Abstract
BACKGROUND: Lung expansion therapy is often ordered after surgery to improve alveolar ventilation and reduce risks of postoperative pulmonary complications. The impact of lung expansion therapy at altering ventilation in patients who are not intubated has not been described. The primary purpose of this study was to determine if there is a difference in dorsal redistribution of ventilation and incidences of postoperative pulmonary complications when comparing incentive spirometry (IS) with EzPAP lung expansion therapy after upper abdominal surgery. Our a priori null hypothesis was that there are no differences.
METHODS: This randomized controlled trial enrolled adult human subjects after upper- abdominal surgery from January 2017 to November 2018. The subjects were allocated to receive IS or EzPAP 3 times a day on postoperative days 1–5. An electrical impedance tomography device was connected to the subjects for a single lung expansion therapy session on postoperative days 1, 3, and 5 to measure the change in post-lung expansion therapy dorsal end-expiratory lung impedance (ΔEELI%). Lung expansion therapy sessions with electrical impedance tomography included 2 min of normal breathing, 3 cycles of 10 breaths, and 2 min of normal breathing after cycle 3. Postoperative pulmonary complications were screened until hospital discharge. Mann-Whitney, chi-square, and Fisher exact tests were applied. Data were reported as count (n), percentage, and median (interquartile range) for primary and secondary outcomes. Alpha (2-tailed) was < 0.05.
RESULTS: A total of 112 subjects were enrolled to receive IS (n = 56) or EzPAP (n = 56). Baseline characteristics were equal. Post-lung expansion therapy dorsal ΔEELI% increased for both groups, but the dorsal ΔEELI% for IS versus EzPAP on postoperative day 1 (16% versus 12%, P = .39), postoperative day 3 (6% versus 6%, P = .68), and postoperative day 5 (9% versus 6%, P = .46) was not significantly different. Hospital length of stay (4 d; P = .30) and incidence of postoperative pulmonary complications (3.6% versus 7.1%, P = .19) were similar.
CONCLUSIONS: There was no significant post-lung expansion therapy dorsal ΔEELI% or postoperative pulmonary complications among the adults who received IS or EzPAP 3 times a day after upper abdominal surgery. (ClinicalTrials.gov registration NCT02892773.)
- incentive spirometry
- EzPAP
- PEP therapy
- atelectasis
- hypoxemia
- lung expansion therapy
- upper abdominal surgery
- Whipple
- hepatectomy
- electrical impedance tomography
Introduction
Pulmonary function is frequently compromised after upper abdominal surgery. Lung expansion therapy, including incentive spirometry (IS) or positive expiratory pressure (PEP) therapy adjuncts are commonly ordered prophylactically in attempts to improve pulmonary function and reduce postoperative pulmonary complications. Lung expansion therapy is often intended to target the dependent region of the lungs because this region is commonly associated with a risk of developing postoperative atelectasis.1,2 The incidence of postoperative pulmonary complications after upper abdominal surgery is 5.8% in the United States, and it is a common cause of morbidity and mortality.3 Postoperative pulmonary complications have been shown to be one of the most significant factors associated with poor patient outcomes, longer hospital stay, increased readmissions, and increased mortality.4 Postoperative pulmonary complications are also associated with significant additional hospital costs.5 In spite of the intended target goals, numerous studies have yet to demonstrate that IS is effective in preventing or reducing postoperative complications.6 There is low-quality evidence that demonstrates that there is no difference in the incidence of postoperative complication when IS was compared with either deep breathing or no respiratory intervention, or with other forms of chest physiotherapy in subjects who had undergone upper abdominal surgery.7
Measuring the immediate impact of lung expansion maneuvers on lung function at the bedside is difficult because standard bedside pulmonary function testing does not provide information on functional residual capacity (FRC). The evaluation of lung expansion therapy effectiveness has often relied on global parameter measures such as pulse oximetry oxygen saturation, arterial blood gas measurements, chest-wall auscultation, and radiographic imaging.6 These global parameters do not provide the sensitivity necessary to objectively evaluate regional redistribution of ventilation response to lung expansion therapy. Chest radiography and computed tomography produce images that clinicians may use to evaluate for the change in regional lung volume, but these techniques have limitations. Standard portable chest radiographs are static, and they may produce delayed detection of dynamic lung-volume changes. Computed tomography imaging is costly, time consuming, and requires patient relocation. Both imaging techniques expose patients to radiation.
Electrical impedance tomography provides real-time, breath-by-breath dynamic imaging that is radiation free. Electrical impedance tomography provides an opportunity to view breath-to-breath changes in regional distribution of ventilation before, during, and after lung expansion therapy. The validity and reproducibility of electrical impedance tomography for assessing distribution of ventilation has compared favorably with other lung imaging and diagnostic techniques, such as computed tomography, positron emission tomography, and pulmonary function tests that are used to measure FRC.8–11 This technique can provide quantifiable data that will help us to determine the effectiveness of lung expansion therapy in our subject population.
The primary purpose of this study was to determine if there is a significant difference in post–lung expansion therapy change in dorsal end-expiratory lung impedance (EELI) percentage (ΔEELI%) when comparing IS lung expansion therapy with EzPAP lung expansion therapy (EzPAP Positive Airway Pressure with a pressure manometer [Smiths Medical, Minneapolis, Minnesota]) in adult human subjects after upper abdominal surgery on postoperative days 1, 3, and 5. We were also interested in evaluating whether there was a difference in lung expansion therapy group association with the incidence of postoperative pulmonary complications. Our primary null hypothesis was that there would be no significant difference in post–lung expansion therapy change in dorsal EELI% when comparing IS with EzPAP lung expansion therapies in adult human subjects after upper abdominal surgery on postoperative days 1, 3, and 5. Our secondary null hypothesis was that there would be no significant association between the incidences of postoperative pulmonary complication when comparing IS with EzPAP lung expansion therapies in adult human subjects after upper abdominal surgery.
QUICK LOOK
Current knowledge
Incentive spirometry and positive airway pressure therapy are commonly ordered prophylactically in an attempt to improve distribution of ventilation and reduce postoperative pulmonary complications. There is low-quality evidence reported of the effectiveness of these therapeutic interventions when used after upper abdominal surgery.
What this paper contributes to our knowledge
Incentive spirometry and PEP therapy increased global lung recruitment after surgery. Both therapies seemed to primarily recruit dorsal regions of the lung within the initial 24 h after surgery. Ventral regions of the lung were predominately recruited on subsequent postoperative days. This study showed no difference in dorsal recruitment between therapies after upper abdominal surgery.
Methods
Study Design
This study was a single-center randomized controlled clinical trial conducted in a 54-bed medical-surgical acute-care ward at the University of Virginia Medical Center, which is a 600-bed tertiary-care facility located in Charlottesville, Virginia. The University of Virginia Institutional Review Board for Health Science Research approved the study protocol (IRB-HSR 19029), and it was registered with the United States National Institutes of Health.
Subjects were screened perioperatively by a study investigator (JLD) who reviewed a daily list of preoperative procedures to identify potential study participants. Respective electronic medical records were reviewed for inclusion and exclusion criteria. Eligible participants were adult human subjects between 18 and 79 y of age who had upper abdominal surgery, an anticipated postoperative hospital length of stay of >3 d, and did not require assisted spontaneous breathing. Subjects were excluded from the study if they met any of the criteria listed in supplementary material (see the supplementary materials at http://www.rcjournal.com). A study investigator (DDR, JLD, RMS, DUG) approached all eligible patients perioperatively to invite participation. Written informed consent was obtained from all the subjects within 24 h after upper abdominal surgery and before study enrollment.
Randomization
We used a simple parallel randomization scheme to allocate subjects to one of two equal (1:1) groups (Fig. 1). A computer-generated random numbers generator (http://randomization.com/, Accessed December 14, 2016) list was used to randomly allocate study subjects into an IS or EzPAP lung expansion therapy group. Group allocation was generated before study commencement. A person not affiliated with the study printed, shuffled, and inserted each of the randomly generated numbers into a sequentially numbered opaque envelope, which was then sealed. Study group allocation was concealed from the investigators, subjects, and all health-care providers until written informed consent was obtained, at which point a study investigator (DDR, TPM, JDP, RMS, DUG) was given a sequentially numbered and sealed envelope to open and reveal the group assignments.
Flow chart. BMI = body mass index; LOS = length of stay; POD = postoperative day; EELI = end-expiratory lung impedance.
Lung Expansion Therapy
An institutional Medicare Severity-Diagnosis Related Group data warehouse summary report of upper abdominal surgical procedures performed before institutional review board submission at University of Virginia Medical Center indicated that we could reasonably anticipate a postoperative hospital stay of 1 to 5 d for our target population. The subjects were randomly allocated to receive lung expansion therapy with volumetric IS (Voldyne 2500, Teleflex, Morrisville, North Carolina) or EzPAP Positive Airway Pressure with a pressure manometer by a respiratory therapist 3 times a day on postoperative days 1–5. For the IS group, the subjects were coached to take 10 breaths through the IS mouthpiece, followed by 1 min of normal breathing. This breath cycle was repeated 2 more times as subjects were encouraged to inhale to a target inspiratory capacity volume obtained from the predictive inspiratory capacity nomogram table of the IS.
The subjects assigned to receive EzPAP were coached to breathe through the EzPAP mouthpiece for 10 consecutive breaths while exhaling to a target expiratory pressure of 15 cm H2O. This breath cycle was repeated 3 times, with 1 min of eupneic breathing occurring between each 10-breath cycle. A medical air or oxygen gas flow meter was adjusted to inspiratory flows of 5–12 L/min by a respiratory therapist to assist the subjects in their effort to reach a target expiratory pressure of 15 cm H2O during lung expansion therapy sessions. Adherence to lung expansion therapy was documented by the respiratory therapists in each subject's electronic medical record.
Electrical Impedance Tomography
Electrical impedance tomography measurements were performed during one of the 3 lung expansion therapy sessions on postoperative day 1, 3, and 5. EELI represents a relative regional distribution of ventilation parameters on the PulmoVista 500 electrical impedance tomography device (Dräger Medical, Lübeck, Germany). Distribution of ventilation is displayed on the electrical impedance tomography device as regions of interest 1 (ventral), 2 (mid ventral), 3 (mid dorsal), and 4 (dorsal). Measured changes in lung impedance is directly related to the change in lung volume. For the purpose of this study, we partitioned the 4 regions of interest horizontally and combined regions of interest 1 and 2 to represent ventral and regions of interest 3 and 4 to represent dorsal regions to quantify changes in respective regional redistribution of ventilation.
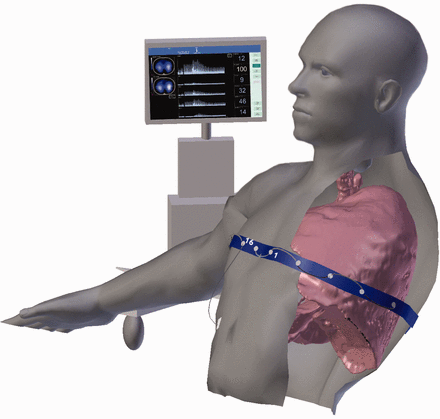
The PulmoVista 500 device was calibrated and self-tested according to the manufacturer's specification. Each subject was positioned in a chair or bed to the standard Fowler position. Chest circumference was measured to determine an appropriate electrode belt size according to manufacturer's recommendations. The electrode imbedded belt was wetted with water to improve electrode conductivity and was placed around the subject's thorax at the level of the fourth-sixth intercostal spaces at the mid clavicular position. The posterior side of the belt's mid position was located over the spine, equidistant from the inferior border of the scapula, with the anterior side electrodes 1 and 16 equally bordering the sternum. A reference electrode was placed on the upper right quadrant of the subject's abdomen (Fig. 2). High-signal quality was confirmed before proceeding with measurement recordings. The electrical impedance tomography monitor was positioned out of view from the subjects and the respiratory therapists who were performing lung expansion therapy to minimize risk of performance bias.
Cross-sectional view of electrode belt position and electrical impedance tomography monitor.
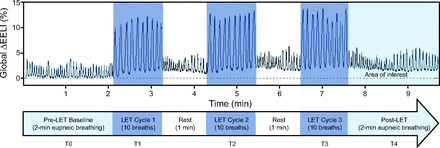
Baseline EELI measurement was recorded for 2 min during eupneic breathing. Each subject was then coached by a respiratory therapist to take 10 breaths during each of 3 lung expansion therapy cycles. One minute of rest breathing occurred between each cycle, and then 2 min of eupneic breathing with EELI measurements was recorded before the session ended. The electrode belt and reference electrode were removed after electrical impedance tomography measurement sessions. The subjects continued to receive scheduled lung expansion therapy 3 times per day until subsequent electrical impedance tomography measurements on postoperative days 3 and 5, unless discharged.
Valid EELI measurements require a closed-circuit constant electrode contact, and minimum patient movement. Removing the electrode belt from study subjects between postoperative day electrical impedance tomography measurements increases the risk of violating validity of repeated measures with EELI comparisons. We chose to analyze and report regional changes in baseline EELI separately for each of the respective postoperative days. The subjects were considered to have completed the study at the completion of postoperative day 5 or at discharge. Continuation of lung expansion therapy was at the discretion of the primary health-care provider team.
Regional distribution of ventilation measurements were recorded on the PulmoVista 500 during monitoring sessions. Each recording was transferred to a USB storage device. Data were downloaded from the USB storage device to a Window's (Microsoft, Redmond, Washington) based personal computer for impedance measurement reconstruction (Electrical Impedance Tomography Data Analysis Tool v6.3, Dräger Medical). Reconstruction allowed for us to isolate our primary areas of interest; the first 2 min (pre–lung expansion therapy baseline) and the last 2 min (post–lung expansion therapy) of the recording (Fig. 3). These isolations were used to quantify relative percentage changes in EELI in response to lung expansion therapy. Examples of a subject on IS and a subject on EzPAP with numeric output and corresponding tomographic images as generated by the Electrical Impedance Tomography Data Analysis Tool are provided (Fig. 4).
Global end-expiratory lung impedance (EELI) changes during a lung expansion therapy (LET) session.
Examples of incentive spirometry (IS) and EzPAP tomographic images and line graph distribution of ventilation change in end-expiratory lung impedance (ΔEELI) after measurement reconstruction. Tomographic image color scale: gray = no/minor changes; light blue = positive deviations; orange = negative deviations. FRC = functional residual capacity.
Outcome Measures
Baseline demographic and clinical characteristics were collected from each subject's electronic medical record, which includes age, sex, height, body mass index, Assess Respiratory Risk in Surgical Patients in Catalonia risk index, pain level, hospital stay, history of smoking, cardiorespiratory comorbidities, and primary surgical procedure. The primary outcome measure was the group difference in respective post-lung expansion therapy dorsal EELI on postoperative days 1, 3, and 5.
Secondary outcome measures of interest were the incidence of postoperative pulmonary complications and patterns of global and regional redistribution of ventilation. Postoperative pulmonary complications included hypoxemia, atelectasis, and pneumonia. Hypoxemia was defined as SpO2 < 90%, with a need to administer or increase supplemental oxygen to maintain SpO2 ≥ 90%. A chest radiograph and a radiologist diagnosis of atelectasis and pneumonia were required for a diagnosis of these complications.
Statistical Analysis
A priori power analysis was performed to determine the study sample size before commencement of the study. A review of the literature showed a 20% FRC increase among healthy adult human subjects who breathed normally at atmospheric pressure and then against 15 cm H2O expiratory positive pressure while seated in a body plethysmograph box.12 We believed that a 15% group difference in dorsal EELI response to lung expansion therapy was reasonable and clinically important. With α set at .05 and 32 β set at .2, we determined that 50 subjects per group would be necessary to power our study at 80%. When considering the possibility of up to 20% attrition, up to 120 subjects could be enrolled in an effort to maintain our desired statistical power with 50 subjects per group on each of the postoperative days.
Continuous variables with normal distribution were presented as mean ± SD and were compared with the use of the independent sample t test. Continuous variables with non-normal distribution were presented as median (interquartile range [IQR]) and compared with the use of Mann-Whitney test. Categorical variables were compared with the use of the chi-square test of independence, with normal approximation or the Fisher exact test if the expected value of a group was <5, as appropriate. We assessed normality of distribution of our data by using the Kolmogorov-Smirnov test and by visually inspecting continuous variable histograms. Primary outcome P (2-tailed) ≤ .05 was considered to be statistically significant. A Bonferroni adjustment was applied when multiple repeated measures were applied for exploratory outcome comparisons, at which point P (2-tailed) ≤ .016 was considered significant. Statistical analysis was performed by using SPSS 25 for Windows (IBM, Armonk, New York) and graphs were generated with GraphPad Prism 8 (GraphPad, La Jolla, California).
Results
Study Population
A total of 180 patients were screened and invited to participate in the study. A total of 112 subjects (62.2%) provided written informed consent to participate. The subjects (N = 112) were equally randomized and allocated at a ratio of 1:1 to receive IS (n = 56) or EzPAP (n = 56) lung expansion therapy. Study enrollment ended after a minimum of 50 subjects per group were obtained, up through postoperative day 3. Continuing to recruit up to an additional 8 subjects would have resulted in unnecessary use of subjects' time and study resources because study attrition precluded us from maintaining enrollment of 50 subjects per group up to postoperative day 5. Sixty eight of the 180 eligible patients (37.8%) were excluded, the majority of whom either declined to participate (n = 33 [49%]) or had a projected hospital stay of <3 d (n = 10 [15%]). Mean ± SD age (61 ± 13 y) was similar between the groups (P = .60) and females represented 58% of the study population.
Both groups had an intermediate risk for developing postoperative complications as determined by the Assess Respiratory Risk in Surgical Patients in Catalonia risk index (median [IQR], 41 [41–41], P = .98). Major respiratory comorbidities consisted of COPD (7.1%), asthma (5.4%), and obstructive sleep apnea (5.4%). The most-common upper abdominal surgical procedures were a Whipple (36.6%) and a hepatectomy (30.4%) (Table 1). A total of 1,126 lung expansion therapies were administered between the IS group (571) and EzPAP group (555). The total subject adherence to lung expansion therapy was 97.4% (1,126/1,156), and adherence to therapy was similar between the IS group (571/585 [97.6%]) and the EzPAP group (555/571 [97.2%]) (P = .66).
Baseline Characteristics
Distribution of Ventilation Dorsal ΔEELI%
The IS and EzPAP groups experienced similar post–lung expansion therapy increases in median dorsal EELI% on each of the postoperative days (Fig. 5). Dorsal EELI% increase was greatest on postoperative day 1 for both groups (16% IS versus 12% EzPAP, P = .39), whereas it was least on postoperative day 3 (6% IS versus 6% EzPAP, P = .68). Similar to postoperative day 1, increased dorsal EELI% was greatest among subjects who received IS (9%) when compared with subjects who received EzPAP (6%) on postoperative day 5 (P = .46).
Change in dorsal end-expiratory lung impedance (ΔEELI) after lung expansion therapy. A: Postoperative day 1. B: Postoperative day 3. C: Postoperative day 5. Boxes represent interquartile range (IQR), with center lines showing the median.
Global ΔEELI%
Exploratory analysis of global ΔEELI% revealed median increases in distribution of ventilation after lung expansion therapy on postoperative days 1, 3, and 5 for both groups (Fig. 6; Table 2). The subjects who received IS experienced a greater global EELI% increase when compared with the subjects who received EzPAP on postoperative day 1 (31% IS versus 25% EzPAP, P = .11), postoperative day 3 (28% versus 16%, P = .08), and postoperative day 5 (26% versus 8%, P = .09), respectively. The greatest regional EELI% increase was in the dorsal region for both groups on postoperative day 1, whereas it was greatest in the ventral region on postoperative day 3 and postoperative day 5. The change in EELI% with IS was greatest in the ventral region of interest when compared with EzPAP on each postoperative day, but these changes in ventral distribution of ventilation were not significant after statistically adjusting for multiple repeated measures (Table 2).
Global and regional change in end-expiratory lung impedance (ΔEELI) after lung expansion therapy. A: Postoperative day 1. B: Postoperative day 3. C: Postoperative day 5. Data are shown as median (interquartile range). IS = incentive spirometry.
Global and Regional Changes in Distribution of Ventilation After Lung Expansion Therapy
Inspiratory Capacity
The IS group experienced a progressive increase in measured inspiratory capacity on each of the postoperative days. The median (interquartile range) inspiratory capacity on postoperative day 1 was 1.15 (0.75–1.50) L, on postoperative day 3 was 1.25 (1.00–1.50) L, and on postoperative day 5 was 1.25 (0.75–2.50) L. Minimum inspiratory capacity was 0.50 L on postoperative day 1, and maximum median inspiratory capacity was 2.5 L on each of the 3 postoperative days. Inspiratory capacity mode was 0.75 L on postoperative day 1 and postoperative day 3, whereas it was 1.25 L on postoperative day 5.
Postoperative Pulmonary Complications
Six subjects (5.4%) developed postoperative pulmonary complications: 2 in the IS group and 4 in the EzPAP group (relative risk 0.50, 95% CI 0.09–2.62; P = .68). Postoperative pulmonary complications included atelectasis (n = 1) and hypoxemia (n = 5), and all 6 occurred on postoperative day 3 (Table 3). No occurrences of pneumonia were documented. Age, Assess Respiratory Risk in Surgical Patients in Catalonia risk index, and respiratory comorbidity were similar between the subjects who did and those who did not develop postoperative pulmonary complications. Postoperative pulmonary complications were more common among the subjects who received EzPAP (n = 4), had a smoking history (n = 4), and were female (n = 5), but these associations with postoperative pulmonary complications were not significant. No adverse events or unintended effects outside of postoperative pulmonary complications were identified. The median (interquartile range) hospital stay was equal between the groups (IS, 4 [3–6], versus EzPAP 4 [3–6]; P = .30).
Incidence of Postoperative Pulmonary Complications
Discussion
Our findings may expand the understanding of the regional distribution of ventilation when comparing IS with EzPAP lung expansion therapies measured by electrical impedance tomography in adult human subjects after upper abdominal surgery. The major outcome of our study found no significant difference in regional distribution of dorsal EELI between the 2 lung expansion therapies. In addition, we did not observe a difference in the postoperative pulmonary complication incidence between the groups. These findings supported our null hypothesis that there is no difference between these 2 postoperative lung inflation techniques in the promoting of dependent lung recruitment or the incidence of postoperative pulmonary complications.
Various factors contribute to reductions in lung volume in the patient after abdominal surgery. Less-resistive and less-compliant regions of the lung will preferentially fill first during a short inflation, with areas of slower time constants filling sequentially thereafter.13 Lung expansion therapy is intended to enhance lung volume and increase the low postoperative FRC.14 The 2 devices used in this study require different lung expansion therapy performance techniques. IS encourages the prolonged, slow, and sustained inspiratory maneuver, with the premise that subsequent lung expansion will recruit atelectatic regions of the lung. PEP therapy incorporates tidal breathing against elevated airway pressure. Patients are instructed to exhale actively but not forcefully against the prescribed pressure level. Variability with expiratory efforts can influence the amount of achieved airway pressure and impact EELI tidal variations that are associated with lung volume.
Patients who undergo upper abdominal surgery have a potential for developing atelectasis, particularly within the first few postoperative days.2,15,16 Surgical procedure, residual anesthetic effects, pain, surgical duration, supine positioning, and shallow tidal breathing, each has the potential to contribute to the development of atelectasis. In our study, both IS and EzPAP groups demonstrated increases in global EELI after lung expansion therapy on each of the postoperative day measurements. A change in EELI reflects a change in lung volume. The increased EELI indicates a sustained increase in lung volume for at least 2 min after lung expansion therapy session completion.
On postoperative day l, both groups demonstrated the greatest increase in EELI in the dorsal regions. We interpreted that both IS and EzPAP were equivalent in increasing distribution of ventilation to the dorsal regions measured after lung expansion therapy on postoperative day 1. This was the region that is typically associated with atelectasis. On postoperative days 3 and 5, ventral regions were the predominant distribution location for IS. We speculated that this may be attributable to the following 2 reasons: a greater regional compliance of ventral anterior regions in the subjects in a Fowler position, and the progressively increasing inspiratory capacity observed through postoperative days 3 and 5, as previously mentioned. PEP therapy requires active exhalation effort. Excessive expiratory effort can result in reduced lung volume. This may persist in some regions of the lung after lung expansion therapy. We speculated that the negative ΔEELI% in the ventral regions of interest identified in Figure 6 reflected a loss of FRC that resulted from excessive expiratory effort. Clinician assessment for excessive expiratory efforts and lung expansion therapy technique re-education may prevent loss of lung volume.
The results of this study were consistent with other published literature on the impact of lung expansion therapy on postoperative lung function. A Cochrane review of randomized clinical trials compared IS with no therapy or physiotherapy, including coughing and deep breathing, in adult subjects admitted to hospital for upper abdominal surgery.7 The review revealed that there was low-quality evidence that showed a lack of effectiveness of IS in the prevention of pulmonary complications. A 2017 single-center randomized controlled trial that compared IS to no IS found no difference in the development of postoperative hypoxemia and pulmonary complications after bariatric surgery.17 A systematic review of PEP therapy administered after abdominal surgery identified only 4 randomized controlled trials, all published before 1993, conducted with PEP therapy in subjects who had abdominal surgery.18 One study revealed positive differences; a higher PaO2 on days 2 and 3, and a lower incidence of atelectasis at day 3.19 A systematic review of 35 clinical trials reported no significant efficacy of various lung expansion therapies in preventing postoperative pulmonary complications among adult subjects after abdominal surgery.20 Only 2 articles referenced PEP therapy.21,22 Few trials supported the use of prophylactic lung expansion therapy. The investigators concluded that prophylactic use of lung expansion therapy seemed to be unjustified for this patient population.
Changes to global EELI in IS and EzPAP have been described in a randomized crossover study in healthy subjects after an endorological procedure.23 This study found an increase in global ΔEELI% during the lung expansion therapy maneuver, with no remnant effect up to 5 min after lung expansion therapy and no difference from the baseline measurement. We reported an increase in global ΔEELI% from the baseline measurements, and this increase was sustained for up to 2 min of post–lung expansion therapy monitoring.
There were several limitations to our study. This was a single-center study. There was not a subject group that did not receive lung expansion therapy, which would have served as a control for both IS and EzPAP comparators. Our routine postoperative orders included IS up to 10 times per hour while awake, a practice consistent with other institutions.24,25 The intent of our study was to compare this standard of IS 3 times per day with EzPAP.
Our standard postoperative upper abdominal surgery methodology uses an enhanced recovery after surgery protocol. The enhanced recovery after surgery multimodal, evidenced-based pathway also incorporates anesthetic techniques, including local, non-narcotic, neuraxial blockade, and neuropain modulation medications administered to limit the application of opioids and maintain a pain score of <4 (1–10 scale). These factors were not studied and may have contributed to the low pulmonary complication rates observed. The subjects in our study had a median pain score of 4. It is possible that the pain management score influenced the reliability of inspiratory capacity measurements for subjects by using IS. Pain may have produced variability in consistently achieving a targeted expiratory pressure level of 15 cm H2O when using EzPAP. Both limitations may have influenced the change in EELI measurements. Early mobility is also encouraged as soon as the day of surgery as a component of the enhanced recovery after surgery protocol. Adherence to the mobility protocol was not recorded and may be a confounding variable directly related to our outcomes.
Although we reported a low incidence of postoperative pulmonary complications, this outcome may have underestimated the true occurrence of atelectasis and pneumonia. Physicians did not order chest radiographs in our patient population, unless there was a reported change in respiratory status. Most of our subjects did not develop hypoxemia or other evidence of worsening respiratory status. Another limitation of our study related to electrical impedance tomography monitoring. Changes in EELI during electrical impedance tomography measurement reflected one cross-sectional slice of the lung that transverses the thorax across the electrode plane.26 Recruitment and de-recruitment are not necessarily evenly distributed throughout the entire lung. The possibility of changes that occurred in other transverse planes was not accounted for by the single electrode plane measured with electrical impedance tomography.
We attempted to reduce the risks of bias for the study. Random sequence generation and allocation concealment were controlled, and incomplete outcome data were reported. Attrition bias due to study dropout, as opposed to attrition due to hospital discharge, was low. Although the study design did not allow for blinding of lung expansion therapy devices, the subjects and the respiratory therapists who administered and coached lung expansion therapy were concealed from viewing the electrical impedance tomography monitor during data recordings. The monitoring schedule required removal of the electrode belt between sessions. This had the potential to lead to variability and reproducibility questions with the belt position. Previously published studies that used electrical impedance tomography on healthy subjects during spontaneous tidal breathing and vital capacity maneuvers in sitting, supine, and lateral positions demonstrated good reproducibility. In this study, the subjects were placed in a Fowler position while sitting in a chair or in bed. The dorsal measurements in this position orientation reflect a posterior positioning, with ventral measurements being anterior. This alignment may be a factor in the observed results.
This study shed additional insight into the current published literature on the impact of lung expansion therapy in patients after upper abdominal surgery. The utilization of electrical impedance tomography to monitor lung volume before, during, and after lung expansion maneuvers provides new information on temporal changes to specific regions of the lung as well as the sustainability of the impact of lung expansion maneuvers. This work provided insight on the reliability and reproducibility of electrical impedance tomography technology on measuring lung expansion maneuvers in this subject population. Recommendations from Cochrane reviews and other recently published articles6,14 on IS highlight the urgent need for additional well-designed clinical trials.6,7,14,24 We attempted to design our study to address some of these shortcomings. Our rate of adherence to IS or EzPAP scheduled frequency, targeted volume or pressure, and the number of breaths/session, was standardized, recorded, and reported. Respiratory therapists followed up the 2 subject groups with equal adherence to lung expansion therapy up to study completion or dropout. Close monitoring of adherence to lung expansion therapy may not be common in patients outside of a study protocol. Respiratory therapists who deliver lung expansion therapy and monitor subject adherence to scheduled therapies could have contributed to our low level of postoperative pulmonary complications.
Conclusions
There was no significant difference in dorsal change in EELI% after lung expansion therapy when comparing IS with EzPAP lung expansion therapies among adult human subjects after upper abdominal surgery on postoperative days 1, 3, and 5. There was no association with the development of postoperative pulmonary complications when comparing IS with EzPAP lung expansion therapy administered 3 times per day to this patient population. Future studies should compare no lung expansion therapy with other lung expansion therapy interventions to determine if there is a difference in global and regional distribution of ventilation and incidence of postoperative pulmonary complications after upper abdominal surgery.
Acknowledgments
We thank the respiratory therapists, nurses, and surgeons who provided clinical support for this study. We thank the subjects who allowed us to perform this work.
Footnotes
- Correspondence: Daniel D Rowley MSc RRT RRT-ACCS RRT-NPS RPFT FAARC, Pulmonary Diagnostics & Respiratory Therapy Services, University of Virginia Medical Center, PO Box 800686, Charlottesville, VA 22908. E-mail: ddr8a{at}virginia.edu.
Financial support was provided by the Respiratory Therapy Department, University of Virginia Medical Center.
Mr Rowley presented a version of this paper as an Editors' Choice abstract at the AARC Congress 2018, held December 4-7, 2018, in Las Vegas, Nevada.
Dräger provided an electrical impedance tomography device and electrode belts.
Mr Rowley and Mr Sharkey disclose relationships with Dräger and Mallinckrodt. The remaining authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
See the Related Editorial on Page 1314
- Copyright © 2019 by Daedalus Enterprises