Article Text
Abstract
Objectives To compare the agreement, precision and repeatability of end tidal carbon dioxide (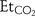 ) and transcutaneous carbon dioxide (
) and transcutaneous carbon dioxide (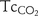 ) with partial pressure of arterial CO2 (
) with partial pressure of arterial CO2 ( ) in postoperative neonates.
) in postoperative neonates.
Patients Fifty mechanically ventilated neonates without lung disease, and with no contraindications for either  or
or  monitoring.
monitoring.
Interventions Paired  and
and  values were recorded with three consecutive
values were recorded with three consecutive 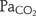 measurements within the first 48 h of surgery.
measurements within the first 48 h of surgery.
Main outcome measures  ,
, 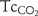 and
and  triplets were compared using Bland-Altman plots.
triplets were compared using Bland-Altman plots.
Results One hundred thirty-two triplet measures of CO2 were recorded with mean  43.5 (7.3) mm Hg,
43.5 (7.3) mm Hg,  38.8 (6.4) mm Hg and
38.8 (6.4) mm Hg and  43.8 (8.8) mm Hg (p<0.0001 for
43.8 (8.8) mm Hg (p<0.0001 for 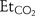 against
against  ; paired t test). The
; paired t test). The  −
− bias±2SD was 4.1±9.0 mm Hg and −0.8±13.0 mm Hg for
bias±2SD was 4.1±9.0 mm Hg and −0.8±13.0 mm Hg for 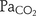 −
−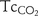 . 56.1% of
. 56.1% of  , and 60.6% of
, and 60.6% of  values were within ±5 mm Hg of paired
values were within ±5 mm Hg of paired  .
.
Conclusions In postoperative neonates,  and
and  demonstrated a clinically acceptable agreement with
demonstrated a clinically acceptable agreement with  .
.
Statistics from Altmetric.com
What is already known on this topic
-
Transcutaneous carbon dioxide (
 ) is widely used to monitor partial pressure of arterial CO2 (
) is widely used to monitor partial pressure of arterial CO2 ( ) trends in the neonatal intensive care unit (NICU) but rarely during or after surgical procedures.
) trends in the neonatal intensive care unit (NICU) but rarely during or after surgical procedures. -
End tidal carbon dioxide (
 ) monitoring is an accepted and reliable method of indicating
) monitoring is an accepted and reliable method of indicating  in the anaesthetised patient.
in the anaesthetised patient. -
 is rarely used in the NICU due to variable accuracy in preterm neonates and neonates with severe respiratory failure.
is rarely used in the NICU due to variable accuracy in preterm neonates and neonates with severe respiratory failure.
What this study adds
-
Transcutaneous carbon dioxide (
 ) demonstrated good agreement with partial pressure of arterial CO2 (
) demonstrated good agreement with partial pressure of arterial CO2 ( ) in postsurgical neonates without lung disease, but lacked precision over time.
) in postsurgical neonates without lung disease, but lacked precision over time. -
End tidal carbon dioxide (
 ) underestimated
) underestimated  by about 4 mm Hg but demonstrated better precision than
by about 4 mm Hg but demonstrated better precision than  .
. -
This study suggests that both
 and
and  are feasible methods of tracking
are feasible methods of tracking  trends over time in the postsurgical neonate.
trends over time in the postsurgical neonate.
Introduction
Non-invasive monitoring carbon dioxide (CO2) is frequently advocated in the care of the ventilated neonate.1 Transcutaneous CO2 ( ) monitoring and end tidal CO2 (
) monitoring and end tidal CO2 ( ) are the two most commonly used techniques. Although
) are the two most commonly used techniques. Although  monitoring is a standard of care during anaesthesia,2 the use in infants is limited due to conflicting results regarding accuracy.3–7 In contrast,
monitoring is a standard of care during anaesthesia,2 the use in infants is limited due to conflicting results regarding accuracy.3–7 In contrast,  is known to provide a good agreement with partial pressure of arterial CO2 (
is known to provide a good agreement with partial pressure of arterial CO2 ( )4 and accurately trend over time.4 Consequently,
)4 and accurately trend over time.4 Consequently,  monitoring is used more in the neonatal intensive care unit (NICU) environment than
monitoring is used more in the neonatal intensive care unit (NICU) environment than  , although neither has gained universal acceptance, mainly due to technical limitations.
, although neither has gained universal acceptance, mainly due to technical limitations.
Previous studies of side-stream  identified a clinically unacceptable underestimation of
identified a clinically unacceptable underestimation of  .4 ,6 ,7 These studies predominantly involved preterm neonates with lung disease.
.4 ,6 ,7 These studies predominantly involved preterm neonates with lung disease.  sensors cannot identify an alveolar CO2 plateau in the large ventilation-perfusion mismatching and fast rate, small tidal volume states characteristic of this population.1 ,2 Neonates are also ventilated after surgery, often without lung disease, and also need strict control of the postoperative course to minimise long-term morbidity. No study has specifically investigated continuous CO2 monitoring in the surgical neonate despite the suitability of this population to non-invasive techniques.
sensors cannot identify an alveolar CO2 plateau in the large ventilation-perfusion mismatching and fast rate, small tidal volume states characteristic of this population.1 ,2 Neonates are also ventilated after surgery, often without lung disease, and also need strict control of the postoperative course to minimise long-term morbidity. No study has specifically investigated continuous CO2 monitoring in the surgical neonate despite the suitability of this population to non-invasive techniques.
The aim of this study was to compare the agreement, precision and repeatability of sidestream  and
and  with
with  in mechanically ventilated postoperative neonates without lung disease.
in mechanically ventilated postoperative neonates without lung disease.
Methods
This study was performed at the Royal Children's Hospital (Melbourne, Australia), a regional surgical NICU, and approved by our research ethics committee. Mechanically ventilated neonates, without primary respiratory failure, <12 h postoperative were studied if they had an indwelling arterial line. Neonates with clinical states known to limit the accuracy of either device were not studied, including congenital diaphragmatic hernia and cyanotic heart disease, fragile skin, significant shock, hypotension, metabolic acidosis, inotropes likely to significantly impair skin perfusion or endotracheal tube leak >20%. Mechanical ventilation was applied using either synchronised intermittent mandatory ventilation or synchronised intermittent positive pressure ventilation with or without volume-targeted modes at the discretion of the clinical team.
On completion of surgery, a microstream FilterLine neonatal sidestream  system (Oridion Medical Inc., Needham, Massachusetts, USA; deadspace <0.5 ml, sampling rate 50 ml/min) was incorporated into the ventilator circuit distal to the flow sensor. A
system (Oridion Medical Inc., Needham, Massachusetts, USA; deadspace <0.5 ml, sampling rate 50 ml/min) was incorporated into the ventilator circuit distal to the flow sensor. A  probe (TINA, Radiometer Medical, Brønshøj, Denmark) was secured to the abdomen or chest, preferably the right upper chest. The sensor was allowed at least 20-min to achieve thermostability at 43°C before arterial sampling, and repositioned at least 4 hourly. This system does not allow manual calibration to
probe (TINA, Radiometer Medical, Brønshøj, Denmark) was secured to the abdomen or chest, preferably the right upper chest. The sensor was allowed at least 20-min to achieve thermostability at 43°C before arterial sampling, and repositioned at least 4 hourly. This system does not allow manual calibration to  . The capnographic waveform and numerical values for
. The capnographic waveform and numerical values for  and
and  were displayed with the arterial pressure waveform in real-time (MP70 monitor, Philips Medical, Boeblingen, Germany). Clinicians were not blinded to either measure and were allowed to make ventilation changes based on the displayed
were displayed with the arterial pressure waveform in real-time (MP70 monitor, Philips Medical, Boeblingen, Germany). Clinicians were not blinded to either measure and were allowed to make ventilation changes based on the displayed  values. Arterial blood gas samples were taken when clinically indicated. The results were recorded and the alveolar to arterial oxygen tension (A/a) ratio (Partial pressure of alveolar oxygen
values. Arterial blood gas samples were taken when clinically indicated. The results were recorded and the alveolar to arterial oxygen tension (A/a) ratio (Partial pressure of alveolar oxygen  ; where 0.8=respiratory quotient) calculated. Severe lung disease was defined as an A/a ratio<0.3.
; where 0.8=respiratory quotient) calculated. Severe lung disease was defined as an A/a ratio<0.3.
At the time of blood gas analysis, the highest  and
and  during the 10 consecutive inflations immediately before and after arterial sampling were recorded. Only
during the 10 consecutive inflations immediately before and after arterial sampling were recorded. Only  values with a distinct alveolar plateau on the capnographic waveform were included. This was repeated for three consecutive arterial samples unless the arterial line was removed, extubation or ineligibility criteria occurred.
values with a distinct alveolar plateau on the capnographic waveform were included. This was repeated for three consecutive arterial samples unless the arterial line was removed, extubation or ineligibility criteria occurred.
Statistical analysis
The Bland-Altman technique8 was used to determine  −
− and
and  −
− agreement. Limits of agreement (precision) defined as two SDs of the bias. A bias of ±5 (10) mm Hg was deemed clinically acceptable.4 ,5 Subgroup analysis to determine the influence of tidal volume,
agreement. Limits of agreement (precision) defined as two SDs of the bias. A bias of ±5 (10) mm Hg was deemed clinically acceptable.4 ,5 Subgroup analysis to determine the influence of tidal volume,  site, time since
site, time since  re-position and order of arterial sampling was made.
re-position and order of arterial sampling was made.
Results
Fifty neonates were studied and summarised in table 1. A total of 132 arterial blood gases (56% postductal) were performed, 45 neonates had two or more measurements with 37 neonates having three. The sensor site or duration since re-position did not influence the  results.
results.
Subject characteristics
The respective mean (SD)  ,
,  and
and  values were 43.6 (7.0) mm Hg, 39.4 (6.3) mm Hg and 44.3 (8.8) mm Hg, with
values were 43.6 (7.0) mm Hg, 39.4 (6.3) mm Hg and 44.3 (8.8) mm Hg, with  being lower than
being lower than  and
and  (both p<0.0001, paired t test). Overall,
(both p<0.0001, paired t test). Overall,  underestimated
underestimated  , with a
, with a  −
− bias (2SD) of 4.1 (9.0) mm Hg (figure 1A).
bias (2SD) of 4.1 (9.0) mm Hg (figure 1A).  approximated
approximated  but with wider limits of agreement: bias (2SD) −0.8 (13) mm Hg (figure 1B). These biases were independent of
but with wider limits of agreement: bias (2SD) −0.8 (13) mm Hg (figure 1B). These biases were independent of . 56.1% of
. 56.1% of  values, and 60.6% of
values, and 60.6% of  were within ±5 mm Hg of the paired
were within ±5 mm Hg of the paired  (p=0.533, Fisher's exact test). Only 27.3% and 35.6% of
(p=0.533, Fisher's exact test). Only 27.3% and 35.6% of  and
and  values were within ±2 mm Hg of
values were within ±2 mm Hg of  (p=0.420).
(p=0.420).
Bland-Altman plots of the difference between partial pressure of arterial carbon dioxide ( ) and end tidal carbon dioxide (
) and end tidal carbon dioxide ( ) (A) and transcutaneous carbon dioxide (
) (A) and transcutaneous carbon dioxide ( ) (B) against average CO2. Values obtained at the first arterial blood gas are shown with closed circle, second with diamonds and third with open circles. The solid line represents the overall bias with ±2 SD being shown in the dashed lines. The
) (B) against average CO2. Values obtained at the first arterial blood gas are shown with closed circle, second with diamonds and third with open circles. The solid line represents the overall bias with ±2 SD being shown in the dashed lines. The  −
− bias was 4.1 mm Hg and
bias was 4.1 mm Hg and  −
− bias −0.8 mm Hg.
bias −0.8 mm Hg.
Subgroup analysis found that tidal volumes ≥10 ml, or ≥4.5 ml/kg (table 2), and sampling order resulted in better agreement between both  and
and  with
with  (table 3).
(table 3).
Relationship between CO2 measurements and expiratory tidal volume (VT).
Bias (limits of agreement) of CO2 measurements at each arterial blood gas sample
Discussion
The benefit of continuous CO2 monitoring in ventilated neonates is well established,1 but not universally applied. This study suggests that both  and sidestream
and sidestream  are reliable methods to describe
are reliable methods to describe  trends in the postoperative period. To our knowledge, this is the largest comparative study of non-invasive CO2 monitoring in ventilated neonates, and the first to selectively identify a population in which neither
trends in the postoperative period. To our knowledge, this is the largest comparative study of non-invasive CO2 monitoring in ventilated neonates, and the first to selectively identify a population in which neither  nor
nor  has significant technical disadvantages.
has significant technical disadvantages.
It is not surprising that there was less discrepancy between  and
and  than
than  ,4 ,6
,4 ,6  is known to be a reliable proxy of
is known to be a reliable proxy of  in neonates.4 ,7 ,9 The precision of
in neonates.4 ,7 ,9 The precision of  , however, was variable, as is evident by the wide limits of agreement. The explanation for this is unclear but likely related to the
, however, was variable, as is evident by the wide limits of agreement. The explanation for this is unclear but likely related to the  system rather than sensor placement1 application temperature10 and skin perfusion.
system rather than sensor placement1 application temperature10 and skin perfusion.
 underestimated
underestimated  compared with
compared with  . Previous studies in neonates found
. Previous studies in neonates found  underestimated
underestimated  by 6.8–11.2 mm Hg.4–7 The better agreement found in our study likely reflects study populations as previous studies included infants with lung disease.
by 6.8–11.2 mm Hg.4–7 The better agreement found in our study likely reflects study populations as previous studies included infants with lung disease.  is known to be more accurate in infants with an A/a ratio >0.3.3 We intentionally choose to limit our investigation, and interpretation, to surgical neonates without lung disease. We found that a sidestream
is known to be more accurate in infants with an A/a ratio >0.3.3 We intentionally choose to limit our investigation, and interpretation, to surgical neonates without lung disease. We found that a sidestream  system, designed for small volume states, showed better precision between the three sample epochs than
system, designed for small volume states, showed better precision between the three sample epochs than . The intrasubject repeatability of the
. The intrasubject repeatability of the  −
− bias over these samples suggests that monitoring CO2 trends would be feasible using
bias over these samples suggests that monitoring CO2 trends would be feasible using  in this population.
in this population.
The improved agreement between  and
and  at higher tidal volumes illustrates the importance of achieving true capnographic alveolar plateau.2 The relatively large circuit deadspace, high rates and low tidal volumes characteristic of neonatal ventilation may not result in the sampled gas representing true
at higher tidal volumes illustrates the importance of achieving true capnographic alveolar plateau.2 The relatively large circuit deadspace, high rates and low tidal volumes characteristic of neonatal ventilation may not result in the sampled gas representing true  .1 ,2 In neonates requiring low tidal volumes
.1 ,2 In neonates requiring low tidal volumes  maybe a better alternative. By contrast, the surgical neonate provides some unique challenges that may limit
maybe a better alternative. By contrast, the surgical neonate provides some unique challenges that may limit  monitoring, including wound dressings, drapes, drains and other monitoring devices limiting access to well perfused
monitoring, including wound dressings, drapes, drains and other monitoring devices limiting access to well perfused  sites. As neither
sites. As neither  nor
nor  were clearly superior in this study, our results suggest that the consideration of each system's limitations, and the clinical environment, will be important in determining the most appropriate method of CO2 monitoring for an individual neonate.
were clearly superior in this study, our results suggest that the consideration of each system's limitations, and the clinical environment, will be important in determining the most appropriate method of CO2 monitoring for an individual neonate.
Limitations
To minimise unnecessary blood loss the timing of arterial sampling was not standardised. Ventilation strategy were also at the discretion of the treating clinician. Not every neonate contributed three arterial gases. This was a pragmatic choice to represent clinical practice but potentially introduces bias, and may explain the  differences over time. We contend that the fact that
differences over time. We contend that the fact that  agreement did not differ between samples suggests that the
agreement did not differ between samples suggests that the  differences were related to other factors. Overall, the postsurgical population is a significant contributor to NICU occupancy, but specific diagnoses are rare and this resulted in a heterogeneous population, limiting subgroup analysis.
differences were related to other factors. Overall, the postsurgical population is a significant contributor to NICU occupancy, but specific diagnoses are rare and this resulted in a heterogeneous population, limiting subgroup analysis.
Conclusion
In postsurgical neonates without lung disease,  underestimated
underestimated  more than
more than  but provided greater precision over repeated arterial blood gases, however it was less accurate at smaller tidal volumes. Both techniques are feasible methods of non-invasively monitoring CO2 trends in the postsurgical neonate. The clinician should be aware of the specific advantages and disadvantages of each.
but provided greater precision over repeated arterial blood gases, however it was less accurate at smaller tidal volumes. Both techniques are feasible methods of non-invasively monitoring CO2 trends in the postsurgical neonate. The clinician should be aware of the specific advantages and disadvantages of each.
Acknowledgments
The authors wish to thank Dr Neil Patel for assistance in preparing this manuscript.
References
Footnotes
-
Contributors All authors made substantial contributions to conception and design, data acquisition, analysis and interpretation. All authors were involved in the drafting of the submitted manuscript and approve of the manuscript in current form. DGT authored the first draft of the manuscript.
-
Financial support DGT is supported by a National Health and Medical Research Council Clinical Research Fellowship (Grant ID 491286) and the Victorian Government Operational Infrastructure Support Programme.
-
Competing interests None.
-
Ethics approval Human Research Ethics Committee of the Royal Children's Hospital, Melbourne, Victoria, Australia.
-
Provenance and peer review Not commissioned; externally peer reviewed.
















