Abstract
BACKGROUND: Noninvasive ventilation (NIV) may reduce the need for intubation in acute respiratory failure (ARF). However, there is no standard method to predict success or failure with NIV. The rapid shallow breathing index (RSBI) is a validated tool for predicting readiness for extubation. We evaluated the ability of the RSBI to predict failure of NIV and mortality in ARF.
METHODS: Prospective, observational trial of patients with ARF treated with NIV. NIV was initiated at the discretion of the clinicians, and an RSBI was recorded on the initial level of support (designated as assisted RSBI [aRSBI]). Patients were categorized by initial aRSBI value as either high (aRSBI > 105) or low (aRSBI ≤ 105). The primary end point was need for intubation, and the secondary end point was in-hospital mortality. Patients in the low and high aRSBI groups were compared using univariate analysis, followed by multivariable logistic regression to determine the association between aRSBI groups and outcome.
RESULTS: A total of 101 patients were included. The majority of patients had an inspiratory pressure of 5–10 cm H2O in addition to an expiratory pressure of 5–8 cm H2O. Of 83 patients with an aRSBI ≤ 105, 26 (31%) required intubation, compared to 10/18 (55%) with an aRSBI > 105 (multivariate odds ratio 3.70, 95% CI 1.14–11.99, P = .03). When comparing mortality, 7/83 patients (8.4%) with an aRSBI ≤ 105 died, compared to 6/18 (33%) patients in the group with an aRSBI > 105 (multivariate odds ratio 4.51, 95% CI 1.19–17.11, P = .03).
CONCLUSIONS: An aRSBI of > 105 is associated with need for intubation and increased in-hospital mortality. Whether patients with an elevated aRSBI could also have benefitted from an increase in NIV settings remains unclear. Validation of this concept in a larger patient population is warranted.
Introduction
Acute respiratory failure (ARF) is a frequent cause of admission to the ICU, with an incidence of 137/100,000 United States residents over the age of 5, or approximately 422,000 cases annually.1 Noninvasive ventilation (NIV) is now being used for the treatment of ARF in ICUs worldwide, with increasing frequency. Upwards of 16% of all patients admitted with ARF are being treated with NIV, and in the COPD population this percentage approaches 50%.2 A 1998 study looking at the use of mechanical ventilation in several countries found that 5% of all adults admitted to the ICU received NIV.2 NIV has been shown to reduce the need for intubation and lead to lower in-hospital mortality in COPD exacerbations and other causes of hypercapnic respiratory failure.3,4 The evidence for the use of NIV for hypoxemic respiratory failure has been less clear, with some studies finding no significant difference between NIV and conventional therapy, and one demonstrating a nonsignificant trend toward increased mortality in the NIV group, which was attributed to delays in intubation.5 More recent studies, however, suggest that NIV can lessen the need for intubation in respiratory failure of all types, as well as reduce the incidence of nosocomial pneumonia, shorten ICU stay, and reduce mortality.6–8
Despite the substantial benefits of NIV, a significant percentage of patients with ARF treated with this modality will fail, eventually requiring endotracheal intubation and mechanical ventilation. There are currently no well defined, easy to use criteria that clinicians can use to predict when a patient with ARF of any type is not likely to do well with NIV, and therefore when intubation should be undertaken early. Prior studies have delineated factors, including disease severity score, PaO2/FIO2, arterial pH, and nutritional status, that have been somewhat predictive of NIV success.9–13 Most of this work has been done in COPD patients, and all of the predictive indicators studied previously have required a 1–2 hour trial of NIV, or specific blood work and information regarding the patient's comorbidities. No single, at-the-bedside clinical parameter has been identified that could be measured at the time of NIV initiation to help predict the likelihood of NIV failure regardless of the cause of ARF. The need for such a tool is clear, as delays in intubation are associated with increased risk of mortality, and earlier triage to determine which patients should be intubated sooner could lead to improved outcomes.14,15
The rapid shallow breathing index (RSBI) was developed by Tobin and Yang as part of a study looking for objective indices to aid in decision making about patient-readiness for ventilator weaning.16 The RSBI is calculated by dividing the respiratory rate (f) by the tidal volume (VT) in L, and in the original trial it was measured while patients were intubated but on no support. The investigators found that an RSBI of ≤ 105 was predictive of a successful spontaneous breathing trial and ventilator weaning, while an RSBI > 105 predicted a higher probability of failing a weaning trial. Follow-up studies have reevaluated the utility of the RSBI as a weaning tool, and 105 remains the most widely accepted cutoff value for intubated patients. The RSBI is used by many institutions as one of several parameters to aid in deciding when to discontinue mechanical ventilation.
QUICK LOOK
Current knowledge
Noninvasive ventilation (NIV) reduces the need for intubation in acute hypercapnic respiratory failure, but there are no standard methods to predict NIV success or failure.
What this paper contributes to our knowledge
A rapid shallow breathing index (RSBI: the ratio of respiratory frequency to tidal volume) of > 105, measured while the patient is receiving NIV, is associated with the need for intubation and increased hospital mortality. RSBI may be useful for titrating NIV settings.
We hypothesized that, just as the RSBI can help predict who may be ready for extubation, it may also be useful as an indicator of who will ultimately fail NIV and require endotracheal intubation. To test our hypothesis we performed an observational study to evaluate whether an RSBI > 105, measured on NIV at the time of NIV initiation, was a predictor of impending failure of NIV in patients with ARF.
Methods
Hospital and Setting
We performed a prospective, observational study of consecutive adult patients with acute respiratory distress in the emergency department and ICUs at an urban academic tertiary care center with 490 beds, approximately 50,000 annual emergency department visits, and over 15,000 admissions yearly. The study protocol was carried out during the period from July 2009 to July 2010, and was approved by the hospital's institutional review board. After review by the board, due to the observational nature of the study, a waiver for the requirement for informed consent was provided.
Study Subject Selection
Subjects were screened in our hospital's emergency department and ICUs. Inclusion criteria consisted of age > 18 and the diagnosis of acute respiratory distress, of any etiology, requiring initiation of NIV. The decision to initiate NIV was made by the clinical team, based on the patient's respiratory status. Patients were excluded if they had a “do not intubate” and/or “do not resuscitate” order at the time of initiation of NIV.
Data Collection
Data were collected on a standardized data collection form and subsequently entered into an electronic database (Access, Microsoft, Redmond, Washington). Pertinent demographics (age, sex), height, weight, comorbid conditions and primary admitting diagnosis, as well as initial vital signs and arterial blood gas (if available) were recorded at the time of NIV initiation. All patients received care for the underlying etiology of their respiratory distress per hospital guidelines and the judgment of the medical team, which may have included but was not limited to the use of bronchodilators, corticosteroids, diuretics, or antibiotic therapy. No interventions were performed as part of the study protocol.
The medical team and respiratory therapist caring for the patient determined the NIV settings based on the patient's comfort and respiratory parameters; the settings were in no way influenced or altered by the study protocol. NIV was delivered using Evita XL ventilators (Dräger Medical, Telford, Pennsylvania), and oronasal masks were used, as is the general policy in our institution. Our hospital does not have established formal criteria for initiation of NIV, and thus the decision was made and the settings determined based on the clinical judgment of the treating physician. Respiratory rate, inspiratory VT, and expiratory VT were recorded on the patient's initial NIV settings, 15 min after the initiation of NIV. The assisted RSBI (aRSBI [f/VT]) was then calculated using the f and expiratory VT. Expiratory VT was used; this is thought to be a better reflection of true VT than the inspiratory VT, as it is less affected by air leaks around the NIV mask. Decisions regarding how long to continue NIV and whether to progress to endotracheal intubation were made by the clinical team, based on the individual physician's judgment. To limit the possibility of investigator bias, outcome parameters (NIV outcome and in-hospital mortality) were recorded separately from and subsequent to the initial observation, based on review of the patient's electronic medical record at a later time.
Outcomes
The primary outcome was failure of NIV, as determined by the need for intubation during the patient's hospital stay. The secondary outcome was in-hospital mortality.
Statistical Analysis
Subjects were categorized according to high and low aRSBI at the time of NIV initiation, such that low was classified as aRSBI ≤ 105 and high as aRSBI > 105, based on the numbers arrived at by Yang and Tobin.16 Descriptive statistics, including median values and interquartile ranges, were used to characterize the study population. Continuous variables were compared by non-parametric Wilcoxon rank-sum test. Categorical variables were compared between low and high aRSBI groups using the Fisher exact test.
We used univariate logistic regression to assess potential individual predictor variables for the primary and secondary outcomes. We considered primary admitting diagnosis, medical history, and laboratory data at the time of NIV initiation for univariate modeling, and variables that appeared to be associated with outcomes (P < .10) were carried forward into multivariable analyses. We subsequently performed multivariate logistic regression to determine the association of aRSBI with the primary and secondary outcomes. Statistical significance for multivariate analysis was set at α = .05. Data analysis was performed in statistics software (SAS 9.1, SAS Institute, Cary, North Carolina).
Results
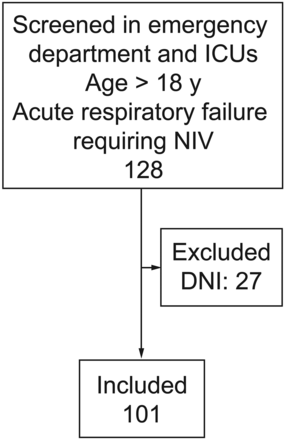
A total of 128 patients with ARF for which NIV was initiated were identified, and 27 were excluded due to their “do not resuscitate/do not intubate” status at the time of NIV initiation. We therefore analyzed data on a total of 101 subjects (Figure). All patients had an aRSBI recorded on their initial NIV settings, designated as high (> 105) or low (≤ 105). Of the 92/101 patients for whom NIV settings were recorded, 58 (63%) were on inspiratory pressure between 5 and 10 cm H2O in addition to an expiratory pressure of 5 cm H2O at the time of aRSBI measurement. Of the remaining patients, the highest inspiratory pressure was 18 cm H2O (a single patient) and the highest expiratory pressure was 10 cm H2O. The average age was 70 ± 16 years, and a wide range of diagnoses were represented. A total of 36 patients (36%) required intubation for the episode of respiratory failure for which they were started on NIV, and 13 (13%) died while in the hospital. Additional baseline characteristics are presented in Table 1.
Enrollment schematic.
Baseline Characteristics
For the primary outcome of need for intubation, univariate logistic regression demonstrated a strong trend toward increased need for intubation in the high aRSBI group, with an odds ratio (OR) for intubation of 2.74 (95% CI 0.97–7.74, P = .06). An admitting diagnosis of pneumonia was also associated with an increased need for intubation (P = .01). Age and COPD exacerbation were inversely associated with intubation (P = .07 and P = .04, respectively). Multivariate logistic modeling therefore included aRSBI group, age, pneumonia, and COPD exacerbation. Controlling for these factors, patients with a high aRSBI were significantly more likely to be intubated (OR 3.70 with 95% CI 1.14–11.99, P = .03) than patients with a low aRSBI (Table 2).
Multivariate Analysis for aRSBI as a Predictor of Intubation*
Our secondary outcome was in-hospital mortality. An unadjusted high aRSBI was associated with an increased risk for in-hospital mortality (OR 5.43, 95% CI 1.56–18.93, P = .008). Additional variables independently associated with in-hospital mortality included age (P = .04) and admission for sepsis (P = .10). Controlling for these factors in multivariate modeling, patients with a high aRSBI were significantly more likely to die during the hospital stay (OR 4.51, 95% CI 1.19–17.11, P = .03), compared to patients with a low aRSBI (Table 3).
Multivariate Analysis for aRSBI as a Predictor of In-Hospital Mortality*
Discussion
In our study, 36/101 patients placed on NIV for the treatment of ARF failed this treatment, with failure classified as requiring invasive mechanical ventilation. This NIV failure rate of 36% is similar to that found in other studies, when all causes of ARF are included. An aRSBI > 105 was found to be significantly associated with both an increased need for intubation and a higher mortality.
NIV is now well established as a treatment for hypercapnic respiratory failure, with studies showing that in many cases outcomes are better with NIV than with invasive mechanical ventilation, both short- and long-term.6,17 Our data were consistent with these prior findings, with an OR for intubation in patients with a COPD exacerbation of 0.23 (95% CI 0.06–0.88, P = .03). In contrast, pneumonia in our patient population was predictive of need for intubation, with an OR of 3.56 (95% CI 1.38–9.19, P = .009). There are comparatively few studies evaluating NIV for hypoxemic respiratory failure, and although some studies have demonstrated efficacy, our increased failure rate in patients with pneumonia is in agreement with prior data suggesting NIV outcomes are not as good for hypoxemic respiratory failure as they are for hypercapnic respiratory failure.6,18
In all of these studies, as in ours, a percentage of patients failed NIV and required endotracheal intubation. To our knowledge, there are few prior studies looking at how to predict NIV failure. Being able to determine which patients will not do well on NIV is potentially important, as delaying intubation for a patient who will ultimately require it may cause undue strain on the respiratory and cardiovascular systems, increased discomfort for the patient, and possibly a worse outcome. Although the amount of available data is small, a few studies have evaluated the effect of delays in intubation. Esteban et al randomized patients who went into respiratory distress after extubation to standard therapy or to NIV and found that NIV did not help prevent reintubation. More importantly, among all patients who eventually required reintubation, the mortality rate in the NIV group was significantly higher. The time between onset of respiratory failure and reintubation was also significantly longer, suggesting a negative impact of the delay in intubation.19 In a similar study, Epstein et al found a significant association between an increased length of time between extubation and eventual reintubation and an increase in mortality.14 A third study examined factors that affected outcomes in patients with respiratory failure secondary to pneumocystis pneumonia and found that delayed intubation was significantly associated with mortality.20 The average time elapsed before intubation among survivors was 4 hours, compared to 48 hours in the group of patients who expired. Of the patients who were initially treated with NIV, a longer time on NIV before being intubated was significantly associated with a higher mortality.
Some prior studies have attempted to delineate predictors of failure of NIV in COPD patients. Putinati et al found that in a group of 59 COPD patients admitted with ARF, a low serum albumin and a high Acute Physiology and Chronic Health Evaluation (APACHE) II score were predictive of failure of NIV, as was failure of the PCO2 to improve after 2 hours on NIV.21 Anton et al looked at 36 patients with an exacerbation of obstructive disease and reported that good level of consciousness and improvement in PCO2 and pH after 1 hour were predictive of success of NIV, but that APACHE II scores were not predictive.22 Anton's group derived a multiple regression model that was able to predict failure of NIV in 93% of a small group of patients, but this model required a complex calculation that would be difficult to carry out at the bedside. Confalonieri et al studied over 1,000 COPD patients who required NIV for an acute decompensation, and found that pH < 7.25, f > 30 breaths/min, and APACHE II score > 29 were somewhat predictive of NIV failure. They included patients who were “do not intubate,” however, and a substantial number of their NIV failures died without being intubated, due to their “do not intubate” status, making the results somewhat difficult to interpret.11
Antonelli et al conducted a prospective cohort study of 147 patients admitted to an ICU for ARDS, all of whom were initially treated with NIV.10 In their analysis, only Simplified Acute Physiology Score (SAPS II) > 34 and PaO2/FIO2 ≤ 175 mm Hg after 1 hour of NIV were found to be significantly associated with the need for intubation. Their study also did not include the patients' code status, making predictors of need for intubation and mortality more difficult to interpret.
To our knowledge, there has been only one previous study that has examined this question in patients with all types (both hypoxemic and hypercapnic) of ARF. Lin et al attempted to identify respiratory indices that would predict failure of NIV in 86 patients with ARF from any cause.12 They calculated the APACHE II score for each patient at the time of enrollment, and over the course of one hour after NIV was started they checked a RSBI, f, VT, and maximal inspiratory and expiratory pressures. The RSBI and the inspiratory and expiratory pressures were measured using a hand-held spirometer, requiring a brief discontinuation of NIV. All measurements were taken at the time of NIV initiation, then at 30 min and at 60 min. This group reported that APACHE II scores were significantly lower in the success group, but of all the respiratory indices measured, they found that only f was significantly different in the 2 groups, with the failure group having a higher f at both 30 and 60 min. They did not find a statistically significant difference in the RSBI between the 2 groups at any of the time points.
Two key differences between Lin's study and ours were that they measured an RSBI on no support, requiring an interruption in NIV, and that they analyzed the RSBI as a continuous variable. We evaluated the aRSBI as a dichotomous variable, based on the data presented previously by Tobin and Yang, which indicated that a cutoff of 105 was most clinically useful for predicting successful weaning from mechanical ventilation. As we were using the RSBI for a purpose other than that for which it was initially developed, we went back to further analyze this, to determine if 105 was truly the best number. We graphically inspected the receiver operating characteristic curves for RSBI as a continuous variable for each of the outcome variables. We used the Youden index (the distance between the receiver operating characteristic curve and a 45° line) to suggest an optimal cut-point. This index score identified approximately 105 as the optimal cut-point in both models. With the a priori identification of 105 as a potentially relevant cutoff, it was determined that this would be both statistically important as well as a clinically relevant cut-point for our outcomes.
We also chose to measure an assisted RSBI on the patient's current level of support, whereas in the Lin study patients were taken off NIV briefly so that the RSBI measurement could be performed. Although Tobin and Yang developed the RSBI as a measure of spontaneous, unsupported breathing, it was also used to predict a patient's success on a spontaneous breathing trial. As we were attempting to use this measure to predict a patient's success on NIV, not on a spontaneous breathing trial, we chose to measure it while the patient was being supported with NIV, as an indicator of how a patient was likely to do on the amount of support currently being delivered.
The significant associations found between age and intubation and mortality in our cohort are intriguing, although the differences were small. Older patients were slightly less likely to be intubated, but in fact more likely to die in-hospital. That all patients included in this observational trial were confirmed to be full code at the time of initiation of NIV and that there was an increased trend toward mortality in older subjects suggests that we may treat older patients less aggressively, irrespective of code status.
Our investigation is limited by its small size and observational nature. The percentage of patients with an aRSBI > 105 was also quite small, and to validate our findings a larger study is warranted. This was also a single-center study, and as such is limited by differing approaches to NIV use among medical centers. The NIV settings recorded for the majority of patients in the study were fairly low (with > 50% being on settings of 10/8 or less), which raises the questions of whether additional up-titration of NIV settings after an initially elevated aRSBI might have helped some patients evade the need for intubation. Our hospital also does not have a clearly delineated protocol for when to initiate NIV, so it is possible that our patient population might differ from the populations treated with NIV at other hospitals. In spite of these limitations, our data suggest that the aRSBI, measured on NIV, is a potentially useful tool for risk-stratifying patients in ARF who are initially treated with NIV.
Conclusions
An aRSBI of > 105 was associated with need for intubation and increased in-hospital mortality in our patient population. Validation of this concept in a larger patient population is warranted.
Acknowledgments
The authors would like to thank Francesca Montillo for her administrative support in preparing the final version of the paper.
Footnotes
- Correspondence: Katherine M Berg MD, Beth Israel Deaconess Medical Center-Pulmonary and Critical Care, 330 Brookline Ave, KB23, Kirstein Basement, Boston MA 02215. E-mail: kberg{at}bidmc.harvard.edu.
Mr Lang presented preliminary data from this paper at the Open Forum of the AARC Congress 2010, December 6–9, 2010, in Las Vegas, Nevada.
The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 1685
- Copyright © 2012 by Daedalus Enterprises Inc.