Abstract
BACKGROUND: Exposure of respiratory therapists (RTs) during aerosol-generating procedures such as endotracheal intubation is an occupational hazard. Depending on the hospital, RTs may serve as laryngoscopist or in a role providing ventilation support and initiating mechanical ventilation. This study aimed to evaluate the potential exposure of RTs serving in either of these roles.
METHODS: We set up a simulated patient with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in an ICU setting requiring endotracheal intubation involving a laryngoscopist, a nurse, and an RT supporting the laryngoscopist. All participants wore appropriate personal protective equipment (PPE). A fluorescent marker was sprayed by an atomizer during the procedure using 3 different methods for endotracheal intubation. The 3 techniques included PPE alone, a polycarbonate intubating box, or a coronavirus flexible enclosure, which consisted of a Mayo stand with plastic covering. The laryngoscopist and the supporting RT were assessed with a black light for contamination with the fluorescent marker. All simulations were recorded.
RESULTS: When using only PPE, both the laryngoscopist and the RT were grossly contaminated. When using the intubating box, the laryngoscopist’s contamination was detectable only on the gloves: the gown and face shield remained uncontaminated; the RT was still grossly contaminated on the gloves, gown, neck, and face shield. When using the coronavirus flexible enclosure system, both the laryngoscopist and the RT were better protected, with contamination detected only on the gloves of the laryngoscopist and the RT.
CONCLUSIONS: Of the 3 techniques, the coronavirus flexible enclosure contained the fluorescent marker more effectively during endotracheal intubation than PPE alone or the intubating box based on exposure of the laryngoscopist and supporting RT. Optimizing containment during aerosol-generating procedures like endotracheal intubation is a critical component of minimizing occupational and nosocomial spread of SARS-CoV-2 to RTs who may serve as either the laryngoscopist or a support role.
- COVID-19
- coronavirus
- SARS-CoV-2
- intubation
- viral exposure
- aerosol-generating procedure
- respiratory therapists
- endotracheal intubation
- occupational exposure
Introduction
A new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged from China in 2019 and caused a pandemic.1 Data regarding the potential of aerosol-generating procedures to transmit SARS-CoV-2 to health care workers, and more specifically to respiratory therapists (RTs), during these procedures are accumulating, but they are still incomplete. Recent experiments have reported that SARS-CoV-2 remained viable in aerosols generated throughout 3 h of an experiment and remained stable on certain surfaces (eg, plastics and stainless steel) for up to 72 h after application to these surfaces.2 Although these experimental conditions may not exactly replicate clinical conditions,3-5 transmission of SARS-CoV-2 to care team members during aerosol-generating procedures is an occupational hazard for RTs. A review of patients with SARS-CoV-2 kept in airborne isolation rooms indicated that, in 1 patient’s room, even the air outlet fan high on the wall behind the patient was contaminated with SARS-CoV-2, raising concerns for potential airborne virus transmission.6
Specific aerosol-generating procedures that have the potential for virus transmission to health care workers during the procedure include bag-valve-mask ventilation, noninvasive ventilation, endotracheal intubation, bronchoscopy, cardiopulmonary resuscitation, and tracheostomy.7 These high-risk procedures are often performed in critical care units involving RTs, whether they are participating as the laryngoscopist or assisting with airway management and instituting mechanical ventilation.8,9 A recent review reported that involvement with endotracheal intubations increased health care worker infection risks from SARS-CoV-2, SARS-CoV-1 (which causes SARS-1), and MERS-CoV (which causes Middle East respiratory syndrome).10
The transmission of the coronavirus infection to health care workers represents a serious threat, as approximately 4% of patients hospitalized with SARS-CoV-2 in China and Italy were health care workers.11 During the SARS-CoV-1 outbreak, health care workers comprised approximately 20% of all infected individuals globally.12 Virus transmission to health care workers can occur through direct, indirect, or close contact with patients through fluids such as saliva or respiratory secretions.13,14 Possible modes of SARS-CoV-2 transmission to health care workers performing or assisting with a procedure involving infected airway secretions such as endotracheal intubation include contact, droplet, airborne caused by droplet nuclei (aerosols), and fomites entailing contact with a contaminated surface or object.2,6 The infectivity of SARS-CoV-2 is increased by the moderate protein concentration in droplets of respiratory secretions that may enhance the virus persistence and transmission via contaminated fomites.15 Preventing virus transmission from patients to health care workers who can cause subsequent infections to other members of the care team remains a crucial strategy to reduce or prevent secondary transmission of the virus and thus disease-related morbidity and mortality, as well as prevent shortages of medical manpower and expertise needed to care for these critically ill patients.16,17
Strategies that provide a barrier that limits health care worker exposure to the virus remain a critical goal to minimize cross-contamination as well as occupational and nosocomial spread of SARS-CoV-2. The SARS-CoV-2 pandemic has led to the development of novel devices and techniques aimed at protecting health care workers from the risks associated with virus transmission during care of these patients and during any aerosol-generating procedures. These novel devices have included polycarbonate aerosol boxes, which are also termed intubating boxes, for endotracheal intubation.18,19 Many of these devices and techniques are primarily aimed at decreasing the risk to the proceduralist (eg, the laryngoscopist during intubation) and thus provide detailed operational instructions with sparse data on the technical impact on the clinician performing the procedure.20-22 When the devices are evaluated for their impact on health care workers, the emphasis is on the proceduralist alone or on a sole member of the team (eg, the individual performing chest compressions) without considering other essential members of the care team, such as RTs.15,16,23,24 These non-proceduralist members of the care team provide necessary assistance during these procedures in an ICU setting and are also at risk for being contaminated and infected, especially because more than half of reported tracheal intubations were performed in the ICU.25
We evaluated a novel technique aimed at further limiting exposure to all those in a hospital setting during an intubation.23 To create the coronavirus flexible enclosure, we intentionally used readily available equipment and materials. This transparent coronavirus flexible enclosure was evaluated during an endotracheal intubation simulation. We evaluated the exposure to the entire team (ie, a proceduralist, an RT, and a nurse) in 3 scenarios: using personal protective equipment (PPE) alone, using PPE and an intubating box, or using PPE and the coronavirus flexible enclosure.
Methods
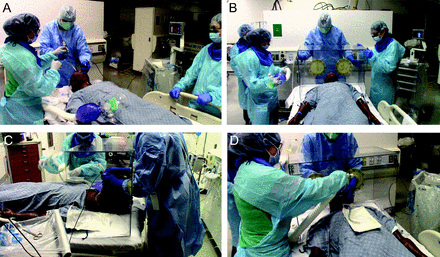
This study was approved by the University of Florida Institutional Review Board, and written informed consent was obtained from all participants. This simulation study evaluated the impact of 3 methods of intubation of a simulated patient with severe SARS-CoV-2 in the ICU. For the study, 2 critical care team members were considered essential for performing the procedure: the provider performing the intubation (who may or not be an RT) and the provider serving as the assisting RT. All individuals wore PPE consistent with local guidelines for intubation of a patient with SARS-CoV-2. PPE consisted of 2 masks (an N95 mask that was covered with a regular surgical mask to protect the N95 supply), a face shield, a hat, an impervious long-sleeved plastic gown, and gloves (Fig. 1). A 7.0-endotracheal tube with a malleable stylet was provided to the intubating proceduralist. Depending on the trial, the simulated patient was enclosed in nothing, in an open-ended polycarbonate intubating box with proceduralist-facing portholes for the laryngoscopist’s hands, or in a clear plastic coronavirus flexible enclosure that draped over the mannequin (HAL S3201 Advanced Multipurpose Patient Simulator, Gaumard, Miami). During intubation, the critical care team included the laryngoscopist and the assisting RT. The RT assisted with mask ventilation and connected the ventilator circuit after intubation and stood to the patient’s right side at the level of the patient’s thorax.
Two different intubation techniques: intubation with PPE only or intubation with a polycarbonate intubation box. A: Intubation with PPE only. B: Polycarbonate airway box position. C: Intubation with polycarbonate intubation box. D: Assisting respiratory therapist at the open end of the polycarbonate intubation box. PPE = personal protective equipment.
All simulations used the C-MAC videolaryngoscope (Karl Storz, Tuttlingen, Germany) with a Mac-3 blade. To approximate coughing and labored breathing, a fluorescent marker (Glo Germ, Glo Germ Company, Moab, Utah) was used for each of the 3 test scenarios. The Glo Germ solution was prepared with 80% isopropyl alcohol and 20% Glo Germ, which was delivered with a 30-mL syringe through small-bore intravenous tubing to 2 MADgic laryngo-tracheal mucosal atomization devices (Teleflex, Wayne, Pennsylvania) attached to the mannequin’s chin. Delivery of the Glo Germ began before the placement of the intubating box or the coronavirus flexible enclosure, and delivery continued at 20-s intervals until the mannequin was “paralyzed,” with the amount delivered ranging from 20 to 30 mL per scenario. After each of the intubation techniques, the area around the patient and the 3 providers was illuminated with a black light, allowing visualization of any contamination by the fluorescent Glo Germ as a proxy for virus contamination.
During the simulation exercise examining the effects of using no additional protective equipment beyond the wearable PPE to shield the laryngoscopist from the patient, the mannequin was positioned in the ICU bed with the head of bed elevated to approximately 20° and the bed height as low as possible, as would be typical for a patient in the ICU. When the mannequin was activated, the Glo Germ delivery began, at which time the care team approached the patient bed and prepared the intubation equipment for use. While the ICU nurse prepared the equipment, the laryngoscopist and the assisting RT provided supplemental oxygen to the patient via bag-valve-mask ventilation. During this time, the bed height and position were also adjusted to a position the laryngoscopist believed would best facilitate the intubation. Once the equipment was ready, the team requested the administration of intubation drugs, and a 90-s timer was started. After 90 s, the mannequin was intubated, and the ventilator circuit was connected to the endotracheal tube. Afterward, the endotracheal tube was secured in place using cloth tape 25 mm wide as per our respiratory therapy standard operating procedures.
During the simulation exercise examining the effects of the open-ended polycarbonate intubation box, the above procedure was modified such that the polycarbonate box was placed around the head and shoulders of the mannequin by the team immediately upon reaching the bedside (Fig. 1). The laryngoscopist inserted their hands through the portholes, and the RT reached around the open end of the box facing the foot of the bed to assist with mask ventilation, intubation, and ventilator circuit connection. Once the ventilator circuit was connected, the box was removed, and the endotracheal tube was secured as described above.
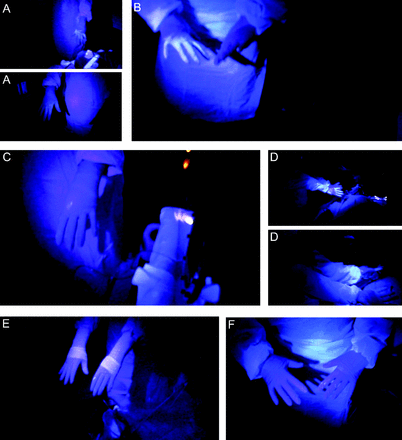
During the simulation exercise examining the effects of the plastic coronavirus flexible enclosure, the above procedure was modified such that the coronavirus flexible enclosure was deployed by the team immediately upon reaching the bedside.26 The coronavirus flexible enclosure consisted of an open-frame Mayo stand with the tray removed, positioned above the thorax of the mannequin at a height sufficient to provide adequate working space for intubation (approximately 61 cm) between the stand and the thorax of the mannequin (Fig. 2). A large plastic sheet was draped over the Mayo stand. The plastic sheet used in this simulation exercise was a bed drape typically used to allow patients in the burn ICU to shower (Medline, Northfield, Illinois). This medical-grade plastic material sheet is approximately 2.3 × 2.7 m and 0.25 mm thick. When arranged with the long axis of the sheet across the short axis of the bed, it was sufficient to cover the mannequin from the head of the bed to approximately waist level and to cover the entire width of the mattress (Fig. 2). Equipment that was needed for intubation was placed on or near the mannequin, under the drape, where it could be easily reached when needed. The edges of the drape were tucked under both sides and the top of the bed. Next, 10 × 15 cm occlusive dressings (Tegaderm, 3M, St. Paul, Minnesota) were placed on the plastic drape at locations where the laryngoscopist and assisting RT intended to insert their hands to act as rip-resistant windows. Importantly, the paper application tape around the outer edge of the occlusive dressings should be left in place to facilitate easy and rapid identification of their location. Small holes were cut through the occlusive dressing and underlying drape with scissors, allowing hands to be inserted through the drape. These hand insertion holes also provided a porthole to pass unanticipated additional small equipment (eg, a different laryngoscope blade) for placement underneath the drape without lifting it. Intubation proceeded as described above. If additional larger equipment is needed, it can easily be passed under the drape by the ICU nurse assisting with the procedure without the need for either the laryngoscopist or assisting RT to remove their hands from under the drape until the procedure has been completed and the ventilator circuit is connected to the endotracheal tube. Each approach was studied once.
The technique to set up the coronavirus flexible enclosure. A: Positioning of the portable Mayo stand. B: Clear drape to cover the portable stand. C: Drape placement over the Mayo stand. D: Addition of occlusive dressings for the laryngoscopist’s hands. E: Position of the occlusive dressing for the assisting respiratory therapist’s hands. F: Intubation using the coronavirus flexible enclosure.
Results
We found that a single person can easily deploy the coronavirus flexible enclosure system in approximately 2 min. The intubation box can also be deployed in a short time (∼ 2 min), but its deployment requires 2 people to position it because of its weight and its rigid external dimension (Fig. 1). Likewise, the rigid placement of the arm holes at a predetermined location created a cumbersome operating environment. These arm holes were positioned for the laryngoscopist only, with none on the side for the assisting RT.
When intubation was performed using only PPE without any additional protective enclosure (Fig. 1), both the laryngoscopist and the assisting RT were grossly contaminated. Glo Germ was found on the front of the gown, gloves, and face shield of both operators (Fig. 3, Fig. 4).
Contamination of the body of the laryngoscopist and the assisting respiratory therapist. A: Laryngoscopist after intubation with PPE only. B: Assisting respiratory therapist after bagging with PPE only. C: Laryngoscopist after intubation with polycarbonate intubation box. D: Assisting respiratory therapist after bagging with the polycarbonate intubation box. E: Laryngoscopist after intubation with coronavirus flexible enclosure. F: Assisting respiratory therapist with coronavirus flexible enclosure. PPE = personal protective equipment.
Contamination of the hands and forearms of the laryngoscopist and the assisting respiratory therapist. A: Laryngoscopist after intubation with PPE only. B: Assisting respiratory therapist after bagging with PPE. C: Laryngoscopist after intubation with the polycarbonate intubation box. D: Assisting respiratory therapist after bagging with polycarbonate intubation box. E: Laryngoscopist after intubation with the coronavirus flexible enclosure. F: Assisting respiratory therapist after bagging with the coronavirus flexible enclosure. PPE = personal protective equipment.
When intubation was performed using the rigid polycarbonate intubation box (Fig. 1), the laryngoscopist was well protected, with Glo Germ contamination detectable only on the gloves (Fig. 4). The assisting RT however was grossly contaminated, with Glo Germ detected on the gloves, gown, neck, and face (Fig. 3, Fig. 4). The laryngoscopist was able to successfully intubate the mannequin but reported that the preset arm holes constricted movement. When intubations were performed using the coronavirus flexible enclosure (Fig. 1), both the primary laryngoscopist and the assisting RT were well-protected, with Glo Germ contamination detectable only on the gloves (Fig. 4). The laryngoscopist was able to successfully intubate the mannequin and reported being able to maneuver easily with the arm holes created at the location chosen by him. During the performance of each of these scenarios, neither the ICU nurse nor the surrounding area were contaminated. Intubation was performed on the first attempt, and no breaches (eg, tears) in the PPE gowns or gloves were noted.
Discussion
Our PubMed search indicates that this pilot study is the first to examine the potential exposure of RTs as part of a critical care team during an aerosol-generating procedure. In this study, we used the recommended technique of PPE (ie, N95 mask, face shield, hat, impervious gown, gloves, and videolaryngoscopy) for endotracheal intubation, an aerosol-generating procedure commonly performed in the ICU.11,27 Traditional videolaryngoscopy without the use of any other novel protective techniques resulted in the highest exposure to both the laryngoscopist and the assisting RT. The intubation box visibly decreased exposure for the laryngoscopist, but not for the assisting RT. The lowest degree of contamination of the care providers was with the flexible coronavirus enclosure, and the contamination was limited to just their gloves. The considerable exposures of both laryngoscopist and RT in our simulation visually demonstrated the vulnerability of the RT in either role during this representative aerosol-generating procedure. Furthermore, the results indicate that not all provider-protective strategies are similarly protective. This was found to be especially true for the laryngoscopist and assisting RT. The use of transparent occlusive film dressings over entry points through the plastic drape in this coronavirus flexible enclosure model for both the laryngoscopist and the RT allowed for personalized positioning of the arm portholes (Fig. 2).
Although the mechanisms have yet to be clearly elucidated for SARS-CoV-2 transmission to health care workers, infectious risk to RTs while caring for patients infected with SARS-CoV-2 has been proposed to involve the inoculum of virus during exposure.28 Factors influencing this inoculum include time exposed, proximity to the patient’s airway, viral dose (eg, viral load, time airborne), environmental factors (eg, room volume, air exchange rates for the room, local air currents), fomites, contact with mucus membranes, and volume, number, and diameter of the particles.28 Protection of the RT during the time at risk for exposure involves many layers analogous to Reason’s “Swiss cheese” model of safety (Fig. 5).29 Using this analogy, each slice of Swiss cheese provides a layer of defense between the RT and the SARS-CoV-2 virus. The coronavirus flexible enclosure detailed in this report provides another layer of protection to the “Swiss cheese model,” which along with the other measures helps decrease exposure and transmission risks to RTs while caring for patients with SARS-CoV-2. Each layer buttresses potential failures in other layers. As an example, we now know that N95 mask filtering reliability is markedly reduced after 4 uses30 and are an example of a breach in the Swiss cheese of defense layers. In an international, multicenter, prospective study of health care workers participating in tracheal intubation of patients with suspected or confirmed SARS-CoV-2 infection, 12% of cases reported insufficient PPE.25 Shortage of PPE has resulted in the reuse of disposable N95 respirators,31-33 which can lead to fit failure of these devices and thus can potentially expose health care workers to the virus.30
Reason’s "Swiss cheese" model. In an ideal world, each defensive layer would be intact. In reality, they are more like slices of Swiss cheese, having many holes; however, unlike Swiss cheese, these holes are continually opening, closing, and shifting their location. The presence of holes in any one "slice" does not normally cause a bad outcome. Usually, this happens only when the holes in many layers momentarily line up to permit a trajectory of accident opportunity, bringing hazards into damaging contact with victims. PPE = personal protective equipment (gown, N95 mask/PAPR/CAPR, face shield, goggles, hat, hood, and gloves). Modified from Reference 29.
The use of a clear plastic drape has been described previously in non-ICU settings.21,24,34 One technique involves construction of a device with the clear plastic drape attached to the bed before patient arrival and does not describe hospital or biomedical approval of its use.21 Once the device is constructed, that technique involves lifting the drape to position hands for the procedure, potentially causing more exposure than our technique, which creates portholes for hand placement using transparent occlusive adhesive film dressings in our model. The second technique34 for patient care involves 3 plastic drapes positioned under the head, covering the torso, and over the head to cover the patient and would not be suitable for performing many procedures that require a proceduralist, such as endotracheal intubation. The use of the clear plastic drape for use during chest compression has been described previously in an in situ ICU simulation23 and focused on the contamination the individual performing chest compressions. Another technique24 evaluated in a simulation environment involves using a precut, specifically shaped, clear, flexible plastic sheet that requires folding for assembly before use, which then adds additional plastic sleeves and a torso drape; the precut flexible sleeves allow for full range of motion for the laryngoscopist and an assistant. This device also utilized high-flow suction to aid aerosol removal, such as what is seen in operating rooms; this method removed more aerosols than the low-flow suction typically available in an ICU or hospital room.24
The use of an intubation box, also termed an aerosol box, to serve as a barrier during intubation has been described previously18-20; this box is a transparent plastic cube designed to cover the patient’s head and has 2 circular apertures that allow the clinician to reach into the box to perform intubation. In simulated environments, the use of this box during endotracheal intubation limited contamination to the proceduralist, with the gloved hands and gowned forearms that entered the box showing contamination as well as the interior of the box18,19; this box allowed less contamination than laryngoscopy without its use, regardless of whether a direct or video-assisted technique was used.
In a recent study, the use of intubation boxes by an experienced airway specialist slowed intubation times and resulted in a higher failure rate, with contributing factors including reduced ability to maneuver arms and hands, limited manipulation of devices placed within the box, as well as increased cognitive load for the proceduralist.35 Several breaches of PPE occurred while using the box, including tears of PPE and exposure of the skin on the wrist when the glove was pulled forward and down when hands and forearms were inserted though the box apertures.35 These polycarbonate boxes are rigid and heavy, which at times makes positioning or repositioning challenging.36 The arm apertures are predetermined and may not work equally well for different body habitus for the various patients or proceduralists. Since the time of this study, polycarbonate boxes are now being created with arm holes on the side as well, which warrants additional study.37 One of the other issues with this type of box is that its configuration requires 2 people to remove and takes longer to access the airway than the coronavirus flexible device should an urgent need arise. The coronavirus flexible enclosure can be removed quickly by pushing the Mayo stand to the side and removing the drape from the upper torso, which took 5 s in our study, should an unfettered approach be required due an unanticipated difficult airway.
The polycarbonate intubation boxes also show substantial air movement from the open side of the box to the room as well as air escape from the arm apertures38; the addition of a clear drape over the open end of the box decreased air flow entering the room from the open side. During a simulated tracheal intubation evaluating aerosol-containment devices, the intubation box showed an increase in airborne particle exposure to the laryngoscopist compared to no device use.39 This simulation also evaluated horizontal and vertical drapes and found no difference in aerosol particle exposure compared to no device.39 However, during tracheal intubation the laryngoscopist reached under the drapes to perform the procedure, which may have resulted in aerosol escape and requires further evaluation with the use of small apertures for insertion of the laryngoscopist’s arms as used in the coronavirus flexible enclosure.39 One limitation of these interventions is that the exposures of the other team members, including the assisting RT, were not evaluated during intubation using these boxes.40 Our study used dispersion of an atomized spray to evaluate splash and splatter contamination of the laryngoscopist and assisting RT. This technique did not evaluate aerosol dispersion, which remains an area for future evaluation. Evaluation of contamination as increasing exposure is important because doffing errors represent another source of inadvertent and often unrecognized transmission to health care workers.41
The coronavirus flexible enclosure has been used for tracheal intubations in the operating room, ICU, and hospital rooms, as well as in hospital-based in situ simulations.23 One demonstration showing how to drape the Mayo stand and establish the arm holes was typically required for proper use. A common mistake was placing the drape with the longest side from head to toe rather than the longest side draped from the side to side across the Mayo stand to allow sufficient height to perform intubation. No breaches in PPE have been reported using this system. The coronavirus flexible enclosure has been successfully used in patients under investigation to perform bronchoscopy as well.
Our study demonstrated that rigid polycarbonate intubation boxes offer minimal protection to the assisting RT during bag-valve-mask ventilation, endotracheal tube securement, and initiation of mechanical ventilation. For the assisting RT to perform these functions, they must reach into the box through the opening toward the foot of the bed (Fig. 1). This places them directly in the highest-risk area during this procedure, with no barrier between the assisting RT and the patient except for their PPE.
Conclusions
We report on the use of a flexible draping system that demonstrates a decrease in contamination for a RT whether serving as the laryngoscopist or as support staff during an intubation procedure for a patient with coronavirus (SARS-CoV-2). Previous work exploring the utility of adjunctive devices to protect medical workers during aerosol-generating procedures has mainly focused on protecting the laryngoscopist. Minimal attention has been paid to the protection of assistants, such as RTs, who are also vulnerable during aerosol-generating procedures.
ACKNOWLEDGMENTS
Thanks to Savanna Mahn who helped during the simulation and Mike DiLena for his assistance with the video recording. Thanks to Vera Barnes, Leah Buletti, and Corey Astrom, ELS, for their assistance with manuscript preparation.
Footnotes
- Correspondence: Brenda G Fahy MD, Department of Anesthesiology, University of Florida College of Medicine, 1600 SW Archer Rd, M509, PO Box 100254, Gainesville, FL 32608. E-mail: bfahy{at}anest.ufl.edu
The authors have disclosed no conflicts of interests.
- Copyright © 2020 by Daedalus Enterprises