6(2): 93–108
Cystic Fibrosis Foundation. Patient Registry 2001 annual data report. Bethesda (MD): Cystic Fibrosis Foundation, 2002
Sermet-Gaudelus I, Le Cocguic Y, Ferroni A, et al. Nebulized antibiotics in cystic fibrosis [published erratum appears in Pediatr Drugs 2003; 5 (1): 40]. Paediatr Drugs 2002; 4(7): 455–67
Robinson P. Cystic fibrosis. Thorax 2001 Mar; 56(3): 237–41
Cystic Fibrosis Committee. Clinical practice guidelines for cystic fibrosis. Bethesda (MD): Cystic Fibrosis Foundation, 1997
Ramsey BW. Management of pulmonary disease in patients with cystic fibrosis [published erratum appears in N Eng J Med 1996 Oct; 335 (15): 1167]. N Engl J Med 1996 Jul 18; 335(3): 179–88
Denton M, Wilcox MH. Antimicrobial treatment of pulmonary colonization and infection by Pseudomonas aeruginosa in cystic fibrosis patients. J Antimicrob Chemother 1997 Oct; 40(4): 468–74
Rosenfeld M, Cohen M, Ramsey B. Aerosolized antibiotics for bacterial lower airway infections: principles, efficacy, and pitfalls. Infect Dis Clin Pract 1998 Feb; 7(2): 66–79
Weber A, Smith A, Williams-Warren J, et al. Nebulizer delivery of tobramycin to the lower respiratory tract. Pediatr Pulmonol 1994 May; 17(5): 331–9
Eisenberg J, Pepe M, Williams-Warren J, et al. A comparison of peak sputum tobramycin concentration in patients with cystic fibrosis using jet and ultrasonic nebulizer systems. Aerosolized Tobramycin Study Group. Chest 1997 Apr; 111(4): 955–62
Smith AL, Ramsey BW, Hedges DL, et al. Safety of aerosol tobramycin administration for 3 months to patients with cystic fibrosis. Pediatr Pulmonol 1989; 7(4): 265–71
Steinkamp G, Tummler B, Gappa M, et al. Long-term tobramycin aerosol therapy in cystic fibrosis. Pediatr Pulmonol 1989; 6(2): 91–8
MacLusky I, Levison H, Gold R, et al. Inhaled antibiotics in cystic fibrosis: is there a therapeutic effect? J Pediatr 1986; 108 (5 Pt 2): 861–5
Ramsey BW, Dorkin HL, Eisenberg JD, et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med 1993 Jun 17; 328(24): 1740–6
Stephens D, Garey N, Isles A, et al. Efficacy of inhaled tobramycin in the treatment of pulmonary exacerbations in children with cystic fibrosis. Pediatr Infect Dis 1983 May–Jun; 2(3): 209–11
Weber A, Williams-Warren J, Ramsey B, et al. Tobramycin serum concentrations after aerosol and oral administration in cystic fibrosis. Am J Ther 1995 Feb; 2(2): 81–7
Touw DJ, Jacobs FAH, Brimicombe RW, et al. Pharmacokinetics of aerosolized tobramycin in adult patients with cystic fibrosis. Antimicrob Agents Chemother 1997 Jan; 41(1): 184–7
Ratjen F, Doring G, Nikolaizik WH. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis [letter]. Lancet 2001 Sep 22; 358(9286): 983–4
Wiesemann HG, Steinkamp G, Ratjen F, et al. Placebo-controlled, double-blind, randomized study of aerosolized tobramycin for early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Pediatr Pulmonol 1998 Feb; 25(2): 88–92
Govan J. TOBI: reducing the impact of pseudomonal infection. Hosp Med 2002 Jul; 63(7): 421–5
CHIRON Corporation. TOBI Prescribing Information. Emeryville (CA): CHIRON Corporation, 2001
Shawar RM, Van Dalfsen JM, MacLeod DL, et al. Comparison of the activity of tobramycin and 6 other antimicrobials against Pseudomonas aeruginosa from cystic fibrosis patients at 69 US centers (1995–1996) [Poster]. 12th Annual North American Cystic Fibrosis Conference; 1998 Oct 15–18; Montreal
Baltch AL, Smith RP, Ritz W. Comparative antimicrobial activity of FK037, cefpirome, ceftazidime and cefepime against aminoglycoside-sensitive and aminoglycoside-resistant Pseudomonas aeruginosa and Pseudomonas spp. Chemotherapy 1994; 40(6): 391–8
Chin N-X, Neu HC. Synergy of new C-3 substituted cephalosporins and tobramycin against Pseudomonas aeruginosa and Pseudomonas cepacia. Diagn Microbiol Infect Dis 1989; 12: 343–9
Benmebarek D, Lapointe JR. In vitro activity of biapenem vs imipenem, in combination with tobramycin, in cystic fibrosis P. aeruginosa [abstract no. 1204]. Can J Infect Dis 1995; 6 Suppl. C: 329
Baltch AL, Ritz W, Smith RP. Comparative antibacterial activity of aztreonam (AZ), piperacillin (Pip), ceftazidime (CZ), ciprofloxacin (Cip) and tobramycin (TB), singly and in combination, against recent isolates of Pseudomonas aeruginosa (P.a.), Klebsiella pneumoniae (K.pn.) and Serratia marcescens (S.m.) [abstract no. E38]. 34th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1994 Oct 4–7; Orlando, 51
National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; twelfth informational supplement. Wayne (PA): NCCLS, 2002: 21. Publication no. M100-S12
Burns JL, Van Dalfsen JM, Shawar RM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis 1999 May; 179(5): 1190–6
Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis: Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med 1999 Jan 7; 340(1): 23–30
Ansorg R, Muller K-D, Wiora J. Comparison of inhibitory and bactericidal activity of antipseudomonal antibiotics against Pseudomonas aeruginosa isolates from cystic fibrosis patients. Chemotherapy 1990; 36(3): 222–9
Ciofu O, Giwercman B, Pedersen SS, et al. Development of antibiotic resistance in Pseudomonas aeruginosa during two decades of antipseudomonal treatment at the Danish CF Center. APMIS 1994; 102(9): 674–80
Gerding DN, Larson TA. Resistance surveillance programs and the incidence of gram-negative bacillary resistance to amikacin from 1967 to 1985. Am J Med 1986 Jun; 80 Suppl. 6B: 22–8
Young LS, Hindler J. Aminoglycoside resistance: a worldwide perspective. Am J Med 1986 Jun 30; 80 Suppl. 6B: 15–21
Schulin T. In vitro activity of the aerosolized agents colistin and tobramycin and five intravenous agents against Pseudomonas aeruginosa isolated from cystic fibrosis patients in southwestern Germany. J Antimicrob Chemother 2002 Feb; 49(2): 403–6
Moss RB. Long-term benefits of inhaled tobramycin in adolescent patients with cystic fibrosis. Chest 2002 Jan; 121(1): 55–63
Moss RB. Administration of aerosolized antibiotics in cystic fibrosis patients. Chest 2001 Sep; 120 (3 Suppl.): 107S–13S
Ichimiya T, Yamasaki T, Nasu M. In-vitro effects of antimicrobial agents on Pseudomonas aeruginosa biofilm formation. J Antimicrob Chemother 1994 Sep; 34(3): 331–41
Geers TA, Baker NR. The effect of sublethal levels of antibiotics on the pathogenicity of Pseudomonas aeruginosa for tracheal tissue. J Antimicrob Chemother 1987 May; 19(5): 569–78
Grimwood K, To M, Rabin HR, et al. Inhibition of Pseudomonas aeruginosa exoenzyme expression by subinhibitory antibiotic concentrations. Antimicrob Agents Chemother 1989 Jan; 33(1): 41–7
Cantin A, Woods DE. Protection by antibiotics against myeloperoxidase-dependent cytotoxicity to lung epithelial cells in vitro. J Clin Invest 1993 Jan; 91(1): 38–45
MacDonald NE. Pseudomonas aeruginosa and cystic fibrosis: antibiotic therapy and the science behind the magic. Can J Infect Dis 1997; 8(6): 335–42
Geller DE, Pitlick WH, Nardella PA, et al. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest 2002 Jul; 122(1): 219–26
Rosenfeld M, Gibson R, McNamara S, et al. Serum and lower respiratory tract drug concentrations after tobramycin inhalation in young children with cystic fibrosis. J Pediatr 2001 Oct; 139(4): 572–7
Gibson RL, Emerson J, McNamara S, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med 2003 Mar; 167(6): 841–9
Hodson ME, Gallagher CG, Govan JRW. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur Respir J 2002 Sep; 20(3): 658–64
LeLorier J, Perreault S, Birnbaum H, et al. Savings in direct medical costs from the use of tobramycin solution for inhalation in patients with cystic fibrosis. Clin Ther 2000 Jan; 22(1): 140–51
Iles R, Legh-Smith J, Prevost T, et al. Economic evaluation of tobramycin nebuliser solution in cystic fibrosis. J Cystic Fibrosis 2003; 2: 120–8
Ramsey BW, Schaeffler B, Montgomery AB, et al. Survival and lung function during 2 years treatment with intermittent inhaled tobramycin in CF patients [abstract no. 237]. Neth J Med 1998; 54 Suppl.: S83–4
Fiel SB. Long term effect of tobramycin solution for inhalation on reduction of hospitalization of CF patients [abstract]. Eur Respir J Suppl 2000 Aug; 16 Suppl. 31: 154s
Quittner AL, Buu A. Effects of tobramycin solution for inhalation on global ratings of quality of life in patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatr Pulmonol 2002 Apr; 33(4): 269–76
Chiron Corporation. Protocol summary study: EU-C-TOB-03-001. Cranford, UK: Chiron, 2003
Mukhopadhyay S, Baer S, Blanshard J, et al. Assessment of potential ototoxicity following high-dose nebulized tobramycin in patients with cystic fibrosis. J Antimicrob Chemother 1993 Mar; 31(3): 429–36
Lucidi V, Salerno T, Bella S, et al. Side effects of inhaled tobramycin in patients with cystic fibrosis. Eur Respir J Suppl 2002 Sep; 20 Suppl. 38: 526
Canadian Cystic Fibrosis Foundation. Canadian consensus statement on aerosolized antibiotics in cystic fibrosis. Toronto (ON): Canadian Cystic Fibrosis Foundation, 1999
Nikolaizik WH, Trociewicz K, Ratjen F. Bronchial reactions to the inhalation of high-dose tobramycin in cystic fibrosis. Eur Respir J 2002 Jul; 20(1): 122–6
Recent events in the EU market. Inpharma 2000; 1253: 22
Foca M, Rajan S, Saiman L. Rational treatment of pulmonary infections in patients with cystic fibrosis. Curr Opin Infect Dis 1999; 12(3): 257–63
Tonelli MR, Aitken ML. New and emerging therapies for pulmonary complications of cystic fibrosis. Drugs 2001; 61(10): 1379–85
Beringer PM. New approaches to optimizing antimicrobial therapy in patients with cystic fibrosis. Curr Opin Pulm Med 1999 Nov; 5(6): 371–7
O’Riordan TG. Inhaled antimicrobial therapy: from cystic fibrosis to the flu. Respir Care 2000 Jul; 45(7): 836–45
Bellingham C. Tackling infection in cystic fibrosis. Hosp Pharmacist 2001; 8: 258–9
Bush A. Decisions facing the cystic fibrosis clinician at first isolation of Pseudomonas aeruginosa. Paediatr Respir Rev 2002 Mar; 3(1): 82–8
Touw DJ, Brimicombe RW, Hodson ME, et al. Inhalation of antibiotics in cystic fibrosis. Eur Respir J 1995 Sep; 8(9): 1594–604
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: A. Bush, Imperial School of Medicine and Royal Bromptom Hospital, London, UK; M.E. Hodson, Department of Cystic Fibrosis, Royal Brompton & Harefield NHS Trust, London, UK; B. Ramsey, Children’s Hospital & Regional Medical Center, Seattle, Washington, USA; F. Ratjen, Children’s Hospital, University of Essen, Essen, Germany; T. Schülin, University of Nijmegen, Nijmegen, The Netherlands; A. Smith, Seattle Biomedical Research Institute, Seattle, Washington, USA; G. Steinkamp, CF Centre Hamburg-Altona, Hannover, Germany.
Data Selection
Sources: Medical literature published in any language since 1980 on tobramycin, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘tobramycin’ and (‘cystic fibrosis’ or ‘inhaled’ or ‘inhalation’ or ‘aerosol’ or ‘aerosolized’). EMBASE search terms were ‘tobramycin’ and (‘cystic fibrosis’ or ‘inhaled’ or ‘inhalation’ or ‘aerosol’ or ‘aerosolized’). AdisBase search terms were ‘tobramycin’ and (‘cystic fibrosis’ or ‘inhal*’ or ‘aerosol*’). Searches were last updated 6 October 2003.
Selection: Studies in patients with cystic fibrosis who received tobramycin solution for inhalation (TOBI®). Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Tobramycin solution for inhalation, cystic fibrosis, Pseudomonas aeruginosa, pharmacodynamics, pharmacokinetics, therapeutic use.
Rights and permissions
About this article
Cite this article
Cheer, S.M., Waugh, J. & Noble, S. Inhaled Tobramycin (TOBI®). Drugs 63, 2501–2520 (2003). https://doi.org/10.2165/00003495-200363220-00015
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200363220-00015