Abstract
BACKGROUND: This was an evaluation of intra-individual variation of the cuff-leak test (ΔCLT) immediately post-intubation and pre-extubation, as a predictor of post-extubation stridor.
METHODS: Prospective, clinical investigation in the ICU of a non-university hospital. CLTs were performed immediately after intubation (T0) and before extubation (T1) to evaluate the differences in cuff leak (ΔCLT = CLT1 − CLT0).
RESULTS: We included 104 mechanically ventilated subjects in the study over a 12-month period. The incidence of post-extubation stridor was 6.7%. Stridor was more common in females of short stature. ΔCLT was considered as significant when CLT1 − CLT0 was negative. The sensitivity and the specificity of the test were 86% and 48%, respectively. When we tested the pre-extubation CLT alone with a threshold of 130 mL as a predictor of post-extubation stridor, the sensitivity and the specificity of the test were 86% and 76%, respectively.
CONCLUSIONS: The intra-individual variation of CLT immediately post-intubation and pre-extubation does not improve the accuracy of a standard pre-extubation CLT to predict post-extubation stridor. Moreover, the standard pre-extubation CLT did not appear in our study to be an ideal test to detect post-extubation stridor. Larger studies should be performed before generalizing these preliminary results.
- cuff-leak test
- mechanical ventilation
- weaning
- post-extubation stridor
- upper-airway obstruction
- critically ill patient
Introduction
Stridor following extubation in the ICU occurs in 2–25% of all patients, leading to reintubation in approximately 1% of the cases.1–6 In a recent study, corticosteroids were proposed as a preventive treatment of laryngeal edema before planned extubation, when administered to subjects mechanically ventilated for > 36 hours.6 Despite the identification of additional risk factors (female, short stature), post-extubation laryngeal edema remains a poorly predictable complication.
In order to select patients who might best benefit from preventive treatment, the cuff-leak test (CLT) has been proposed as a simple and noninvasive procedure to identify high-risk patients.7 Despite improvements made to the original qualitative test, by quantification of the leak volume (inspired minus exhaled tidal volume during mechanical ventilation) or the fraction of the leak volume,8 the positive predictive value of a failed CLT remains fairly low in adults. Indeed a recent meta-analysis looked at 11 prospective studies on CLT, including more than 50 patients. It concluded that there was a possible but limited use for this test for predicting post-extubation airway obstruction, with a sensitivity of 0.56 (95% CI 0.48–0.63) and a specificity of 0.92 (95% CI 0.90–0.93).9
The ratio of the diameter of the endotracheal tube to that of the larynx on leak volumes has never been studied, but might influence CLT accuracy. A negative pre-extubation CLT might be explained either by a large endotracheal tube in a small larynx or by laryngeal edema. To examine these potential confounding factors, we tested the hypothesis that the intra-individual variation of the CLT between immediate post-intubation and pre-extubation periods (ΔCLT) might be a better predictive factor of post-extubation stridor than simply the pre-extubation CLT. A secondary objective was to compare the accuracy of the ΔCLT to the accuracy of a standard pre-extubation CLT in predicting post-extubation stridor.
QUICK LOOK
Current knowledge
Stridor following extubation in the ICU occurs in 2–25% of all patients, leading to reintubation in approximately 1% of the cases The cuff-leak test has been proposed as a simple and noninvasive procedure to identify patients at high risk for post-extubation stridor.
What this paper contributes to our knowledge
The intra-individual variation of cuff-leak testing immediately post intubation and pre extubation does not improve the accuracy of a standard pre-extubation cuff-leak test to predict post-extubation stridor. The standard pre-extubation cuff-leak test does not reliably detect post-extubation stridor.
Methods
Study Design
This prospective study was conducted over a 12-month period in a 10-bed medical and surgical ICU at Fleyriat Hospital, a general hospital in Bourg-en-Bresse, France. The ICU staff includes 6 intensivist-trained physicians. There is always at least one of them present in the ICU. This study protocol was approved by the ethical committee of the Société de Réanimation de Langue Française, which waived the requirement for informed consent.
Study Subjects
The eligibility criteria for the study were: age > 18 years, necessity for intubation after ICU admission, and planned extubation during ICU stay. Patients were excluded if they died before the weaning process was achieved, if they needed a tracheostomy, if the duration of mechanical ventilation was shorter than 48 hours, or if there was unplanned extubation.
Procedures
All subjects were intubated and ventilated in accordance with standard-of-care practices, or at the discretion of the attending physician. Endotracheal tubes were all of 7–8 mm internal diameter, and cuff pressure was maintained below 30 cm H2O. For each subject the following characteristics were recorded: age, sex, height, weight, reason for admission, severity of illness, organ failure score (Simplified Acute Physiology Score II), duration of intubation, internal diameter of the cuffed tube, and results of both CLTs (following intubation and preceding extubation). Evaluation for extubation readiness was made according to national guidelines, independent of CLT results.10 The occurrence of post-extubation stridor within 48 hours of extubation was recorded. Post-extubation stridor was clinically defined as the development of laryngeal dyspnea after extubation, associated with respiratory distress. Subjects developing post-extubation stridor were first treated with epinephrine and corticosteroids nebulization before reintubation was considered by the physicians in charge. Incidence of reintubation due to post-extubation stridor was also recorded.
CLT was performed on 2 occasions for each subject included in the study: the first CLT was acquired within the hour following intubation (T0) and the second within the hour preceding extubation (T1). CLT was performed by all physicians of the unit, as initially described by Miller and Cole.7 The subject was placed in the semi-Fowler position, oral and tracheal suction were performed, and the subject was placed on assist-control ventilation mode with the following parameters: respiratory rate 15 breaths/min, FIO2 1.0, PEEP 0 cm H2O, and tidal volume 10 mL/kg ideal weight.11 A few respiratory cycles were needed to equalize inspired and exhaled volumes with an acceptable error margin of 20 mL. The endotracheal tube cuff was then deflated, and the operator waited for a few respiratory cycles (to allow the subject to cough) before performing the measurements. The exhaled tidal volume was recorded over 6 respiratory cycles. The cuff leak (CL) was calculated as the difference between the mechanical inspired volume and the average of the 3 lowest exhaled volumes. The variation of CLT for each subject, between T0 and T1 was defined as ΔCLT = CLT1 – CLT0.
Statistical Analysis
Discrete variables were expressed as percentage, and continuous variables as mean ± SD for variables normally distributed, and as medians with interquartiles for non-normally distributed variables. Differences between subjects with and without post-extubation stridor leading to reintubation were compared using the Student t test for continuous and normally distributed variables. The Mann-Whitney U test was used to compare continuous and non-normally distributed variables, and the Fisher exact test was used for categorical data. Subjects presenting with missing CLT were considered as censored data and were not included in the statistical analysis. Significance was reported at a P value of .05 or less.
The variation of CLT was defined as a negative test when ΔCLT was positive (ie, when CL was higher at the time of post-intubation than at the time of pre-extubation, and positive test when ΔCLT was < 0). Receiver-operating characteristic (ROC) curves were created for ΔCLT and single pre-extubation CLT, and permitted calculation of sensitivity, specificity, positive predictive value, and negative predictive value and their 95% confidence intervals. Sensitivity, specificity, positive predictive value, and negative predictive value for ΔCLT were calculated with this threshold of 0 mL. The single pre-extubation CLT threshold value for optimum test discrimination was determined by ROC curves.12 Sensitivity, specificity, positive predictive value, and negative predictive value of the single pre-extubation CLT were calculated with this threshold. Data were analyzed with commercially available software (MedCalc 10.4, MedCalc Software, Mariakerke, Belgium).
Results
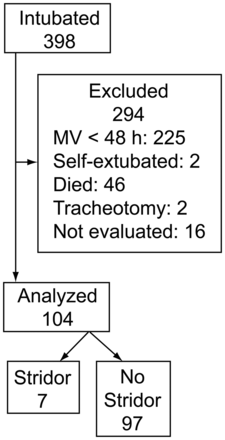
Figure 1 shows the trial profile. During the study period, 398 subjects were intubated and mechanically ventilated in the ICU. Reasons for exclusion from the study included: duration of mechanical ventilation < 48 hours (n = 225), death before achievement of weaning process (n = 46), unplanned extubation (n = 5), tracheostomy (n = 2), and missing data (n = 16). Data on the remaining 104 subjects were included in the analysis. Table 1 shows the baseline characteristics of the study population. The main reasons for admission to ICU were medical (n = 85). Post-extubation stridor occurred in 7 cases (6.7%), all within the 12 hours following extubation. Six subjects required tracheal reintubation within the 48 hours following extubation in the stridor group (n = 7), and 23 subjects in the no-stridor group (n = 97). Table 1 shows the results of univariate analysis undertaken to identify risk factors for post-extubation stridor. Subjects who developed this complication were mainly women. Duration of mechanical ventilation was similar in both groups, with a median of 5 days (IQR 3–9 d) in the overall population.
Distribution of intubated subjects during the study period.
Characteristics of Subjects
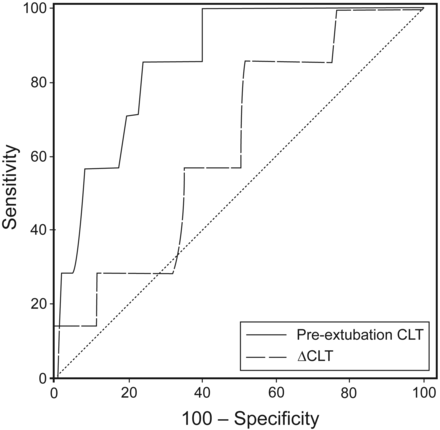
Table 2 shows the main characteristics of the post-extubation stridor group and their individual pre-extubation CLT and ΔCLT values. ROC curves for ΔCLT and single pre-extubation CLT are represented in Figure 2. The diagnostic abilities of the ΔCLT and single pre-extubation CLT in predicting post-extubation stridor were not similar, with an area under the ROC curve of 0.63 (95% CI 0.53–0.72) for the ΔCLT and 0.86 (95% CI 0.78–0.92) for the single pre-extubation stridor (P = .01). Sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio of ΔCLT were calculated with the threshold of 0 mL and were of 86% (95% CI 42–100%), 48% (95% CI 38–59%), 1.7 (95% CI 1.2–2.4), and 0.3 (95% CI 0.1–1.8), respectively. Positive predictive value and negative predictive value raised to 11% (95% CI 4–22%) and 98% (95% CI 89–100%), respectively.
Main Characteristics, Values of Pre-extubation Cuff-Leak Test, and Individual Cuff-Leak Test Volume Variations for the 7 Cases of Stridor
Receiver operating characteristic (ROC) curves for variation in cuff-leak test (ΔCLT) and single pre-extubation CLT. True positive and true negative fractions are plotted on the Y axis and X axis, respectively.
As far as single pre-extubation CLT values were concerned, an absolute volume < 130 mL was found to predict post-extubation stridor and was considered as a positive CLT, with a sensitivity of 86% (95% CI 42–100%), a specificity of 76% (95% CI 67–84%), a positive likelihood ratio of 3.6 (95% CI 2.6–5.0), and a negative likelihood ratio of 0.2 (95% CI 0.03–1.2). In our study, with an incidence of post-extubation stridor of 6.7%, the positive and negative predictive values of a single pre-extubation CLT were 21% (95% CI 8–40%) and 99% (95% CI 93–100%), respectively.
Discussion
In this study we evaluated the individual variation of CLT between post-intubation and pre-extubation as a predictor of post-extubation stridor. This composite test proved to be of limited value, with a poor positive predictive value and a good negative predictive value. A single pre-extubation CLT predicted better which subjects would require an escalation of care, possibly culminating in reintubation, which was comparable to previous reports.4,5,7,8,13 The incidence of post-extubation stridor in our population was 6.7% (7 subjects out of 104), which was low, although in agreement with most clinical studies.1–6 Although CLT sensitivity was high, its poor specificity and the low incidence of post-extubation stridor explained why the CLT had a poor positive predictive value. If used at all, the CLT should therefore be used in a high-risk population to select patients that may benefit from a preventive treatment. Because of the limited number of subjects, we could not make any post-hoc analysis of groups presenting one or more of the following risk factors: female sex, high organ failure and severity, prolonged over-inflated cuff, high duration of mechanical ventilation, and history of laryngeal trauma.4
We made the hypothesis that measuring CLT immediately after intubation would demonstrate the possible influence of the ratio of endotracheal tube diameter to laryngeal size on leak volumes. One reason to explain the lack of superiority of ΔCLT over a single pre-extubation CLT could be the difficulty in achieving a standardized CLT at 2 different times during the subject's ICU stay: use of sedatives and neuromuscular blockade for induction, as opposed to awareness and active participation of the subject at the time of the second CLT, could lead to an uninterpretable ΔCLT value. Another reason could be changes in pulmonary compliance between intubation and extubation. Indeed, Prinianikis et al suggested in 2005 that cuff leak could be influenced by compliance of the respiratory system and inspiratory flow.14 As these results were not released when our study was designed, data regarding compliance were not collected.
Moreover, in our study, the diagnosis of post-extubation stridor remained clinical and therefore subjective. Laryngeal edema documented at laryngoscopy during reintubation is a more objective end point, with a reported incidence of between 37% and 60%.15,16 Non-relevant laryngeal lesions could possibly be clinically undetectable but still demonstrated by CLT, leading to a false interpretation of the test.
In addition, 6 subjects were reintubated within 48 hours after extubation in the stridor group, while one subject remained free from reintubation in this group. These results are comparable to those of previous studies.17,18 In the no-stridor group, 23 of the 97 subjects required reintubation within the 48 hours following extubation. The cause of reintubation was not laryngeal edema, as attested by laryngoscopy. Lastly, CLT reproducibility has often been questioned, and may be a limiting factor in our study. Pettignano et al found that a standardized procedure, performed after sedation and curarization of the patient, guaranteed a reliable cuff-leak measure.19 However, these ideal conditions for measurement can never be obtained in the hour preceding planned extubation. In an attempt to exclude erroneous values, leak volumes were observed and averaged over several respiratory cycles, but in clinical practice pre-extubation CLT may be inaccurate due to agitation and coughing after cuff deflation.
In our population, female sex and short stature were associated with a higher occurrence of stridor after extubation, as reported in other studies.1,7 Yet our results did not confirm the relationship between post-extubation stridor and other reported risk factors such as medical admission, traumatic intubation, Simplified Acute Physiology Score II, and duration of intubation.1,4,8 This can be explained by the fact that, in our study, all of the subjects were intubated according to the same protocol, and were all mechanically ventilated for longer than 48 hours.
Conclusions
The intra-individual variation of the CLT between immediate post-intubation and pre-extubation is an unreliable predictor of post-extubation stridor and does not improve the accuracy of the standard pre-extubation CLT to predict post-extubation stridor. Due to the small number of stridor cases the standard pre-extubation CLT does not appear in our study to be an ideal test. Larger investigations are needed before these results can be generalized.
Footnotes
- Correspondence: Antoine Gros MD, Intensive Care Unit, René Dubos Hospital, 6 Avenue de l'Ile de France, BP 79, 95303 Cergy-Pontoise Cedex. E-mail: antoinegros6{at}gmail.com.
The authors have disclosed no conflicts of interest.
Dr Gros presented a version of this paper at the annual congress of the Société de Réanimation de Langue Française, held October 13, 2005, in Paris, France.
See the Related Editorial on Page 2136
- Copyright © 2012 by Daedalus Enterprises Inc.