Abstract
BACKGROUND: Cough is part of life in patients with cystic fibrosis (CF). Weak coughing may add to increased respiratory disease that affects the mechanical properties of cough in these patients. In this study, we investigated cough characteristics in relation to forced expiratory flow/volume indices in CF.
METHODS: This prospective study included 54 subjects (26 ± 10 y old) with CF. Similar indices of the maneuvers were compared. Additionally, other cough characteristics revealed by the maneuvers were investigated. Cough was considered efficient with 6 or more secondary spikes at a flow of > 2.67 L/s.
RESULTS: Cough peak flows similar to peak expiratory flows (regardless of FEV1 severity level) were found in 98% of subjects. The secondary spike flows deteriorated alongside the percent-of-predicted FEV1 (r2 = 0.17, P = .002), yet inefficient secondary spike flows could also be found when FEV1 was within normal range. Mean efficient spike number was low (2.5 ± 1.2 spikes/maneuver). Most cough spike flows were very small (< 0.9 L/s), resembling vibration that may contribute to the propulsion of mucus toward the central airways. Cough maneuver duration ended faster than forced expiration duration (3.7 ± 1.7 s vs 6.8 ± 2.5 s, P < .001), resulting in a smaller cough vital capacity compared with expiratory vital capacity (2.1 ± 0.9 l vs 3.1 ± 0.7 l, P < .001). Inspiratory volume below 2.23 ± 0.07 L reduced efficient secondary spike number.
CONCLUSIONS: The cough flow/volume maneuver reveals abnormalities in cough velocities and volume. A low secondary spike correlates with FEV1 severity level. The method may lead to earlier intervention to assist cough in CF.
Introduction
Cough, the natural defense mechanism for airway clearance, is part of life for a patient with cystic fibrosis (CF), and frequent coughing is observed in 50% of infants with CF by the age of 10 months.1,2 Weak coughing is perceived as an additional cause of recurrent respiratory infections, leading to respiratory disease characterized by deterioration of lung function in CF.1–4
The sequence of events that lead to an effective cough, which has long been known, consists of inspiratory, compressive, and expiratory phases.5–8 The initial phase of cough is characterized by inhalation of gas that may be as little as 50% of the tidal volume or as great as 50% of the vital capacity (VC).9 However, inhalation of a large volume of gas will produce greater lengthening of the expiratory muscles, which will generate greater positive intrathoracic pressures for a given expiratory phase.9
The second phase of cough depends on expiratory muscle strength and adequate control of the upper airway to provide an explosive release of peak expiratory flow (PEF).5–8 The commonly used parameters to assess this phase are the cough peak flow (CPF) and CPF/PEF ratio.10 These parameters were found to be normal in most patients with CF and to correlate with patient body mass index (BMI) level.11
The third phase of cough is continuation of exhalation, where the bronchi and trachea are compressed and narrowed in the intrathoracic space during coughing (or forced expiration). In healthy subjects, the narrowing is evenly distributed, and the expiratory decay is smooth. In patients with airway obstruction such as in CF, the distribution of narrowing may be greater, more irregular, and sometimes the cause of near collapse of the main bronchi.5–8 Moreover, the high pressures developed during the expiratory explosion may cause airway collapse in the presence of airway obstruction and changes in airway structure.8
The expiratory decay of the cough flow consists of transient supramaximal flow (higher than PEF) cough spikes. The number of spikes/cough epoch ranges from 8 to 14 in a healthy adult.5–8 Cough spike flows between 160 and 180 L/min (2.67–3.0 L/s) were found to provide effective airway clearance in adult patients with stable neuromuscular disease who still had bulbar function.12
The forced expiratory and cough flow/volume maneuvers link in several indices. These include the inspiratory capacity, peak flows, and VC. We hypothesized that although CPFs may be normal, early airway collapsibility during voluntary cough maneuvers may pathologically affect secondary cough spike flows in these patients.
The aim of this study was to investigate the similarity and differences between the cough and forced expiratory flow/volume maneuver indices. Additionally, we explored the cough flow/volume index characteristics in relation to actual anthropomorphic data retrieved from subjects with normal lung function and from subjects with bacterial infections. Indices were also related to the retrospective FEV1 yearly deteriotation rate.13
QUICK LOOK
Current knowledge
Cough is both a symptom and protective mechanism in cystic fibrosis (CF). Weak or ineffective cough results in secretion retention, infection, and deterioration of lung function. Cough effectiveness is linked to the degree of FEV1 impairment.
What this paper contributes to our knowledge
The measurement of the cough flow/volume maneuver in CF demonstrates abnormalities in cough velocity and volume. A low secondary spike in the maneuver correlates with the severity of FEV1 impairment and can identify patients for early intervention to improve cough and secretion clearance.
Methods
Subjects
Patients with CF who were treated at The National Center for Cystic Fibrosis at The Edmond and Lily Safra Children's Hospital (Ramat Gan, Israel) were enrolled in this study. For inclusion, subjects had to be older than 10 y and to be able to comply with both the FVC and cough flow/volume maneuvers. Subjects were excluded if they had respiratory exacerbations, or were hospitalized for other reasons.
Clinical charts were reviewed for the following data: age, BMI, age and gender percentiles, and genetics.
Study Design
This was a prospective and cross-sectional study. It was approved by the Sheba Medical Center Helsinki Review Board (IRB 8709-11-SMC, NCT01636219). Measurements were performed after each subject signed an informed consent form. Tests were performed before physiotherapy, and no inhaled medications (such as bronchodilators, hypertonic saline, and antibiotic inhalations) were given 12 h before the tests.
Measurements.
Both forced expiratory flow/volume and cough flow/volume maneuvers were measured with the KoKo spirometer (nSpire Health, Longmont, Colorado): data sampling rate, 200 samples/s; flow range, ± 16 L/; volume range, 0–19.9 L; and accuracy, ± 2%. Measurements were performed while the subject was standing to standardize both measurements because the cough maneuver can be performed better while standing.
Forced Flow/Volume Maneuver Test.
The forced expiratory maneuvers were performed according to guidelines for quality control.14,15 During the test, the subject was asked to wear a nose clip, and the mouth was sealed around the mouthpiece. The subject was then asked to take a full inspiration from residual volume to total lung capacity, considered inspiratory VC, and blow into a pneumotachograph. At least 3 maneuvers were performed. The best curve (FEV1 + FVC) was stored for further analysis.
Cough Flow/Volume Maneuver Tests.
Tests were performed ∼5 min after spirometry.16 While wearing a nose clip and with the mouth sealed around a mouthpiece, the subject took a full inspiration from residual volume to total lung capacity (cough inspiratory VC) and coughed as forcefully as possible through the sealed mouthpiece into the pneumotachograph without intervening inspirations between the coughing spike effort. Up to 3 cough maneuvers were performed. The cough maneuver chosen for analysis was the one with the highest cough inspiratory VC and the best CPF. Other cough flow/volume parameters were defined similarly to the conventional expiratory flow/volume indices.
Data Analysis
Analysis of the cough maneuver included the cough inspiratory capacity volume, CPF, cough spike flows, and the number of spikes. An efficient cough was defined as having at least 6 spikes at a flow of > 160 L/min (2.67 L/s).12 Analysis of the flow/volume indices included inspiratory capacity, PEF, FVC, and FEV1. Reference values of the spirometry (FEV1)17 were also used to predict cough inspiratory VC and cough VC. Correlations were sought between severity of airway disease classified by the percent-of-predicted FEV1 and other cough mechanical properties (number of spikes, spike flows, and cough inspiratory VC). In addition, we sought to determine whether the FEV1 yearly deterioration rate13 affects some cough characteristics.
Statistics
We used the Prism statistical software package (GraphPad Software, San Diego, California). The data were tested for normal distribution by the Kolmogorov-Smirnov test. Normally distributed variables were reported as mean ± SD and range. When data had no normal distribution, variables were also reported as median and range and were analyzed using the paired-sample t test or the Wilcoxon signed-rank test. Significant differences between cough flow/volume maneuver indices and FVC indices were compared by paired t tests (Mann-Whitney tests). Correlations between continuous variables were assessed using Pearson correlation coefficient tests when variables were normally distributed or the Spearman test. Correlations between cough parameters were also studied in relation to BMI and other CF comorbidities and in relation to yearly deterioration in FEV1.13 P < .05 was considered significant.
Results
Fifty-four subjects with CF (31 males) participated in this study. The mean age was 26 ± 10 y (median age of 26 y with 95% confidence limit, range of 23–29 y). The mean BMI was 26 ± 23 kg/m2. Mutation severity distribution included 33 subjects with severe mutation (classes I–III) and 21 subjects with milder mutation (classes IV and V). Twenty-six patients (48%) were infected with Pseudomonas.
Several typical superimposed curves of forced expiratory and cough maneuvers are presented in Figure 1. The cough flow/volume curve contains several spikes. Figure 1 shows that the peak flows of cough and forced expiration were similar, regardless of FEV1 severity. However, the flow decay of the curves differs. Cough flow decay was faster compared with expiratory flow decay in relation to FEV1 severity. Cough spikes with very small flow amplitudes (> 0.9 L/s) resembling vibration also increased with FEV1 deterioration.
Representative scanned forced flow/volume curves and cough flow/volume curves. The cough flow/volume curve presented has several spikes. A: FEV1 = 95% of predicted; B: FEV1 = 73% of predicted; C: FEV1 = 40% of predicted; D: FEV1 = 25% of predicted.
The actual measured values for the cough and forced expiratory flow/volume maneuvers for the entire group are presented in Table 1. The median FEV1 was 65.8% of predicted (range of 19.8–116.1). Similarities between maneuvers were limited to inspiratory capacity before CPF and to the peak flows. The ratio between the peak flows was normal. The cough VC significantly differed from the FVC. A cough should include 6 spikes; however, not all subjects could produce all 6 spikes. All subjects could perform one spike; but there was a decline in the number of subjects able to perform 2, 3, or more spikes. Coughing bout duration was significantly shorter than expiration time. Cough maneuver duration was shorter than forced expiration time, resulting in a smaller cough VC compared with expiratory VC.
Measured Values of Cough and Forced Expiratory Indices
The effect of inspiratory cough capacity values on the number of efficient spikes is presented in Figure 2. Third and fourth efficient secondary spikes were initiated mostly when inspiratory capacity was > 2.23 ± 0.07 L.
Effect of inspiratory cough capacity values on the number of efficient spikes (r2 = 0.35, P < .001). Third and fourth sufficient secondary spikes were initiated mostly when inspiratory capacity prior to CPF was above 2.23 ± 0.07 L.
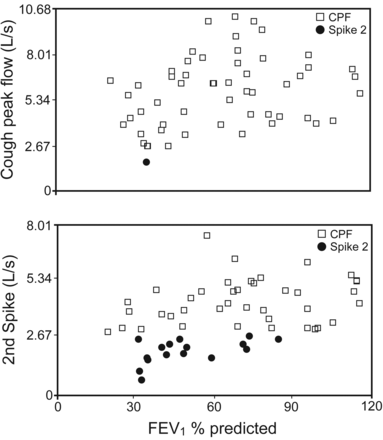
The effect of percent-of-predicted FEV1 on CPF and secondary spike flows is presented in Figure 3. There was no correlation between percent-of-predicted FEV1 and CPF or secondary cough spike flow. CPF was reduced in a single subject who had an FEV1 of 35% of predicted. Secondary spike flow values were reduced across the percent-of-predicted FEV1.
Effect of percent-of-predicted FEV1 on cough peak flow (CPF) and secondary spike flow. Correlations did not reach significance, yet CPFs were reduced in a single subject who had an FEV1 of 35% of predicted, whereas secondary spike flows were reduced across percent-of-predicted FEV1.
The effect of the yearly deterioration rate for FEV1 and CPF or secondary spike flows is presented in Figure 4. Both CPF and secondary spike flows significantly correlated with FEV1 yearly deterioration rate (r2 = 0.33, P < .001; and r2 = 0.36, P < .001, respectively). However, the actual CPF values were not reduced below efficiency, whereas the secondary spike flow values deteriorated below efficiency along with the FEV1 yearly deterioration rate. Subjects with FEV1 yearly deterioration rates of 3.0% and higher tended to have low secondary spike flows.
Effect of the yearly deterioration rate in FEV1 and cough peak flow (CPF) or secondary spike flows (r2 = 0.32, P = .002).
We found a positive correlation between BMI (percentile) and secondary spike flow (r2 = 0.12, P = .01). When the BMI was < 20th percentile, CPF was reduced in 2 subjects, and secondary spike flows were reduced in 15 subjects. Three subjects with BMI in the 50th, 65th, and 85th percentiles also had reduced secondary spike flows. We did not find a correlation between secondary spike flow efficiency and mutation severity. We could not establish a correlation between secondary spike flow values and Pseudomonas infection. Seven of 28 subjects without Pseudomonas and 10 of 26 subjects with Pseudomonas had low secondary spike flows, but the differences did not reach significance.
Discussion
In this study, we investigated cough characteristics in relation to lung function in subjects with CF. The findings indicate that although cough inspiratory VC, CPF, and CPF/PEF ratios were relatively preserved in our subjects, other tested parameters were obstructed. These included decrease in secondary spike flows, limited number of efficient spikes/cough maneuver, and short duration of each coughing bout leading to reduced cough VC. These findings correlated with the decrease in percent-of-predicted FEV1, FEV1 yearly deterioration rate, and BMI (percentile). However, the impaired parameters could also be observed when the percent-of-predicted FEV1 and BMI were within the normal range.
Our findings that CPF is efficient in CF are strengthened by former studies. All but one subject could produce CPF values above the lower limit for efficient cough capacity. Indeed, CPF and CPF/PEF values were within the expected range to produce an efficient cough. Apparently, these values were preserved despite deterioration in percent-of-predicted FEV1 and despite inefficiently low inspiratory VC. The findings imply that the expiratory muscles are well preserved. Dassios et al9,18 likewise found that subjects with CF have adequate maximal expiratory pressures, CPF, and CPF/PEF ratios, and they suggested that the expiratory pressures needed for CPFs are preserved in patients with CF.
We also found that the cough inspiratory VC volume did not limit the CPF values, as found in a former study,10 but the amount of air (L) inhaled before the cough (cough inspiratory VC) did affect the number of efficient secondary spikes (see Fig. 2). This finding may be related to the volume used during the CPF, but we could not measure that volume. Chaudri et al19 studied the secondary spike efficiency in motor neuron diseases and found an association between the absence of efficient cough spikes and increased mortality.
Our findings suggest that secondary spike flows elucidate cough deterioration better than the CPF or CPF/PEF ratio. Indeed, secondary spike flow values correlated with percent-of predicted FEV1 deterioration (see Fig. 3), but inefficient low values were obtained even when FEV1 was normal. Interestingly, we also found that most subjects with a yearly deterioration rate higher than 3% also had low secondary spike flows (see Fig. 4). The dependent variable here has yet to be discovered.
Furthermore, we found that secondary spike flows were inefficient when BMI was ≤ 18 kg/m2 (20th percentile), but low secondary spike flows were likewise seen even when BMI was normal. The connection between low BMI and lung function deterioration has been established.11 The secondary cough spike characteristics in relation to FEV1 reinforce the need to study not only CPF but also other cough parameters.
The decay of cough spike flows was parallel to the decay of the forced expiratory flow maneuver only at the beginning of expiration, which included the portion between the PEF and forced expiratory flow at 25% of VC. After that, the spike flows rapidly decreased (see Fig. 1) compared with the expiratory maneuver, whereas the cough epoch duration was shorter compared with the forced expiratory duration. We therefore attribute the short cough time to a smaller cough FVC volume compared with FVC (see Table 1), as found in our study and corroborated previously.5–8 The pathophysiology underlying our findings may be explained by the high collapsibility of airways, diffuse and severe inflammation, thickening of airway walls, presence of bronchiectasis, and abnormal quantity or quality of mucus lodged in airways, which are all found in CF patients with moderate-to-severe airway disease.20,21
Rapid very small spikes with flows below 0.9 L/s (seemingly vibration-like amplitudes) were apparent from mid-expiratory flows to the end of the cough maneuver (FEV1 is below 40% predicted values; see Fig. 1). We suggest that these rapid very small flow amplitudes contribute to the propulsion of small amounts of liquids and mucus toward the central airways. As mucus clearance is directly proportional to mucus rheology (it is inversely proportional to its viscosity and elasticity depending on the depth at which it is situated in the airways),20 it is not surprising that patients with CF may require up to 14–39 cough epochs/h to overcome the sticky mucus in their airways.21,22
The main limitation of this study is that the cough flow/volume maneuver is not yet standardized, and no reference values exist for secondary spike values in the healthy population. Therefore, we had to refer to older publications for values for healthy subjects. However, our findings revealed abnormalities in cough velocity that were connected with the descending portion of the flow/volume curve and are evident in CPF alone.
The cough flow/volume maneuver is always measured after spirometry, and we feel that it would have been better to use a random order. However, the cough flow/volume maneuver is a fatiguing process, and we therefore decided on this fixed order. One could argue that the findings are related to fatigue due to the repetition of spirometry tests before the cough maneuver. We propose that fatigue will manifest as poor CPF, but the expiratory portion will not be influenced by fatigue.
We conclude that the cough flow/volume maneuver used in our study reveals abnormalities in cough characteristics that may not be evident with the CPF method alone. All other parameters (secondary spike flows, number of secondary spikes, cough duration, and cough VC) were found to be low in many of our subjects with CF. Inefficient secondary spikes, in particular, were found to be directly correlated with percent-of-predicted FEV1, FEV1 deterioration rate, and low BMI, but these were also apparent even when FEV1 or BMI was normal. The use of the cough flow/volume maneuver with the measurement may therefore be justified and may be clinically important in CF, but further standardization is required to recommend its use.
Footnotes
- Correspondence: Daphna Vilozni PhD, Pediatric Pulmonary Unit, The Edmond and Lily Safra Children's Hospital, Sheba Medical Center, Ramat Gan 52621, Israel. E-mail: daphna.vilozni{at}sheba.health.gov.il or avi_vil{at}bezeqint.net.
The study was supported by the J Baum Foundation of the Israel Lung Association, Tel Aviv, Israel.
The authors have disclosed no conflicts of interest.
- Copyright © 2014 by Daedalus Enterprises