Abstract
Background:
Current guidelines recommend spirometry to confirm a diagnosis of chronic obstructive pulmonary disease (COPD).
Aims:
To investigate whether a self-reported diagnosis of COPD is associated with prior spirometry and whether a correct diagnosis of COPD is more likely when spirometry was performed.
Methods:
We used data from the population-based Austrian Burden of Obstructive Lung Disease (BOLD) study. Participants were aged >40 years and completed post-bronchodilator spirometry. Reported COPD diagnosis and reported prior lung function test were based on questionnaire. Persistent airflow limitation was defined as post-bronchodilator forced expiratory volume in one second/forced vital capacity ratio <0.7, corresponding with COPD Global initiative for chronic Obstructive Lung Disease (GOLD) grade I+, and GOLD grade II+ was also investigated. A correct diagnosis of COPD was defined as a reported physician's diagnosis of COPD and the presence of persistent airflow limitation.
Results:
68 (5.4%) of 1,258 participants reported a prior physician's diagnosis of COPD. Of these, only 17 (25.0%) reported a lung function test within the past 12 months and 46 (67.6%) at any time in the past. The likelihood for a correct COPD GOLD grade I+ diagnosis was similar among subjects reporting a lung function test during the last 12 months (likelihood ratio 2.07, 95% CI 0.89 to 5.50) and those not reporting a lung function during the last 12 months (likelihood ratio 2.78, 95% CI 1.58 to 4.87). Similar likelihood ratios were seen when GOLD grade II+ was investigated and when lung function was reported at any time in the past.
Conclusions:
One-third of subjects with a reported diagnosis of COPD never had a lung function test. When spirometry was reported, this did not increase the likelihood of a correct COPD diagnosis.
Similar content being viewed by others
Introduction
Persistent airflow limitation is the physiological hallmark of chronic obstructive pulmonary disease (COPD). This can be confirmed by post-bronchodilator spirometry. A lung function test is recommended for subjects presenting with respiratory symptoms and/or exposure to tobacco smoke or other risk factors.1,2
In 2020 COPD will be the third leading cause of death worldwide. The increasing prevalence and mortality of COPD are related to both the ageing of the global population and the exposure to cigarette smoke or other harmful dusts and fumes.3 However, the epidemic of COPD is currently characterised by a high proportion of non-diagnosed cases.4 Underutilisation of spirometry, low test quality, and insufficient interpretation are likely to contribute to the underdiagnosis of COPD.5
We investigated whether subjects with a reported diagnosis of COPD would also report a lung function test in the past. Theoretically, all subjects with reported COPD should have had such a test in the past if guideline recommendations are followed. However, a reported lung function test in the past does not necessarily guarantee that this test was performed and interpreted correctly. To estimate the overall performance of lung function tests, we analysed whether the likelihood for a correct COPD diagnosis was different in subjects reporting and not reporting a prior lung function test.
Methods
Study population
A gender-stratified random sample of 2,200 participants (1,100 men and 1,100 women) from the inhabitants of Salzburg County aged ≥40 years was examined in accordance with the Burden of Obstructive Lung Disease (BOLD) protocol.6 Details of the study population and the prevalence of airways obstruction have been reported elsewhere.7 The study was approved by the local ethical committee and all participants provided informed consent.
Study measures
Spirometry was performed according to American Thoracic Society (ATS) criteria8 by trained and certified technicians using the NDD Easy OneTM spirometer (ndd Medical Technologies Inc, Switzerland) with participants in a seated position. Separate measurements were taken before and at least 15 mins after two puffs of salbutamol (200μg) from a metered dose inhaler, administered using a Volumatic spacer (GlaxoSmithKline, UK). Spirometry data were sent electronically to the Pulmonary Function Quality Control Centre in Salt Lake City, Utah, USA, where each spirogram was reviewed and graded using ATS guidelines.8
Only spirograms that met ATS acceptability and reproducibility criteria were included — that is, at least three trials: two acceptable (free from artifact, sudden stops, and back extrapolated volumes >5.0% of forced vital capacity (FVC)) and reproducible (difference between the largest and second largest values <200mL) for both forced expiratory volume in one second (FEV1) and FVC.
Study technicians were continuously monitored. When a technician's quality score dropped below a pre-set level, he/she had to stop testing and be re-trained and re-certified.
Questionnaire data
The BOLD questionnaires, administered by trained and certified staff, included information on respiratory symptoms, risk factors for COPD, health status, co-morbidities, respiratory diagnoses, and limitation of activity.
Definitions
In accordance with the Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines, persistent airflow limitation was defined as a post-bronchodilator FEV1/FVC ratio of <0.70, which corresponds to GOLD grade I and higher.2 We also report data for GOLD grade II or higher, which corresponds to FEV1/FVC ratio <0.70 and FEV1 <80% predicted. The Third National Health and Nutrition Examination Survey (NHANES III) reference equations were used to calculate predicted values.9 Doctor-diagnosed COPD was defined as a self-reported physician (general practitioners in Salzburg city and Salzburg county) diagnosis of COPD and/or emphysema. Among those who reported a diagnosis of COPD and/or emphysema, we also recorded how many reported a diagnosis of chronic bronchitis. Ever smoking (current or former smoking) was defined as smoking more than 20 packs of cigarettes in a lifetime or more than 1 cigarette/day for a year. A prior lung function test was defined as present when the question “Has a doctor or other health care provider ever had you blow into a machine or device in order to measure your lungs?” was answered with “yes”. This was assessed for both the last 12 months and for any time in the past.
The staff who administered the questionnaire were trained and certified. In case of doubt they were able to explain the difference between a peak flow meter and a spirometer.
Statistical analysis
The statistical analysis was performed with SAS Version 9.1. Logistic regression (log-binomial regression model) analysis (unadjusted and adjusted) was used to investigate likelihood ratios associated with a correctly confirmed COPD diagnosis. Likelihood ratios were adjusted for age, sex, smoking, and reported respiratory symptoms. The level of significance is defined and described as p value, and p<0.05 was considered to be statistically significant.
Results
Of the 1,349 participants with complete questionnaire data and post-bronchodilator spirometry, 1,258 (93%) met the quality control criteria and were included in this analysis. The characteristics of this population are given in Table 1. The prevalence of persistent airflow limitation corresponding to COPD GOLD grade I+ and COPD GOLD grade II+ was 24.2% (304/1,258) and 9.5% (119/1,258), respectively. A prior physician's diagnosis of COPD was reported by 5.4% of the study population in both males and females.
Prior physician's COPD diagnosis and persistent airflow limitation
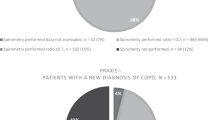
Only 11.5% (35/304) of subjects with persistent airflow limitation reported a prior physician's diagnosis of COPD, while 88.5% (269/304) did not. On the other hand, 3.5% (33/954) of subjects without airways obstruction also reported a physician's diagnosis of COPD. Therefore, 48.5% (33/68) of all reported COPD diagnoses were false positive and 88.5% of subjects with persistent airflow limitation were undiagnosed (Figure 1).
Prior lung function tests and reported diagnosis of COPD
The proportion of subjects who reported having a lung function test either in the last 12 months (Figure 2) or at any time in the past was 12.2% (153/1,258) and 41.2% (518/1,258), respectively. Among subjects with a reported physician's diagnosis of COPD, only 25.0% (17/68) reported having a lung function test within the last 12 months and 67.6% (46/68) at any time in the past.
The likelihood ratio for a correct COPD diagnosis, defined as a reported prior physician's COPD diagnosis and the concurrent presence of persistent airflow limitation (COPD GOLD grade I+), was 2.21 (95% CI 0.89 to 5.50) among those who reported having a lung function test during the last 12 months and 3.39 (95% CI 2.00 to 5.77) among those not reporting a lung function test during the same period (p=0.427 for comparison of the two likelihood ratios).
The corresponding likelihood ratios for a correct COPD diagnosis, defined as a reported prior physician's COPD diagnosis and the concurrent presence of more stringent persistent airflow limitation (COPD GOLD grade II+) was 4.15 (95% CI 1.76 to 9.77) among those reporting having a lung function test during the last 12 months and 3.77 (95% CI 2.08 to 6.81) among those not reporting a prior lung function test during the same period (p=0.855 for comparison of the two likelihood ratios).
When a lung function test at any time in the past was evaluated, the likelihood ratio for a correctly confirmed COPD diagnosis (COPD GOLD grade I+) was 3.19 (95% CI 1.83 to 5.53) in those reporting a test and 2.63 (95% CI 1.15 to 6.05) in those not reporting such a test (p=0.707). For a more stringent spirometric definition of COPD (GOLD grade II+), the likelihood ratio for a correctly confirmed COPD diagnosis was 3.85 (95% CI 2.23 to 6.65) in those reporting a lung function test and 3.59 (95% CI 1.38 to 9.37) in those not reporting a lung function test at any time in the past (p=0.423 for comparison of the two likelihood ratios).
The results were robust when the confounders sex, age, smoking, and respiratory symptoms were taken into account (see Tables 2 and 3).
Discussion
Main findings
Our findings show relevant shortcomings associated with the diagnosis of COPD and the use of spirometry. In the participants of the BOLD study in Salzburg, Austria we observed that: (1) among participants with a reported prior physician's diagnosis of COPD, only 25% reported a lung function test within the last 12 months and only 68% at any time in the past; (2) the likelihood of a correct COPD diagnosis was not different among those reporting or not reporting a prior lung function test; (3) our results suggest that lung function testing is greatly underused to diagnose and monitor COPD and, when used, the quality appears to be of too low to establish a correct diagnosis.
Strengths and limitations of this study
For this analysis we used data from the population-based BOLD study in Salzburg, Austria. Questionnaire data and post-bronchodilator spirometry data were recorded following the highly standardised international BOLD study protocol.6 In Salzburg (one of nine counties of Austria), general practitioners are reimbursed for spirometries by health insurance. In other areas of Austria and in several other European countries, general practitioners are not reimbursed for lung function testing. Our findings may not apply to regions/countries without reimbursement. However, it has to be supposed that the use of spirometry is even less frequent when reimbursement is lacking. Finally, both previous lung function testing and physician diagnosis were based on self-reporting, which can be subject to recall bias. Subjects with a reported previous diagnosis of asthma were excluded from this analysis. However, since reported diagnoses can be subject to recall bias, we cannot be absolutely sure that every case of chronic asthma with persistent airflow limitation has been excluded.
Interpretation of findings in relation to previously published work
Underdiagnosis of COPD has been reported for many countries. For example, the rates of underdiagnosis in Spain and Sweden were reported to be 78% and 89%, respectively.10,11 Ten years after IBERPOC, underdiagnosis in Spain changed from 78% to 73%.12,13 Using data from the BOLD study, we recently reported a rate of underdiagnosis of 88% in Austria.14
On the other hand, our data show that many subjects with a reported diagnosis of COPD do not have persistent airflow limitation when spirometry is performed according to internationally accepted quality control standards. These results indicate that a reported COPD diagnosis is poorly associated with airways obstruction on post-bronchodilator spirometry. On the one hand our data support the notion that many subjects with COPD are undiagnosed. On the other hand, a few others without obstructed airways (in our data 33/68 (48.5%)) are wrongly misdiagnosed and costly and potentially harmful medication may be prescribed to them.15
In current clinical practice COPD is often diagnosed exclusively on the basis of the presence of respiratory symptoms,16 and some general practitioners still believe that spirometry is neither necessary nor helpful to diagnose COPD.17 This is in contrast to current guidelines (GOLD, National Institute for Health and Clinical Excellence, European Respiratory Society/American Thoracic Society) which state that a confident diagnosis of COPD can only be made with spirometry.2,18,19 Although spirometry does not fully capture the impact of COPD on a patient's health, it is still the gold standard for diagnosing the disease and monitoring its progression.2 To date, the BODE index (body mass index, airflow obstruction, dyspnoea, and exercise capacity) and other multicomponent indices used to assess the local and systemic consequences of COPD holistically include spirometry.20,21 Periodic spirometric measurements help to track a patient's decline in lung function, but useful information about lung function decline is unlikely from measurements performed more than once a year.2 In our sample, only 25% of those with a physician's diagnosis of COPD reported a lung function test within the last 12 months. Our data therefore indicate that spirometry is infrequently used for both diagnosing the disease and monitoring its progression.
The infrequent use of spirometry in GP offices and subsequent lack of experience causes uncertainty about both the performance and interpretation of a lung function test,22 as recently emphasised by the UK Primary Care Respiratory Society (PCRS-UK).23 A comparison of lung function test interpretations by family physicians and by pulmonary specialists showed concordant results in only 76% of cases.24 There are several explanations and reasons for the underuse of spirometry, of which time constraints, staffing, and poor training with subsequent lack of confidence in data interpretation are thought to be the most important contributors.25,26 The last of these might explain why spirometry (when performed) was not found to increase the likelihood of a correct respiratory diagnosis. However, well-trained office spirometry can be achieved and training of GP offices has been shown to increase the number of spirometry tests in the three months following training by about 60%, equivalent to an average (median) weekly increase in spirometry from one test every two weeks to one test every week.22
Implications for future research, policy and practice
The results of the BOLD study have shown that quality goals for spirometry tests can be met about 90% of the time in population-based samples of adults from several countries.27 Thus, more frequent use of properly performed lung function tests would be likely to increase the rate of correct COPD diagnoses and help to initiate early interventions (e.g. smoking cessation). This would result in improved patients' health status, slow down the decline in lung function,28 and could avoid exacerbations by prescribing appropriate medication.29,30 Centralisation of spirometry may be an alternative to well-trained office testing. A Community Respiratory Assessment Unit was established in 2004 in West London to provide high-quality spirometry and diagnostic support to primary care physicians. In this case, centralisation of spirometry via a dedicated service has been shown to improve diagnosis and treatment of respiratory disease.31
Conclusions
We observed a significant underutilisation of spirometry among those with a diagnosis of COPD and, when spirometry was used, it was not found to increase the likelihood of a correct COPD diagnosis. These results should not be disregarded in the ongoing debate about screening for COPD, case finding, or selected early detection. However, to reduce underdiagnosis and overdiagnosis of COPD, every effort should be undertaken to establish quality managed spirometry programmes in primary care.
References
Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. GOLD Executive Summary. Am J Respir Crit Care Med 2007;176:532–55. http://dx.doi.org/10.1164/rccm.200703-456SO
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. 2011. Available from: http://www.goldcopd.org/.
Murray CJL, Lopez AD . Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997;349:1498–504. http://dx.doi.org/10.1016/S0140-6736(96)07492-2
Firlei N, Lamprecht B, Schirnhofer L, Kaiser B, Studnicka M . Die Prävalenz der COPD in Österreich — die erwartete Entwicklung bis 2020. Wien Klin Wochenschr 2007;119/17–18:513–18. http://dx.doi.org/10.1007/s00508-007-0867-3
Soriano JB, Zielinski J, Price D . Screening for and early detection of chronic obstructive pulmonary disease. Lancet 2009;374:721–32. http://dx.doi.org/10.1016/FS0140-6736/809/961290-3
Buist AS, Vollmer WM, Sullivan SD, et al. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD 2005;2:277–83. http://dx.doi.org/10.1081/COPD-200057610
Schirnhofer L, Lamprecht B, Vollmer W, et al. COPD prevalence in Salzburg, Austria: first results from the Burden of Obstructive Lung Disease (BOLD) study. Chest 2007;131(1):29–36. http://dx.doi.org/10.1378/chest.06-0365
American Thoracic Society. Statement: Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995;152:1107–36. http://dx.doi.org/10.1164/ajrccm.152.3.7663792
Hankinson JL, Odencrantz JR, Fedan KB . Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 1999;159:179–87. http://dx.doi.org/10.1164/ajrccm.159.1.9712108
Peña VS, Miravitlles M, Gabriel R, et al. Geographic variations in prevalence and underdiagnosis of COPD: results of the IBERPOC multicentre epidemiological study. Chest 2000;118(4):981–9. http://dx.doi.org/10.1378/chest.118.4.981
Lindberg A, Jonsson AC, Rönmark E, Lundgren R, Larsson LG, Lundbäck B . Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor's diagnosis, symptoms, age, gender, and smoking habits. Respiration 2005;72:471–9. http://dx.doi.org/10.1159/000087670
Miravitlles M, Soriano JB, García-Río F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009;64:863–8. http://dx.doi.org/10.1136/thx.2009.115725
Soriano JB, Ancochea J, Miravitlles M, et al. Recent trends in COPD prevalence in Spain: a repeated cross-sectional survey 1997–2007. Eur Respir J 2010;36:758–65. http://dx.doi.org/10.1183/09031936.00138409
Schirnhofer L, Lamprecht B, Firlei N, et al. Using targeted spirometry to reduce non-diagnosed chronic obstructive pulmonary disease. Respiration 2011;81(6):476–82. http://dx.doi.org/10.1159/000320251
Walker PP, Mitchell P, Diamantea F, Warburton CJ, Davies L . Effect of primary-care spirometry on the diagnosis and management of COPD. Eur Respir J 2006;28:945–52. http://dx.doi.org/10.1183/09031936.06.00019306
Halbert RJ, Isonaka S, George D, Iqbal A . Interpreting COPD prevalence estimates: what is the true burden of disease? Chest 2003;123(5):1684–92. http://dx.doi.org/10.1378/chest.123.5.1684
Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis 2005;63(1):6–12.
National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary care and secondary care. London: National Clinical Guideline Centre, 2010. Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/English
Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23(6):932–46. http://dx.doi.org/10.1183/09031936.04.00014304
Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–12. http://dx.doi.org/10.1056/NEJMoa021322
Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 2009;374:704–11. http://dx.doi.org/10.1016/S0140-6736(09)61301-5
Kaminsky DA, Marcy TW, Bachand M, Irvin CG . Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care 2005;50(12):1639–48.
Levy ML, Quanjer PH, Booker R, Cooper BG, Holmes S, Small I; General Practice Airways Group. Diagnostic spirometry in primary care: Proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations: a General Practice Airways Group (GPIAG). Prim Care Respir J 2009;18:130–47. http://dx.doi.org/10.4104/pcrj.2009.00054
Yawn BP, Enright PL, Lemanske RF, et al. Spirometry can be done in family physicians' offices and alters clinical decisions in management of asthma and COPD. Chest 2007;132:1162–8. http://dx.doi.org/10.1378/chest.06-2722
Walters JA . Under-diagnosis of chronic obstructive pulmonary disease: a qualitative study in primary care. Respir Med 2008;102(5):738. http://dx.doi.org/10.1016/j.rmed.2007.12.008
Moore PL . Practice management and chronic obstructive pulmonary disease in primary care. Am J Med 2007;120(8):S23–7. http://dx.doi.org/10.1016/j.amjmed.2007.04.009
Enright P, Vollmer WM, Lamprecht B, et al. Quality of spirometry tests performed by 9893 adults in 14 countries: the BOLD Study. Respir Med 2011;105:1507–15. http://dx.doi.org/10.1016/j.rmed.2011.04.008
Parkes G, Greenhalgh T, Griffin M, et al. Effect on smoking quit rate of telling patients their lung age: the step2quit randomized controlled trial. BMJ 2008;336:598–600. http://dx.doi.org/10.1136/bmj.39503.582396.25
Price D, Crockett A, Arne M, Garbe B, Jones R, Kaplan A, Langhammer A, Williams S, Yawn B . Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J 2009;18(3):216–23. http://dx.doi.org/10.4104/pcrj.2009.00055
Lin K, Watkins B, Johnson T, Rodriguez JA, Barton MB . Screening for chronic obstructive pulmonary disease using spirometry: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2008;148:535–43. http://dx.doi.org/10.7326/0003-4819-148-7-200804010-00213
Starren ES, Roberts NJ, Tahir M, O'Byrne L, Haffenden R, Patel IS, Partridge MR . A centralised respiratory diagnostic service for primary care: a 4-year audit. Prim Care Respir J 2012;21(2):180–6. http://dx.doi.org/10.4104/pcrj.2012.00013
Acknowledgements
Handling editor Irem Patel
Statistical review Gopal Netuveli
We acknowledge the support of the European Respiratory Society, Fellowship STRTF 326–2011.
Funding The BOLD study in Salzburg, Austria was funded by unrestricted grants from the pharmaceutical industry, the local government of Salzburg, and the local public health insurance. BL is the recipient of a European Respiratory Society Fellowship (STRTF 326–201 1). The sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing the report.
Author information
Authors and Affiliations
Contributions
BL collected and analysed the data, drafted the manuscript and is the guarantor. AM analysed the data and helped to draft and revise the manuscript. BK analysed the data and was involved in drafting the manuscript. JBS, ASB, and MS helped to revise the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflicts of interest in relation to this article.
Rights and permissions
About this article
Cite this article
Lamprecht, B., Mahringer, A., Soriano, J. et al. Is spirometry properly used to diagnose COPD? Results from the BOLD study in Salzburg, Austria: a population-based analytical study. Prim Care Respir J 22, 195–200 (2013). https://doi.org/10.4104/pcrj.2013.00032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.4104/pcrj.2013.00032
This article is cited by
-
Diagnostic spirometry in COPD is increasing, a comparison of two Swedish cohorts
npj Primary Care Respiratory Medicine (2023)
-
Time-ordered comorbidity correlations identify patients at risk of mis- and overdiagnosis
npj Digital Medicine (2021)
-
Development and validation of the Salzburg COPD-screening questionnaire (SCSQ): a questionnaire development and validation study
npj Primary Care Respiratory Medicine (2017)
-
Use of spirometry among chest physicians and primary care physicians in India
npj Primary Care Respiratory Medicine (2016)
-
Improving quality of care in general practices by self-audit, benchmarking and quality circles
Wiener klinische Wochenschrift (2016)