Abstract
BACKGROUND: Optimal titration of inspired oxygen is important to prevent hyperoxia in mechanically ventilated patients in ICUs. There is mounting evidence of the deleterious effects of hyperoxia; however, there is a paucity of data about FIO2 practice and oxygen exposure among patients in ICUs. We therefore sought to assess excessive FIO2 exposure in mechanically ventilated patients with acute lung injury and to evaluate the effect on pulmonary outcomes.
METHODS: From a database of ICU patients with acute lung injury identified by prospective electronic medical record screening, we identified those who underwent invasive mechanical ventilation for > 48 hours from January 1 to December 31, 2008. Ventilator settings, including FIO2 and corresponding SpO2, were collected from the electronic medical record at 15-min intervals for the first 48 hours. Excessive FIO2 was defined as FIO2 > 0.5 despite SpO2 > 92%. The association between the duration of excessive exposure and pulmonary outcomes was assessed by change in oxygenation index from baseline to 48 hours and was analyzed by univariate and multivariate linear regression analysis.
RESULTS: Of 210 patients who met the inclusion criteria, 155 (74%) were exposed to excessive FIO2 for a median duration of 17 hours (interquartile range 7.5–33 h). Prolonged exposure to excessive FIO2 correlated with worse oxygenation index at 48 hours in a dose-response manner (P < .001.). Both exposure to higher FIO2 and longer duration of exposure were associated with worsening oxygenation index at 48 hours (P < .001), more days on mechanical ventilation, longer ICU stay, and longer hospital stay (P = .004). No mortality difference was noted.
CONCLUSIONS: Excessive oxygen supplementation is common in mechanically ventilated patients with ALI and may be associated with worsening lung function.
Introduction
Titration of supplemental oxygen is important, but not adequately practiced. Hypoxia and hyperoxia both produce detrimental effects at a cellular level.1–3 Healthcare practitioners are well aware of the catastrophic effects of hypoxia, and this has led to the “more is better” culture of oxygen supplementation. Adverse effects of hyperoxia in healthy adults have been documented since the 1940s.4 Prolonged high FIO2 in patients requiring mechanical ventilation worsens gas exchange, decreases ciliary efficacy, and produces hyperoxic bronchitis and atelectasis.5 Studies have shown adverse effects associated with hyperoxia in various conditions such as COPD, after acute myocardial infarction, and after resuscitation from cardiac arrest.6–8 Acute lung injury (ALI) presents initially with hypoxemia, which might lead to mechanical ventilation and use of high FIO2. If oxygen is continually supplemented without titration, it may inadvertently perpetuate ALI. The microscopic changes from hyperoxia are undifferentiated from those seen in ARDS.5,9
Although there is increasing awareness of the potential harms of high FIO2, this has not translated to change in routine practice. There are reports of unregulated oxygen use in the emergency room and pre-hospital emergency care environment.7,10 The literature on the practice of oxygen supplementation in the ICU is sparse. ICUs harbor critically ill patients, with most requiring mechanical ventilation; thus oxygen titration becomes critical in this setting. In this study we describe FIO2 practice within ICU in patients with ALI, and evaluate the association of excessive FIO2 and pulmonary function.
QUICK LOOK
Current knowledge
Hyperoxemia has been reported to be a common condition in patients on mechanical ventilation when the set FIO2 is ≤ 0.40. The literature on the effect of hyperoxemia on lung function and outcomes is contradictory.
What this paper contributes to our knowledge
Excessive oxygen supplementation is common in mechanically ventilated patients with acute lung injury, and may be associated with worsening lung function at 48 hours. The use of the lowest possible FIO2 to maintain normoxemia may be warranted.
Methods
The study was approved by the Mayo Clinic institutional review board (IRB no. 06–005418). The inclusion criteria included all adult patients with ALI who had invasive mechanical ventilation for > 48 hours at any of the ICUs at the Mayo Clinic, Rochester, Minnesota, from January 1 to December 31, 2008. Those who had a diagnosis of ALI, carbon monoxide poisoning prior to admission, withdrawal of care within 48 hours, or no research authorization were excluded.
A previously validated electronic surveillance tool (ALI Sniffer)11 was used to screen all patients who received ICU services from 2004 to 2008. The ALI Sniffer has a negative predictive value ranging from 98–100%, and sensitivity of 96% (95% CI 94–98%), making it an excellent screening tool. The diagnosis of “sniffer positive” patients was confirmed or excluded by manual review by a team of critical care experts including clinical/research fellows and staff. The inter-observer variability between the reviewers for the year 2006 had a kappa value of 0.86. The criteria for identification of the patients by the ALI sniffer were the following within a single 24-hour period:
Qualifying arterial blood gas analysis: PO2/FIO2 < 200 mm Hg for ARDS and < 300 mm Hg for ALI. In case of multiple arterial blood gas values, the worst value during the 24 h window was selected.
Qualifying chest radiograph report: free text Boolean query containing the words “edema” or “bilateral” and “infiltrate”
Invasive mechanical ventilation for acute respiratory failure or duration of invasive mechanical ventilation > 12 hours following an operative procedure
Mechanical Ventilation Protocol
Respiratory therapy guidelines in our institution suggest tidal volumes of 6–8 mL/kg predicted body weight for all patients with or at risk for ARDS. Predicted body weight charts are available on each machine. Oxygenation goals are not part of the protocol and are practiced at the discretion of bedside clinicians.
Data Collection
Utilizing a preexisting electronic database, the FIO2 and the exact corresponding peripheral SpO2 were noted. The FIO2 and SpO2 were collected through the first 48 hours of mechanical ventilation. These continuous FIO2 values (recorded at each 15 min interval) and corresponding continuous SpO2 values (also recorded at each 15 min interval) were used to define excessive oxygen exposure in the study patients. The first hour after endotracheal intubation was excluded (Fig. 1). In patients with a clinical diagnosis of shock and acute myocardial infarction, the first 6 hours after diagnosis were excluded.
FIO2, SpO2, and PEEP in an example patient.
Baseline Variables
Demographic data, along with admission Acute Physiology and Chronic Health Evaluation (APACHE) III scores were noted. Median FIO2, SpO2, PaO2, SpO2/FIO2, PaO2/FIO2, oxygenation index (OI), and risk factors for ARDS were recorded.
Outcome Variables
The primary outcome variable was duration of exposure to excessive inspired oxygen for each patient during the initial 48 hours of mechanical ventilation. Excessive FIO2 was defined as an FIO2 > 0.5 on mechanical ventilation while maintaining a corresponding SpO2 > 92% (Fig. 2).12 Appropriate exposure to FIO2 was defined as, if SpO2 > 92%, then FIO2 < 0.5 or any FIO2 with SpO2 < 92% during mechanical ventilation.
Outline of the study. MV = mechanical ventilation.
The secondary, exploratory outcome variables were evolution of pulmonary failure (mean change in OI at 48 hours, compared to baseline), and ICU stay (ventilator-free days at day 28 in the ICU, or 28-day mortality, whichever occurred earlier).
Statistical Analysis
Continuous and categorical variables were compared using the Wilcoxon rank sum test and the chi-square test. Univariate and multivariate linear regression were performed to investigate the relation between FIO2 and mean OI at 48 hours. Association between the mean change in OI and duration of exposure was evaluated by multivariate linear regression. Initial OI was added in the model for multivariate analysis in both cases. A P value of .05 was considered significant. Statistics software (JMP 6.0, SAS Institute, Cary, North Carolina.) was used for all data analysis.
Results
Screening of electronic medical records identified 289 patients with ALI, and 210 met the inclusion criteria (Table 1). Over the initial 48 hours of mechanical ventilation, excessive oxygen exposure (FIO2 > 0.5, SpO2 > 92%) was identified in 155 (74%) patients, and, among them, 110 (53%) were exposed to FIO2 > 0.7 (FIO2 > 0.7, SpO2 > 92%) (see Fig. 2). Baseline characteristics, APACHE III scores, and risk factors for ALI/ARDS were similar between patients with and without excessive FIO2 exposure. The initial OI, SpO2/FIO2, and PaO2/FIO2 at admission were similar in both groups. Both groups were ventilated with comparable tidal volumes (7.2 vs 7.6 mL/kg predicted body weight, P = .30) and initial PEEP (5 vs 6.5 cm H2O, P = .20) (Table 2). The nadir hemoglobin was similar in both groups (9 vs 9.2 g/dL, P = .40). Mean cardiovascular Sequential Organ Failure Assessment scores in the exposed and non-exposed groups were noted to be 3 versus 2.5 (P = .80). Use of sedation in both groups was as per the pre-designed protocol for the ICU. The median duration of excessive oxygen exposure was 17 hours (interquartile range [IQR] 7.5–33 h). Ninety six (62%) patients were exposed to excessive FIO2 for 12 hours or more, and about half among them had exposure for > 30 hours. When considered for SpO2 > 95%, the median duration of excessive exposure was 11 hours.
Baseline Variables
Mechanical Ventilation Related Variables
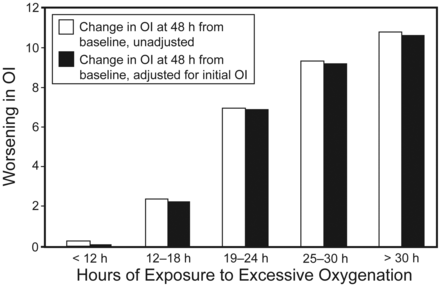
At 48 hours, the exposed group had higher median OI (13.3 vs 5.1, P < .001.) and a higher mean change in OI (4.6 vs −1.5, P < .001.). Figure 3 reflects change in OI for each patient from baseline to 48 hours with severe (FIO2 > 0.7), moderate (FIO2 > 0.5), or no excessive FIO2 exposure. At least 12 hours of excessive FIO2 exposure was required for a significant increase in OI from baseline (2.26 vs 0.4, P < .001.). Thereafter, further exposure was linearly associated with a higher OI at 48 hours. This persisted even after adjusting for initial OI and severity of illness (Fig. 4, Table 3). Correlation between excessive exposure and worsening OI remained significant regardless of definition of excessive exposure (FIO2 > 0.5 or > 0.7) (see the supplementary material at http://www.rcjournal.com.). Over 48 hours the exposed group was found to have a higher median FIO2 (0.6 vs 0.4, P < .001). The FIO2 distribution over the study period in exposed and non-exposed groups is shown in the supplementary material. Both groups had similar PaO2 values to begin with (57 vs 56 mm Hg, P = .80) and that improved in a comparable manner at 48 hours (92 vs 87 mm Hg, P = .60). The mean airway pressures were similar to begin with, but higher in the exposed group at 48 hours (15 vs 12 cm H2O, P = .008). The exposed group had longer duration of mechanical ventilation and consequent ICU and hospital stay. There was no difference in the 28 day mortality between both groups (Table 4). When considering the association of overall FIO2 to outcomes regardless of oxygenation, higher FIO2 correlated with higher OI. In univariate analysis, FIO2 was associated with higher hospital mortality, but when adjusted for severity of illness, the association was not significant.
Change in oxygenation index from initiation of mechanical ventilation (baseline) to 48 h after intubation, versus FIO2.
Mean worsening of oxygenation index 48 h after initiation of mechanical ventilation (baseline), versus hours of excessive FIO2.
Multivariate Linear Regression Analysis for Change in Mean Oxygenation Index at 48 Hours by Hours of Exposure
Outcome Variables
Discussion
In this retrospective study we found that excessive oxygen exposure through mechanical ventilation was prevalent at a tertiary care center. Prolonged excessive FIO2 exposure may be associated with worsening pulmonary function, in a dose-response manner. In this study mortality was not associated with excessive FIO2 exposure.
Our methodology aimed to determine “excessive FIO2 exposure” in ALI patients who had improving oxygenation, for which we have used SpO2 as a surrogate. We defined excessive FIO2 exposure when SpO2 was > 92% and FIO2 continued to be > 0.5. When SpO2 was > 92% and the FIO2 is appropriately titrated to ≤ 0.5, or in situations where SpO2 remained < 92%, receiving FIO2 > 0.5 was categorized as “appropriate exposure” to FIO2. While we used SpO2 levels in conjunction with FIO2 for monitoring excessive oxygen exposure, both SpO2 and PaO2 levels could be used for monitoring oxygenation. Although, PaO2 levels are considered the gold standard for arterial oxygenation, they can be determined only by invasive blood gas measurements. It is neither feasible nor necessary to have multiple blood gas measurements for FIO2 titration. Additionally, they can significantly vary over short periods with constant FIO2, due to agitation and positioning. The noninvasive nature and continuous measurements of peripheral oxygen saturation allow continuous FIO2 titration at the bedside, a standard of care in most ICUs.13 SpO2 levels may have reduced accuracy in conditions of low cardiac output, methemoglobinemia, or skin color and artifacts. SpO2/FIO2 shows good correlation with PaO2/FIO2 in ALI.14 Our cutoff for optimal saturation was 92%. In mechanically ventilated patients, employing a saturation of 92% has a positive predictive value of 80% for a PaO2 ≥ 60 mm/Hg (except in African-American patients, where SpO2 of at least 95% is required to prevent hypoxia).12 The ARDS Network study for lower tidal volumes in ARDS patients used an oxygenation goal of SpO2 88–95%.15
In our study 74% patients had excessive oxygen exposure for 41% of time within the first 48 hours of mechanical ventilation (see the supplementary material). We found that adherence to the ARDS Network maximum target SpO2 values was also incomplete, as in the exposed cohort 90% of patients had SpO2 values higher than 95% for a median duration of 2 hours (1.5–4 h). These findings are not completely surprising. The practice of high oxygen to ensure “safety” and hence “inattention” to timely oxygen titration has been noted in various healthcare settings across the world. A retrospective review of clinicians' response to FIO2 changes after blood-gas analysis in mechanically ventilated patients in a Dutch ICU revealed that hyperoxia (PaO2 > 120 mm Hg) was frequent and led to down-titration of FIO2 in only 25% of the arterial blood gas tests.16 In a study of patients who were prescribed oxygen in the emergency room setting at a tertiary care hospital, oxygen prescription was either not required or excessive in 79% of evaluations, determined by peripheral oxygen saturation of 92% or greater.10
There could be various reasons for this practice of oxygen supplementation. Optimal FIO2 titration requires frequent monitoring. Time constraints for intensivists could be a plausible explanation for inadequate titration in the ICUs. Setting up a protocol-driven titration by registered respiratory therapists might be a reasonable approach to minimize excessive oxygen supplementation. Similar ventilation protocols have been successful for weaning and neonatal ventilation.17,18 The determinants of oxygen delivery include hemoglobin, cardiac output, and oxygen saturation. Therefore, clinicians may tend to hyperoxygenate in the presence of anemia or dysoxic states associated with hemodynamic compromise. In our cohort, the nadir hemoglobin levels were in the range of 9 g/dL and were similar between 2 groups; therefore, excessive FIO2 provision to allow for liberal oxygenation should not have been necessary. Additionally, we chose to exclude the first 6 hours of shock and acute myocardial infarction, considering that these conditions are reflective of generalized and/or local tissue dysoxia. Higher oxygen may be needed to alleviate tissue hypoxia. The practice of using higher FIO2 cannot be considered unreasonable under these settings. These high values could affect outcomes, but according to the contemporary clinical guidelines, they cannot be labeled as “excessive oxygen”; therefore, to stay on a conservative side, we choose to exclude these values. This question or dogma, however, should be explored in future studies
Prolonged FIO2 exposure showed linear correlation with worsening OI. Also, the OI increased irrespective of FIO2 (FIO2 > 0.5 or > 0.7). This was associated with longer duration of mechanical ventilation and ICU stay. OI integrates airway pressure with oxygenation and has shown to be a reliable predictor of worsening lung function in ALI.19–21 Decline in lung function related to duration and concentration of FIO2 has been well defined in various animal studies.22 Prolonged exposure even to moderately high FIO2 (0.6) resulted in alveolar septal edema in baboons.23 Wistar rats demonstrated dose dependent lung toxicity when exposed to higher FIO2.24
Our findings are in conjunction with some previous human studies. Nash et al, in the 1960s, provided the first detailed pathologic review of pulmonary changes after exposure to both higher concentration of oxygen and duration of therapy.5 They found distinct pathological changes of interstitial edema progressing to fibrosis seen with prolonged duration of high FIO2. A pulmonary venous admixture study revealed worsening shunt fraction when FIO2 was increased beyond 0.6.25 Davis et al studied the effects of high FIO2 (0.95) on humans after about 16 hours of exposure, and found increased “alveolar capillary leak,” as well as evidence of induction of fibrosis, through bronchioalveolar fluid assessment.26 Sevitt and colleagues worked to determine the threshold for hyperoxic lung injury.27 They found changes of diffuse pneumonitis after 48 hours of exposure to FIO2 of 0.6–1.0. Prolonged exposure of 7 days to FIO2 of 0.40 was also detrimental, while no worsening was noted with FIO2 < 0.40.
Based on our study results, we may contemplate that, in patients with ALI, further excessive FIO2 may have resulted in some degree of alveolar damage, which reflected as worsening OI. Patients with ALI can be prone to effects of hyperoxia. Santos et al looked at the effects of high FIO2 in ALI patients and found deterioration in pulmonary shunt, probably by collapse of unstable alveolar units.28 Hyperoxia can also augment ventilator-induced lung injury in ALI in the presence of high tidal volumes, by activating signaling pathways.29
Excessive FIO2 did not appear to influence mortality in our cohort. In another large retrospective study of mechanically ventilated ICU patients in the Netherlands, the hospital mortality was independently associated with the mean FIO2 during ICU stay.30 In the same study, high FIO2 in the first 24 hours of admission was linearly related to hospital mortality, along with both high and low PaO2 values. In an Australian pre-hospital setting, liberal oxygenation practices were prevalent in patients with COPD exacerbation, and appropriate titration showed reduction in mortality and hypercapnia.7
There are several limitations that have to be addressed in the interpretation of data obtained for this study. SpO2 measurements may not have complete accuracy. Errors created by equipment, skin color, or artifacts from foreign objects on nails are possible. Our patient population is mostly white, and the results may not be generalizable to other settings. The SpO2 cutoff value of 92% is not optimal for African-American patients and should be extended to 95%.
The association between worsened OI and high FIO2 may not be necessarily causal. Although this association appeared to be independent of potential confounding covariates, it is possible that there might have been some differences in our patients that we may not have accounted for in our multivariate analysis. In addition, in this epidemiologic study, the OI was not obtained under standardized ventilator settings; however, this limitation affected both of the study groups. The OI correlates directly with the product of FIO2 and the mean airway pressure, and inversely with PaO2. Considering this mathematical relationship, the increase in OI could partially be attributed to the high FIO2 rather than to actual worsening in lung function.
In the exposed group, lower PEEP could have resulted in lower OI at 48 hours. However, the presence of higher mean airway pressures at 48 hours, in spite of lower PEEP levels and improvement in arterial oxygen, may be indicative of worsening of OI, as a conjugate function of all the 3 variables, rather than only higher FIO2. Based on the retrospective nature of the study, we can only speculate that the group with excessive FIO2 may have had a higher FIO2 as a consequence of using lower PEEP. This may reflect inadequate use of PEEP in practice.
With the current study design we are unable to show the cutoff value for degree of FIO2 > 0.50 that may be toxic to patients who continue to have prolonged hypoxemia (with SpO2 < 92%. This would help to clarify the targets of “permissive hypoxemia,” which have not been well defined in the literature and need to be studied further.
Conclusions
Optimal oxygen titration is important and not adequately practiced in ICUs, resulting in substantial exposure to unnecessary excessive oxygen. There is a likely association between excessive oxygen and worsening lung function, which needs further study. Optimal titration needs close monitoring and may be a potential area for greater participation of respiratory therapists. The safety and efficacy of FIO2 titration protocols needs to be evaluated in prospective studies.
Footnotes
- Correspondence: Sonal R Rachmale MD, Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester MN 55905. E-mail: rachmale.sonal{at}mayo.edu.
Supplementary material related to this paper is available at http://www.rcjournal.com.
The authors have disclosed no conflicts of interest.
- Copyright © 2012 by Daedalus Enterprises Inc.