Article Text
Abstract
Objective: Randomised controlled trials comparing elective use of high frequency ventilation (HFV) with conventional mechanical ventilation (CMV) in preterm infants have yielded conflicting results. We hypothesised that the variability of results may be explained by differences in study design, ventilation strategies, delay in initiation of HFV, and use of permissive hypercapnia.
Methods: Randomised controlled trials comparing the elective use of HFV with any form of CMV were identified. Trials were classified according to the ventilation strategies used for HFV and CMV and oscillator device employed. For cumulative meta-analyses, trials were arranged by the following covariables: mean duration until randomisation, Paco2 limits, publication date, and sample size. Odds ratios (OR) and 95% confidence intervals were calculated using fixed and random effects models.
Results: Seventeen randomised trials enrolling 3776 patients were included. Unlike previous meta-analyses, there was no significant difference in the incidence of bronchopulmonary dysplasia or death (OR 0.87, 0.75–1.00) and severe intraventricular haemorrhage grade 3–4 (1.14, 0.96–1.37). The incidence of air leaks (OR 1.23, 1.06–1.44) was significantly increased with HFV. Subgroup analyses and cumulative meta-analyses demonstrated that trial results were related to the ventilation strategies used for HFV and CMV. No influence was found for mean time to randomisation, degree of permissive hypercapnia, or sample size.
Conclusions: Heterogeneity among trials of elective HFV compared to CMV in preterm infants is mainly due to differences in ventilatory strategies. Optimising CMV strategy appeared to be as effective as using HFV in improving pulmonary outcome in preterm infants.
- BPD28, bronchopulmonary dysplasia, defined as persistent requirement for supplemental oxygen or mechanical ventilation at a postnatal age of 28–30 days
- BPD36, bronchopulmonary dysplasia, defined as persistent requirement for supplemental oxygen or mechanical ventilation at a postmenstrual age of 36–37 weeks
- CMV, conventional mechanical ventilation
- HFJV, high frequency jet ventilation
- HFOV, high frequency oscillatory ventilation
- HFPPV, high frequency positive pressure ventilation
- HFV, high frequency ventilation
- HLVS, high lung volume strategy
- IVH 3–4, intraventricular haemorrhage grade 3 or 4
- LLVS, low lung volume strategy
- LPVS, low pressure volume strategy for CMV
- OR, odds ratio
- PVL, periventricular leukomalacia
- RDS, respiratory distress syndrome
- bronchopulmonary dysplasia
- high frequency ventilation
- meta-analysis
- preterm infant
- review
Statistics from Altmetric.com
- BPD28, bronchopulmonary dysplasia, defined as persistent requirement for supplemental oxygen or mechanical ventilation at a postnatal age of 28–30 days
- BPD36, bronchopulmonary dysplasia, defined as persistent requirement for supplemental oxygen or mechanical ventilation at a postmenstrual age of 36–37 weeks
- CMV, conventional mechanical ventilation
- HFJV, high frequency jet ventilation
- HFOV, high frequency oscillatory ventilation
- HFPPV, high frequency positive pressure ventilation
- HFV, high frequency ventilation
- HLVS, high lung volume strategy
- IVH 3–4, intraventricular haemorrhage grade 3 or 4
- LLVS, low lung volume strategy
- LPVS, low pressure volume strategy for CMV
- OR, odds ratio
- PVL, periventricular leukomalacia
- RDS, respiratory distress syndrome
An extensive literature supports the concept that ventilation with relatively large tidal volumes can result in lung injury.1 It is possible to preserve the desired minute ventilation by using relatively small tidal volumes and a high ventilatory rate. Three randomised trials have shown that conventional mechanical ventilation (CMV) with higher ventilatory rates (60/min v 20–40/min) reduces the incidence of air leaks and may even decrease mortality.2–4 However, all three studies were performed in the pre-surfactant era. Another way to minimise lung injury may be to reduce tidal volume at the expense of minute ventilation while accepting higher arterial CO2 tension.5–7
The use of high ventilatory rates during CMV is limited by the minimum amount of time needed for a complete inspiration and expiration. New technologies to further increase the ventilatory rate and reduce the tidal volume led to the development of high frequency ventilation (HFV), which has been studied extensively in premature infants but with conflicting results.8–24 Comparability between trials is limited by differences in ventilatory strategies, time between birth and randomisation, ventilator technology, patient population, study end points, and use of antenatal steroids and surfactant. Preferring higher ventilation rates during CMV2–4 and permissive hypercapnia5 may have benefited the control groups in some studies and thereby diminished the advantage of HFV. The most extensively discussed reasons for conflicting results included the ventilation strategies used for HFV25 and CMV,17,26 the age when HFV was started,27 and the ventilator technology and devices used.28
Two opposing strategies are possible when applying HFV. Mean pressure and lung inflation can be minimised, but higher inspired oxygen concentrations may be necessary (low lung volume strategy, LLVS). On the other hand, with higher mean airway pressure, recruitment of alveoli and elimination of atelectasis tend to improve oxygenation by increasing lung surface area and eliminating intrapulmonary shunts (high lung volume strategy, HLVS). Animal experiments indicate better short term results after using an HLVS.29–31
Herein, we present an in depth meta-analysis of all available randomised trials comparing elective HFV with CMV, including three trials19,22,24 not yet included in the latest Cochrane reviews.32,33 We use cumulative34 and recursive-cumulative meta-analyses35–37 to determine if the following covariates and study design differences may have influenced whether or not trials found a significant advantage associated with HFV:
-
Publication date
-
Use of surfactant
-
High frequency ventilation strategy
-
Conventional ventilation strategy
-
Overall outcome
-
Delay before starting randomised ventilation mode
-
Paco2 targets (use of permissive hypercapnia)
-
Sample size of the individual trials
METHODS
A MEDLINE search was undertaken to identify randomised controlled trials that compared HFV with CMV in preterm infants requiring mechanical ventilation mainly due to respiratory distress syndrome (RDS). Further, the abstract books of the recent Pediatric Academic Societies Meetings (1995–2004) were hand searched to identify trials not published in final form.
Trials attempting to include all infants showing a certain degree of RDS as soon as possible after the start of CMV and usually within the first 24 h of life were classified as elective and incorporated into the meta-analysis. Furthermore, the randomly assigned mode had to be sustained for at least 5 days or until extubation. Both criteria limited exposure of participants to the alternate ventilation mode during the early phase of lung disease. Trials including only patients showing signs of treatment failure or developing complications were classified as rescue, and excluded from the meta-analysis.
The following outcomes were selected for further evaluation: mortality until 36 weeks postmenstrual age, bronchopulmonary dysplasia (defined as oxygen and/or ventilator dependency at age 28–30 days (BPD28) or at 36–37 weeks postmenstrual age (BPD36)), BPD28 or death at age 28–30 days, BPD36 or death at 36–37 weeks postmenstrual age, air leaks (pulmonary interstitial emphysema or gross air leaks), intraventricular haemorrhage (IVH) grades 3–4, according to Papile et al,38 and periventricular leukomalacia (PVL).
To determine the influence of ventilatory strategy or technology, trials were subdivided into the following subgroups:
-
High lung volume strategy (HLVS); the ventilatory strategy in the HFV group included lung volume optimisation.
-
SensorMedics 3100A (SM3100); at least 80% of patients randomised to HFV were treated with the SensorMedics 3100A device (VIASYS, Yorba Linda, CA, USA).
-
Low pressure and volume strategy (LPVS); the ventilatory strategy in the CMV group aimed at lowering tidal volumes by specifying
The influence of the continuous covariates, time between birth and randomisation, Paco2 limits, and sample size of the trials was determined by cumulative meta-analyses as described.34 The trials were ordered by ascending time to randomisation, descending Paco2 limits, or ascending sample size. Odds ratios of the risk of BPD36 or death were determined for the first trial of the lists, then for the first and second combined, and so on, until all were included.
The recursive cumulative meta-analysis consisted of two steps.35,36 First, trials were ordered by publication date and a cumulative meta-analysis was done.34 For the recursive analysis, the relative change of the odds ratio incurred with the addition of each individual trial, equivalent to the first derivative of the odds ratio, was calculated and graphically displayed.
Calculations of the odds ratios according to the fixed effect model of Mantel and Haenszel39 were done with SAS Software (SAS Institute, Cary, NC, USA). Review Manager and RevMan Analyses software (The Cochrane Collaboration, Oxford, England) were used for random effects model calculations according to DerSimonian and Laird40 when necessary.
RESULTS
Seventeen trials with a combined total of 3776 patients met the inclusion criteria for the meta-analysis.8–24 Data were generally extracted from the publications, with the following exceptions. Data about additional patients enrolled in the study of Clark et al,11 who had been excluded after randomisation, were found in the Cochrane database.32 We included these data so we could do an intention-to-treat analysis. The third group of patients in this study, receiving HFV only for 72 h, was not included. Additional data from the study of Gerstmann et al12 about the incidence of bronchopulmonary dysplasia at 36 weeks postmenstrual age were found in a review article by the same authors.28 Data from the study of Keszler et al,14 which used different HFV strategies in different study centres, was included in the subgroups as appropriate. Dr Schreiber kindly provided unpublished data from his study.24 The trials differed markedly in patient demographics, time of randomisation, ventilation strategies and devices used in both the HFV and CMV groups, sample size, use of surfactant, and reported outcomes (table 1). A funnel plot did not indicate publication bias.
Studies included in this meta-analysis of elective use of HFV in preterm infants
Overall results are shown in fig 1. As not all outcome parameters were available from all studies, the analysis of some outcomes had to be based on fewer trials. Most importantly, BPD36 was not reported in the HIFI trial,8 which reduced the sample size for this outcome. There was no statistically significant difference regarding mortality, BPD28, BPD28 combined with mortality, severe intraventricular haemorrhages (IVH 3–4), or PVL by either fixed or random effects models. Trends favouring HFV were observed for BPD36 and BPD36 or death, which, however, did not achieve statistical significance. HFV was significantly associated with an increase in air leaks in the fixed and random effects models.
Overall results showing odds ratios and 95% confidence intervals, calculated according to a fixed effect model, for the analysed outcome parameters. The significant difference in the incidence of air leaks remained significant in a random effects model. Air leaks: pneumothorax, pneumomediastinum, or pulmonary interstitial emphysema; BPD28: bronchopulmonary dysplasia, defined as oxygen or ventilator dependency at 28 days postnatal age; BPD28/D: BPD28 or death; BPD36: bronchopulmonary dysplasia, defined as oxygen or ventilator dependency at 36 weeks postmenstrual age; BPD36/D: BPD36 or death; IVH 3–4: intraventricular haemorrhage grade 3 to 4 according to Papile et al38; NNH: number needed to harm; PVL: periventricular leukomalacia.
For brevity, subgroup analyses are only shown for BPD36 or death and IVH 3–4 (fig 2). After limiting the analysis to trials using an HLVS or to trials using the SensorMedics 3100A for HFV, the reduction of BPD or death became marginally significant in the fixed effect model only. However, when the subgroup analysis was limited to studies which also optimised their CMV by using a low pressure or tidal volume strategy (LPVS), there was no longer a significant difference, even though all studies in this subgroup used an HLVS for HFV. When the analysis was further limited to trials using a high rate low tidal volume strategy for CMV (HFPPV), there was a trend in the opposite direction, favouring HFPPV over HFV. A non-significant trend towards more IVH grade 3–4 with HFV in the overall analysis completely disappeared when limiting the analysis to trials using an HLVS.
Results of subgroup analyses for the incidence of bronchopulmonary dysplasia, defined as oxygen or ventilator dependency at 36 weeks postmenstrual age or death (BPD36/D) and intraventricular haemorrhage grade 3 to 4 (IVH 3–4), according to Papile et al,38 in a fixed effect model. The differences in the HFV/HLVS and SM3100 subgroups were no longer significant in a random effects model. All: all available studies included11–24; CMV/LPVS: only studies using a low pressure and tidal volume strategy for CMV17–23; HFPPV: only studies using a high rate low pressure and tidal volume strategy for CMV17,21,23; HFV/HLVS: only studies using an HLVS for HFV11,12,14,16–24; NNT: number needed to treat; SM3100: only studies using the SensorMedics 3100A for HFV.11,12,16,19,20,23,24
Individual study results for the BPD36 or death outcome are shown in fig 3. Only trials using an HLVS for HFV, while limiting CMV to rates lower than 60/min, yielded significant results favouring HFV. Plotting rates of survival without BPD36 against birth weight (fig 4) accounts for varying baseline data, and indicates that studies which show a difference between HFV and CMV11,12,14,16,19,20 invariably had CMV results below the regression line indicating the average outcome, whereas their HFV results were not better than the CMV results of several other studies showing no advantage of HFV.13,15,17,23
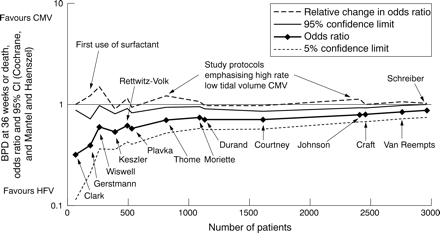
The recursive cumulative meta-analysis shows the relative changes of the odds ratio after stepwise analysis including more and more data (fig 5). Along with fig 3, fig 5 also allows analysis by publication date, as trials are ordered chronologically. It appears that the early advantage seen with HFV diminished as time went on and more and more evidence was added. Therefore, the relative change of the odds ratio (dashed line) is mainly above unity. Three distinct peaks in the relative change can de discerned, indicating a strong movement of the cumulative odds ratio towards unity caused by the studies included at these points. Surfactant replacement therapy and high rate CMV appear to be important factors in this process because their introduction was associated with these rather strong movements of the odds ratio towards unity.
Recursive cumulative meta-analysis showing the relative change of the odds ratio (dashed line), also called cumulative treatment effect ratio, after each additional trial was included. Also shown are odds ratio, calculated according to a fixed effect model, and 95% confidence intervals. With the last study included, the 95% confidence boundary crossed unity. Trials are identified by the first author names.11–24
A cumulative meta-analysis with trials ordered by the time elapsed before randomisation and commencement of the randomised ventilation mode (fig 6) explores whether achieving the best results with HFV is dependent on reducing the preceding exposure to CMV.27 The odds ratio, however, does not show a clear pattern but undulates several times on either side of unity.
Cumulative meta-analysis with trials ordered by the delay before the start of the randomised ventilation mode. In the left panel, the odds ratios and 95% confidence intervals of individual trials are shown. In the right panel, the cumulative results are shown as follows: in the first line the results of the first trial, in the second line the combined results of the first two trials, and so on, until all trials are cumulated at the bottom of the panel. Trials are identified by the first author names and publication years.11–24 Total indicates overall result. Mean values of actual delays were used when reported, protocol limits were used otherwise. One trial not reporting any delay was set to 24 h.22
Following speculation that permissive hypercapnia helps CMV more than HFV to improve pulmonary outcome,17 we also performed a cumulative meta-analysis, with trials ordered by the Paco2 limits (fig 7), which also shows a non-conclusive undulating pattern. Similarly, a cumulative analysis with trials ordered by ascending sample size (fig 8) does not indicate any influence of sample size on trial outcome.
Cumulative meta-analysis with trials ordered first by the lower, then by the upper Paco2 limits as defined by the study protocols. In the left panel, the odds ratios and 95% confidence intervals of individual trials are shown. In the right panel, the cumulative results are shown as follows: in the first line the results of the first trial, in the second line the combined results of the first two trials, and so on, until all trials are cumulated at the bottom of the panel. Trials are identified by the first author names and publication years.11–24 Total indicates overall result.
Cumulative meta-analysis with trials ordered by ascending sample size. In the left panel, the odds ratios and 95% confidence intervals of individual trials are shown. In the right panel, the cumulative results are shown as follows: in the first line the results of the first trial, in the second line the combined results of the first two trials, and so on, until all trials are cumulated at the bottom of the panel. Trials are identified by the first author names and publication years.11–24 Total indicates overall result.
DISCUSSION
This meta-analysis is distinguished from previous ones26,32,33,37,41 by a higher number of included trials and assessment of possible confounders by cumulative techniques. All randomised controlled trials on elective use of HFV for preterm infants available to date have been included. Furthermore, we have extensively used the techniques of cumulative meta-analysis34 and recursive cumulative meta-analysis35,36 to gain additional insight into the influence of covariates, previously claimed to be important for evaluating HFV trials, on the outcomes of individual trials as well as the meta-analyses.
With all 17 trials and 3776 patients included, there were no significant differences except for an increase in air leaks associated with HFV, which is in contrast to previous meta-analyses based on less data.26,32,33,37,41 The trends towards fewer cases of BPD and more cases of severe IVH were not significant despite the high number of trials and patients. Funnel plots do not indicate publication bias, but at least one trial was not included because of lack of access to the data.42 This trial apparently did not find a difference in favour of HFV and thus would probably not change the overall results if it was included.
In the subgroup of trials using HFV with an HLVS and the subgroup using only the SM3100 with an HLVS, the trend towards less cases of BPD or death became significant. This finding, however, must be interpreted with great caution for two reasons. First, when measures were taken to optimise CMV, as in the trials using an LPVS strategy with CMV, the advantage of HFV disappeared, and was even replaced by a trend in the opposite direction in the subgroup of trials using HFPPV, despite the continued use of an HLVS for HFV in these trials (fig 3). A similar pattern was found among the studies using the SM3100 for HFV. Most found an advantage associated with HFV but did not use an LPVS or HFPPV strategy for CMV. The only direct comparison between HFV using the SM3100 device with an HLVS, and CMV with HFPPV, performed by van Reempts et al,23 did not demonstrate any benefit associated with HFV (fig 3) while achieving a very low rate of adverse outcomes (fig 4). Second, statistical significance in the HLVS and SM3100 subgroups was only achieved with the fixed effect model, which assumes that the effect size in all studies is the same. However, a random effects model, which assumes a distribution of effect sizes, may be more appropriate, because different ventilatory strategies were used and significant results were found in the test for heterogeneity. Differences that are not robust to the random effects model may not be relevant under such circumstances.
With time, the odds ratio of BPD or death moved towards unity, thus diminishing the initial advantage of HFV, which may reflect the improvement in conventional respiratory care. In the recursive cumulative meta-analysis (fig 5), the trials of Gerstmann et al, Thome et al, and Johnson et al12,17,21 brought about the strongest changes of odds ratio towards unity. Common features of these trials were the introduction of strategies to improve ventilatory care for infants receiving CMV. Gerstmann et al12 used surfactant, in contrast to their predecessors, which may have benefited infants randomised to CMV more than those receiving HFV. Thome et al17 and Johnson et al21 emphasised the HFPPV strategy for CMV.2–4,43 Taken together, these findings indicate that whenever the CMV strategy was optimised, the advantage associated with HFV dwindled.
Two trials in this meta-analysis used synchronised ventilation with flow triggering for the CMV groups and found significantly better pulmonary outcomes with HFV.19,20 However, when synchronisation is performed by flow triggering, the additional dead space of the flow sensor will lead to larger tidal volumes than would be necessary otherwise, especially in very small infants. Synchronisation has not been found to improve pulmonary outcome in two large randomised trials43–45; the only study suggesting a better outcome by synchronised ventilation did not use flow triggering.46 Therefore, synchronised ventilation with flow triggering and rates of less than 60/min may not be the optimal choice for small premature infants, which may explain why HFV achieved a better outcome in these two trials.
There appeared to be an association between the overall outcome of the trials and the results of the comparisons between HFV and CMV. Studies finding an advantage associated with HFV generally had below average results with their CMV strategies (fig 4). A cumulative analysis with trials ordered by their control group event rate34 may further support this notion but would require correcting all trials by their large differences in baseline demographic variables. We felt that this would introduce too many statistical errors to be valid.
Trials finding no difference between HFV and CMV have been criticised for allowing too much exposure to CMV before starting HFV.27 The cumulative meta-analysis with trials ordered by time to enrolment (fig 6) does not support this view, as the odds ratio values undulate around unity rather than showing clear patterns.34 Speculation that permissive hypercapnia may have influenced the outcome of HFV trials by benefiting infants on CMV more than those on HFV17 has also not been substantiated by a cumulative meta-analysis (fig 7). Likewise, the enrolled sample size was without a clear influence on the outcome difference between HFV and CMV (fig 8).
A significant increase in air leaks was associated with HFV. It was robust to the random effects analysis and also appeared in most subgroup analyses. Therefore, one can safely conclude that HFV as applied in most studies caused an increase in air leaks. Although most of these air leaks were probably radiological diagnoses without clinical sequelae, and were not followed by increased complications affecting long term health, such as severe IVH and BPD, this finding argues against routine use of HFV in the absence of clear benefits. More important than the outcome parameters discussed here would be long term follow up examinations. However, such data were only reported from three trials at different ages,47–51 and reflected the short term outcomes of the same trials.8,12,21
One trial included in this meta-analysis24 used a factorial design randomising not only HFV or CMV but also inhaled nitric oxide and placebo. We included this trial as there was no interaction between the randomised ventilation mode and inhaled nitric oxide.
This meta-analysis also highlights the difficulties arising in situations where the available data consist of very heterogeneous studies. As in previous attempts,26,32,37,41 the results of the significance tests depend on the selection of the studies for the subgroups, and the statistical models. The evidence obtained cannot be regarded as equivalent to level 1a.52
In conclusion, heterogeneity among trials of elective HFV in comparison to CMV in preterm infants appears to arise mainly from differences in ventilatory strategies. The time lag before enrolment and the use of permissive hypercapnia do not appear to influence study outcomes.
Optimising both modes, HFV by using an HLVS and CMV by using a high rate and minimal tidal volumes, appears to lead to comparable outcomes. Therefore, meticulous attention to the ventilator settings seems to be more important than the choice of a particular mode or machine. Purchasing costly HFV ventilators appears to be unnecessary for most neonatal intensive care units. The use of HFV for rescuing patients with severe lung failure was beyond the scope of this review.
What is already known on this topic
-
It is thought that ventilation with relatively large tidal volumes can result in lung injury
-
Randomised controlled trials comparing elective use of high frequency ventilation with conventional mechanical ventilation in preterm infants have yielded conflicting results
What this study adds
-
Optimising conventional mechanical ventilation strategy appeared to be as effective as high frequency ventilation in improving pulmonary outcome in preterm infants
-
Purchasing costly HFV ventilators appears to be unnecessary for most neonatal intensive care units
Acknowledgments
The authors thank Dr Michael D Schreiber for providing unpublished data from his study.
REFERENCES
Footnotes
-
Published Online First 7 June 2005
-
Competing interests: none declared