Abstract
BACKGROUND: Blow-by, a common form of nebulizer therapy, in which the device is held away from a child's face, has been dismissed as ineffective because studies have demonstrated incremental aerosol drop-off with increasing distances from the face. Many of these studies do not take into account differences among nebulizer systems. Using common, commercially available nebulizer systems, we defined the interaction of system components (nebulizer type, face mask configuration, and compressor characteristics) on aerosol delivery with and without blow-by.
METHODS: A pediatric model consisting of a ventilated mannequin fitted with a filter (inhaled mass), and 3 commercial nebulizer/compressor/face mask systems (Pari Sprint, Respironics Sidestream, and Salter 8900) were used to nebulize budesonide (1.0 mg/2 mL) at 0, 2, and 4 cm from the face. Inhaled mass and the deposition on face, eyes, and mask were measured using high-performance liquid chromatography and reported as a percent of nebulizer charge.
RESULTS: At 0 cm, inhaled mass for the Pari, Respironics, and Salter systems was 5.33%, 1.14%, and 3.50%, respectively; at 4 cm from the face, inhaled mass decreased to 1.83%, 0.13%, and 1.14%. Facial (1.12%, 0.63%, and 2.94%) and eye (0.35%, 0.12%, and 0.68%) deposition varied significantly. Pari compressor/nebulizer flow rate was lower than Respironics and Salter (3.5 L/min vs 5.7 L/min and 5.9 L/min), resulting in longer run time (7.7 min vs 4.0 min and 5.3 min).
CONCLUSIONS: At 4 cm, the Pari system delivered more drug than Respironics at 0 cm, suggesting adequate therapy during blow-by for some systems. Our results indicate that pediatric aerosol delivery is a strong function of the nebulizer system as a whole, and not simply a function of blow-by distance from the face or nebulizer efficiency. In uncooperative children, blow-by can be an effective means of drug delivery with the appropriate nebulizer system.
Introduction
Delivering nebulizer treatments to young children can be challenging. For example, younger children may lack sufficient understanding to use a mouthpiece effectively. A traditional alternative, applying a face mask directly to the face, may cause distress. Blow-by is a controversial but common form of nebulizer therapy, where the nebulizer/face mask is held at a distance from an uncooperative child's face.1 Because of incremental decreases in aerosol delivery with increasing distances from the face,2,3 some authorities have dismissed blow-by and condemned it as altogether ineffective.4–6
While blow-by in itself reduces aerosol delivery, other factors might be important. For example, drug delivery can vary by more than 10-fold between different nebulizer systems.7 In addition, face mask design is recognized as an important factor in optimizing aerosol delivery,8 and its potential role in affecting delivery via blow-by has been studied.9 These observations suggest that the efficient delivery of an aerosol to a child is dependent upon multiple factors, some of which may be more important than the decrement involved with the use of blow-by, in ultimately determining the amount of aerosol delivered. If so, then proper choice of nebulizer system may allow for the use of blow-by if device efficiency and design factors compensate for the effect of increasing distances from the face.
At the bedside, practitioners can titrate bronchodilator therapy using the mode of delivery that is best tolerated by the patient, independent of in-vitro studies. However, the effects of some drugs, such as aerosolized steroids, are not easily assessed during clinical aerosol delivery. Therefore, the practitioner must rely on experience with proven delivery systems used in clinical studies, combined with in-vitro measurements determining the effects of modifications to standard methods of delivery. For example, the use of a valved holding chamber combined with a metered-dose inhaler,9 or, as in this case, drug delivery via blow-by.
The purpose of the present study was to determine whether nebulized budesonide, an important steroid commonly used to treat young children, could be delivered in reasonable amounts using the blow-by method. We used 3 commercially available pediatric nebulizer systems (compressor, nebulizer, and mask), chosen based on differences in design. Using a previously described in-vitro model, we measured inhaled mass and aerosol distribution, as well as facial, eye, and mask deposition at 0, 2, and 4 cm from the face, to determine how differences in system components (mask design, flow rates, nebulization times) ultimately influence the effectiveness of drug delivery via blow-by.
QUICK LOOK
Current knowledge
Blow-by, a common form of aerosol therapy, in which the device is held away from a child's face, is often used in pediatrics to improve tolerance. This technique is often dismissed as ineffective, as research demonstrates incremental aerosol drop-off with increasing distance from the face.
What this paper contributes to our knowledge
In this in-vitro model, efficient nebulizer systems can deliver clinically relevant budesonide concentrations, even when the mask is held up to 4 cm from the face. The nebulizer system (nebulizer, mask, compressor) significantly impacts the efficiency of blow-by aerosol delivery.
Methods
In-Vitro Model
We used a modified version of a published model of a ventilated child's face8 (Fig. 1). To receive the face mask, we used a custom-made model of a 2-year-old child's face (Consulting Group, Cambridge Technology Centre, Melbourn, United Kingdom) with an 18 mm orifice for a mouth. A filter (Pari, Starnberg, Germany) was fitted to the mouth between the mannequin head and the breathing simulator, to capture the inhaled budesonide (inhaled mass [IM]). Aerosol deposition over the face was determined by extracting drug from filters placed over the eye regions (Fig. 2),8 and washing the rest of the face and the mask. A lung simulator (ASL 5000, IngMar Medical, Pittsburgh, Pennsylvania) was set at a maximum muscle pressure of 13.5 cm H2O, resistance of 20 cm H2O, and compliance of 5 mL/cm H2O, to achieve a tidal volume of 60 mL and a frequency of 20 breaths/min (inspiratory/expiratory cycle of 1:3), to duplicate settings from prior blow-by studies.2,3
Face model used in study.
Face model with filter paper over eyes.
Nebulizer Systems
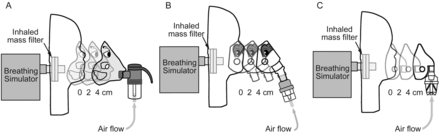
Three commercially available nebulizer systems were chosen to represent different nebulizer-mask configurations (front-loaded, angled, and bottom-loaded): features that prior research suggested were important in influencing drug delivery2,8,9 (Fig. 3). A “nebulizer system” consisted of a typical nebulizer/compressor/mask kit provided to patients in the home setting (Table 1). The Pari LC Sprint nebulizer, with a Bubbles the Fish II mask, powered by a Vios compressor (Pari Respiratory Equipment, Midlothian, Virginia), represented a front-loaded system. The Pari LC Sprint is similar to the Pari LC Jet Plus: the nebulizer used in the original clinical trials with budesonide. The angled Sidestream nebulizer connected to a Tucker the Turtle mask, powered by an OptionHome compressor (Philips Respironics, Murrysville, Pennsylvania), represented a commonly used intermediate mask-nebulizer design, while the Salter 8900 series nebulizer, Aire Plus compressor, and I-Guard pediatric mask (Salter Labs, Arvin, California) represented a bottom-loaded configuration.
Setup for 3 systems tested. A: Pari front-loaded, LC Sprint nebulizer, and Bubbles II mask. B: Respironics angled interface, Sidestream nebulizer, and Tucker Turtle mask. C: Salter bottom-loaded, 8900 nebulizer, and I-Guard pediatric mask.
Components of the 3 Nebulizer Systems
Pre-testing and Characterization of Nebulizer Systems
Six nebulizers of each brand were visually pre-tested using saline. Three of each type were chosen at random (labeled 1, 2, and 3) and used throughout the study. To measure nebulization time, the nebulizers were run to dryness (evidenced by sputtering and the lack of visible aerosol generation) and the run times averaged. To define compressor function, the pressure generated under load was measured for 2 examples from each company, using a mechanical pressure gauge (Checkmate, Bourns Medical Systems, Riverside, California.). Once shown to be comparable, one representative compressor was used for the rest of the study. The volume rate of flow generated by the nebulizer-compressor unit was measured in triplicate for each system by attaching the nebulizer-compressor unit to a Stead-Wells spirometer (Warren E Collins, Braintree, Massachusetts).
Measurement of Particle Distribution
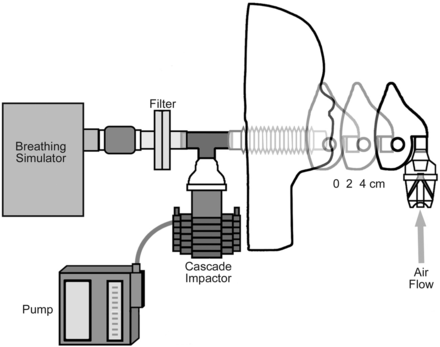
Aerosols were measured by cascade impaction (Marple, Thermo Electron, Waltham, Massachusetts) with a sampling flow rate of 2 L/min. For these experiments the impactor was connected in-line behind the mannequin head, in lieu of the filter, to sample the aerosol distribution of inhaled aerosols (Fig. 4). Particle distribution was measured at 0, 2, and 4 cm for each of the nebulizer systems. To ensure accumulation of enough budesonide for measurement, 2 runs were done at 0 cm, 4 at 2 cm, and 6 at 4 cm before extraction of the budesonide was achieved by washing each stage with 25-mL aliquots of ethanol containing fluocinolone, and analyzing by high-performance liquid chromatography (HPLC).10
Particle distribution setup, using Salter 8900 nebulizer as an example, with the cascade impactor positioned in-line between the lung model and mannequin face.
Inhaled Mass
The amount of drug in the nebulizer at the beginning of each run is referred to as the nebulizer charge. For each system, the 3 representative nebulizers attached to their corresponding masks were charged with 2 mL of budesonide 0.5 mg/mL (Pulmicort respules, AstraZeneca, Westborough, Massachusetts) and run serially for 8 min (in excess of all measured run times, to ensure complete nebulization) at distances of 0 cm (mask on face), 2 cm, and 4 cm away from the face. To provide sufficient budesonide for HPLC analysis, repeated nebulizations were performed for each data point. That is, for each brand of nebulizer, the 3 chosen examples were run in sequence (1, 2, and 3) serially into a single IM filter (eg, to yield one data point). The IM filter (and filter holder) was removed and budesonide extracted in a beaker containing 25 mL of ethanol with fluocinolone (40 mg/L), serving as an internal standard, and analyzed by HPLC, and the budesonide on the filter was divided by 3 to determine IM for a single nebulization. This series of experiments was repeated in triplicate, resulting in 3 data points for IM at each distance. Therefore, there were a total of 9 nebulizations at each distance for each system. This approach reflected nebulizer variability, because, for a given nebulizer brand, 3 devices were used for each point, and it allowed for accurate HPLC measurements, providing sufficient drug as distance increased away from the face.
Residual Nebulizer and Mask Drug Activity
For each individual nebulization, residual nebulizer drug activity was measured by washing the nebulizer cup with 25-mL aliquots of ethanol containing fluocinolone, and analyzing the solution for budesonide by HPLC. Budesonide deposited over the eyes was extracted from filters placed over the eye regions (see Fig. 2). Drug activity over the rest of the face (excluding the eye region) and separately for the face mask (inner surface) was measured by washing each with 25-mL aliquots of ethanol containing fluocinolone, and analyzing solutions for budesonide by HPLC. At 0 cm, the eyes, face, and face mask deposition were analyzed after 3 consecutive runs and divided by 3, as done with the IM filter, to report deposition for each individual run. At distances of 2 and 4 cm from the face, the drug was allowed to accumulate for the duration of the 9 runs, to ensure adequate concentration for analysis, and divided by 9 to approximate deposition for individual runs. This resulted in single data points for deposition of face, mask, and eyes at distances of 2 and 4 cm. Budesonide delivery to the “patient” is reported as percentage of the initial nebulizer charge. To normalize for the wide distribution in particle size generated across the 3 different systems, the respirable mass, which describes particles < 3.5 μm in diameter, was calculated for each system, and is reported alongside the IM values.
Statistics and Analysis
For IM at all distances, and for face, eye, and mask deposition at 0 cm, 3 values at each location were averaged and reported as a mean ± SD. Face, eye, and mask deposition at 2 and 4 cm are reported for illustrative purposes and not included in any statistical analysis. Statistical comparisons were made using 95% CIs and are illustrated on bar graphs. Results were considered statistically significant if the CIs did not overlap.
Results
Pre-testing and System Characterization
Nebulizer run time and compressor flow varied significantly for different commercial systems (Table 2). The Pari system demonstrated the longest run time and the lowest compressor flow.
Device Characteristics
Particle Distribution
Particle distributions were polydisperse and changed with blow-by distance (Fig. 5). The mass median aerodynamic diameter for Salter was > Pari and > Respironics at 0, 2, and 4 cm.
Particle distribution (mass median aerodynamic diameter [MMAD]) with the Pari, Respironics, and Salter nebulizer-mask setups, with the mask at 0 cm, 2 cm, or 4 cm from the mannequin face.
Drug Delivery and Deposition
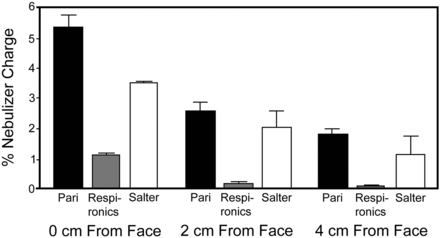
At 0 cm from the face, the Pari system delivered the most drug, at 5.33 ± 0.18% of the nebulizer charge, followed by the Salter system, at 3.50 ± 0.03%, and 1.14 ± 0.03% for the Respironics system (Table 3 and Figs. 6⇓⇓–9). These findings were similar at 2 and 4 cm. As shown in Figure 6, the Pari system delivered more drug at 4 cm than the Respironics system placed at 0 cm. The same patterns observed on Figure 6 were seen when the respirable mass was calculated (see Table 3). Drug delivery from the Pari system was > Salter and > Respironics at all distances, and the respirable mass of the Pari at 4 cm was still higher than Respironics at 0 cm. Nebulizer residual was lowest for the Salter system, at 34.7 ± 5.4% of original charge, indicating that approximately 65% of initial drug was nebulized. Pari and Respironics had roughly equal residuals (63%), with about 37% of the initial nebulizer charge being nebulized.
Drug Delivery
Drug delivered to inhaled mass filter in each nebulizer system, The bars represent mean ± 95% CI.
Facial deposition normalized for inhaled mass for each nebulizer system. A value of 1.0 indicates facial deposition equal to inhaled mass.
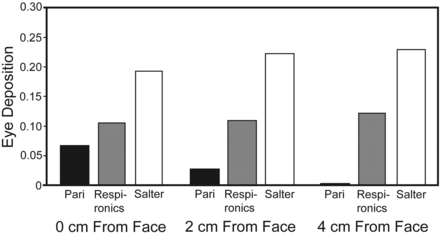
Normalized eye deposition for each nebulizer system. Front-loaded design reduces eye deposition. A value of 1.0 indicates eye deposition equal to inhaled mass
Amount of drug deposited on mask during nebulization for each nebulizer system. The bars represent mean ± 95% CI. At 0 cm the mask losses were similar for each design.
Eye, face, and mask deposition are listed in Table 3, and represented graphically in Figures 7⇑–9. Figures 7 and 8 are normalized for inhaled mass. Facial deposition of budesonide was greatest when the masks were directly against the face, and decreased with increasing distances (see Table 3). However, when normalized for inhaled mass (see Fig. 7), the Pari system deposited the least per unit dose on the face and in the eyes. At 2 and 4 cm the Respironics and Salter systems deposited as much or more aerosol on the face than the IM filter (ratio > 1). Normalized eye deposition for the Pari dropped with distance, while the Respironics and Salter deposition in the eyes was unchanged (see Fig. 8).
Discussion
Our results indicate that pediatric aerosol delivery is a strong function of the nebulizer system as a whole, and not simply a function of blow-by distance from the face. Delivering aerosol treatments to small children with any device can be difficult. A mouthpiece requires cooperation. A common substitute, the valved holding chamber/metered-dose inhaler with mask, must be sealed against the face to deliver any aerosol.9 The requirement of a seal with both the valved holding chamber/metered-dose inhaler and nebulizer-face mask can induce crying.11 Although mask configuration is known to be important in optimizing drug delivery,2,8,9 the ability of a child to cooperate with treatment has been shown to have an even greater effect on daily dose delivery.12,13 When dealing with uncooperative children, caregivers may find themselves either attempting to deliver the treatment by forcing the mask against an uncooperative crying child's face or using blow-by.1 Forcing a mask on an uncooperative child can lead to crying and virtually no drug delivery,14 making blow-by, with a nebulizer/face mask held away from the face, the only other reasonable alternative. Yet opponents of blow-by still advise against its use, citing a 50% reduction of dose with a gap of 1 cm.1,2,5 While our data support the findings of earlier studies indicating reduced aerosol delivery with blow-by, we found that blow-by can be a reasonable treatment alternative if the proper nebulizer system is used. Proper choice of delivery system can provide substantial amounts of drug, even at 4 cm from the face. Further, it is possible to maintain inhaled mass with reduced deposition on the face and eyes: an important consideration, since it is not known whether an exposed child will have increased risks of side effects from long-term inhaled corticosteroids. Inhaled corticosteroids have been associated with an increased risk of cataracts, ocular hypertension, and glaucoma in some studies15,16 but not in others.17 Nonetheless, there are reported cases of patients suffering from acute pupillary dilatation secondary to ocular bronchodilator deposition,18,19 indicating the potential for side effects from all aerosolized drugs. Therefore, we feel it is wise to minimize facial and ocular deposition whenever possible.
In our experiments we studied 3 different widely available nebulizer systems, with corresponding nebulizers, masks, and compressors. Devices were chosen to be representative of the different mask-nebulizer configurations that are commercially available to patients (front-loaded vs angled vs bottom-loaded) and of comparable cost. Our experimental design differed from previous, frequently cited studies that measured blow-by effects using a single nebulizer at increasing distances from the face, or a nebulizer with various masks that are not normally sold together. None of the previous studies looked into the possible role of compressors.
What are the important factors defining drug delivery for budesonide using these devices? From the data in Table 3, it is clear that nebulizer efficiency, per se, is not the determining factor, because both the Salter and Respironics devices were more efficient than the Pari. Nor is alignment of the nebulizer-airway axis the decisive factor in determining the superiority of one system over the other. In fact, further analysis of our study suggests the contrary. The Salter system, which is a bottom-loaded system and aligned at a 90° configuration to our mouth-breathing model, delivered a substantial amount of drug both at 0 cm (mask on face) and at 4 cm via blow-by. If alignment of the nebulizer-airway axis was the major determining factor, we would expect the bottom-loaded Salter to provide the least amount of drug under all circumstances. In stark contrast, the Respironics system, an angled system, which, when fit on our model was directed at the mouth, delivered the least amount of drug of all the systems tested. Particle distribution may be important, but our respirable mass data indicate that adjusting for particle distribution did not affect the differences in delivery between these systems. Deposition of particles directly on the masks did not account for differences in delivery (see Fig. 9).
An important difference between systems was the difference in compressor flow. The low compressor flow of the Pari system resulted in longer treatment times. Aerosol is delivered to the inhaled mass filter only during inspiration. During expiration and in-between breaths, aerosol losses will be affected by the compressor flow, which for continuous nebulizers blows the aerosol into the room and causes waste. Drug delivery will therefore be affected by the total time of inspiration. This variable is a function of the duty cycle and the nebulization time.
Our results for front-loaded masks used with blow-by agree with those previously reported by Lin et al.2 Finally, the Salter system had the highest overall deposition on the face and eyes of the 3 systems tested at 0 cm. This is likely a function of its bottom-loaded design.8 It is ultimately the combination of all these factors (mask, nebulizer, and compressor) that defines overall drug delivery.
There is no in-vitro standard dose of nebulized budesonide that must be delivered to the patient for a nebulizer to be considered “effective.” Because in-vitro comparisons are thus relative to each other, caregivers should be aware of the differences in function between nebulizer systems. The presumption that devices are equally efficacious can lead to highly variable drug delivery. Our data do not justify use of blow-by with all nebulizer systems: only those tested for drug delivery.
Our results apply to our model, which cannot be expected to perfectly represent all pediatric faces, or any living face for that matter. We feel this is appropriate as a model to measure aerosols presented to the child and suffices for our comparative illustration of device function. The 60 mL tidal volume and the short duty cycle represent a worst-case scenario (increases in tidal volume and duty cycle are known to increase inhaled mass).9 The results of this study are in reference to budesonide, which, unlike bronchodilators, is a suspension and not a solution, and behaves differently when nebulized.8,10 Our study emphasizes that testing commercially available systems (all components together) can better define drug delivery and aid physicians in prescribing the optimal system for a desired effect for a given formulation and clinical situation.
Conclusions
With the appropriate nebulizer system one can compensate for the decrement of drug delivery associated with blow-by and can, in fact, deliver as much budesonide using an “efficient” system as other systems with the mask placed directly against the face.
Acknowledgments
The authors thank Lorraine Morra for technical assistance.
Footnotes
- Correspondence: Gerald C Smaldone MD PhD, Pulmonary, Critical Care, and Sleep Division, Stony Brook University Medical Center, T17–040 Health Sciences Center, Stony Brook NY 11794-8172. E-mail: gerald.smaldone{at}stonybrook.edu.
Dr Mansour has disclosed no conflicts of interest. Dr Smaldone has disclosed relationships with Pari, Respironics, and Salter. This study was partly funded by Pari.
See the Related Editorial on Page 2127
- Copyright © 2012 by Daedalus Enterprises Inc.