Abstract
BACKGROUND: Efficacy of high-flow nasal cannula (HFNC) over noninvasive ventilation (NIV) in severe coronavirus disease 2019 (COVID-19) pneumonia is not known. We aimed to assess the incidence of invasive mechanical ventilation in patients with acute hypoxemic respiratory failure due to COVID-19 treated with either HFNC or NIV.
METHODS: This was a single-center randomized controlled trial performed in the COVID-19 ICU of a tertiary care teaching hospital in New Delhi, India. One hundred and nine subjects with severe COVID-19 pneumonia presenting with acute hypoxemic respiratory failure were recruited and allocated to either HFNC (n = 55) or NIV (n = 54) arm. Primary outcome was intubation by 48 h. Secondary outcomes were improvement in oxygenation by 48 h, intubation rate at day 7, and in-hospital mortality.
RESULTS: Baseline characteristics and 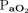 /
/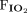 ratio were similar in both the groups. Intubation rate at 48 h was similar between the groups (33% NIV vs 20% HFNC, relative risk 0.6, 95% CI 0.31–1.15, P = .12). Intubation rate at day 7 was lower in the HFNC (27.27%) compared to the NIV group (46.29%) (relative risk 0.59, 95% CI 0.35–0.99, P = .045), and this difference remained significant after adjustment for the incidence of chronic kidney disease and the arterial pH (adjusted OR 0.40, 95% CI 0.17–0.93, P = .03). Hospital mortality was similar between HFNC (29.1%) and NIV (46.2%) group (relative risk 0.6, 95% CI 0.38–1.04, P = .06).
ratio were similar in both the groups. Intubation rate at 48 h was similar between the groups (33% NIV vs 20% HFNC, relative risk 0.6, 95% CI 0.31–1.15, P = .12). Intubation rate at day 7 was lower in the HFNC (27.27%) compared to the NIV group (46.29%) (relative risk 0.59, 95% CI 0.35–0.99, P = .045), and this difference remained significant after adjustment for the incidence of chronic kidney disease and the arterial pH (adjusted OR 0.40, 95% CI 0.17–0.93, P = .03). Hospital mortality was similar between HFNC (29.1%) and NIV (46.2%) group (relative risk 0.6, 95% CI 0.38–1.04, P = .06).
CONCLUSIONS: We were not able to demonstrate a statistically significant improvement of oxygenation parameters nor of the intubation rate at 48 h between NIV and HFNC. These findings should be further tested in a larger randomized controlled trial. The study was registered at the Clinical Trials Registry of India (www.ctri.nic.in; reference number: CTRI/2020/07/026835) on July 27, 2020.
- high-flow nasal cannula
- noninvasive ventilation
- coronavirus disease 2019
- acute respiratory failure
- HFNC
- NIV
Introduction
Patients with severe coronavirus disease 2019 (COVID-19) pneumonia present with acute hypoxemic respiratory failure and require oxygen therapy by face mask as the primary device. Patients who develop significant hypoxia may need endotracheal intubation and mechanical ventilation. However, invasive mechanical ventilation in COVID-19 is associated with high mortality.1,2 Therefore, use of less invasive oxygenation devices like high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) may be a feasible option to avoid intubation and improve outcome.3,4 Avoidance of intubation is desirable to preserve resources in a difficult pandemic setting, but there is clinical equipoise between respiratory support with HFNC and NIV in terms of reducing intubation rate and mortality.
In the FLORALI trial5 performed in subjects with acute hypoxemic respiratory failure, HFNC reduced 90-d mortality compared to standard oxygen therapy and face mask. Although, the intubation rate was not significantly different, there was a trend toward a lower intubation rate in the HFNC group compared to conventional oxygen and NIV group.5 On the other hand, use of NIV may have high failure rate, and patients with delayed intubation may have worse clinical outcomes.6
Based on the initial experience and past data, the Surviving Sepsis Campaign guidelines on the management of critically ill adults with COVID-19 recommended use of HFNC over NIV.7 However, data in acute hypoxemic failure due to COVID-19 are too limited to choose between HFNC and NIV so far. We, therefore, planned to perform a randomized controlled trial to identify the efficacy of HFNC versus NIV in subjects with severe COVID-19. We hypothesized that primary use of HFNC will significantly reduce early intubation rate (within 48 h) compared to NIV.
QUICK LOOK
Current Knowledge
Invasive mechanical ventilation in acute hypoxemic respiratory failure due to severe COVID-19 pneumonia is associated with high mortality. Whether use of high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) reduces the incidence of invasive ventilation in COVID-19 pneumonia is not clear.
What This Paper Contributes to Our Knowledge
We were not able to demonstrate a statistically significant improvement of oxygenation parameters nor of the intubation rate at 48 h between NIV and HFNC. These findings should be further tested in a larger randomized controlled trial.
Methods
Study Design and Participants
This study was designed as a single-center, prospective, randomized, controlled trial and was conducted in the 28-bed COVID-19 ICU in All India Institute of Medical Sciences, New Delhi, from August to December 2020. Inclusion criteria: Subjects with laboratory-confirmed diagnosis of COVID-19 pneumonia, presenting with severe COVID-19 pneumonia, who failed oxygen therapy by face mask, were included in this study after obtaining informed written consent from the subjects or their legally acceptable representatives. Adult subjects of age 18–75 y were considered, and the following definitions were followed.
Severe COVID-19 pneumonia: Subjects presenting with fever, cough, and respiratory distress with frequency > 30 breaths/min and/or room air  < 90%.8
< 90%.8
Failure of oxygen therapy by face mask: Subjects with frequency > 24 breaths/min and/or  < 94% in spite of oxygen by face mask at 10 L/min flow for 30 min.
< 94% in spite of oxygen by face mask at 10 L/min flow for 30 min.
Exclusion criteria: Hemodynamic instability and requirement of high-dose vasopressor therapy; pregnancy; COPD/chronic respiratory failure; morbid obesity; patients with urgent requirement of invasive mechanical ventilation, severe hypoxia ( < 90% with frequency > 40 breaths/min for > 10 min), severe hemodynamic instability (mean arterial pressure < 65 mm Hg in spite of high-dose noradrenaline support) with altered mentation, Glasgow coma scale score < 8, or cardiac arrest were excluded.
< 90% with frequency > 40 breaths/min for > 10 min), severe hemodynamic instability (mean arterial pressure < 65 mm Hg in spite of high-dose noradrenaline support) with altered mentation, Glasgow coma scale score < 8, or cardiac arrest were excluded.
The Institute Ethics Committee, All India Institute of Medical Sciences, New Delhi, India, provided ethical approval on July 15, 2020. The study was registered at the Clinical Trials Registry of India (www.ctri.nic.in; reference number: CTRI/2020/07/026835) on July 27, 2020.
Sample Size, Randomization and Blinding
Previous data from the H1N1 pandemic in 2009 indicated that around 75% of patients who received NIV ultimately required invasive mechanical ventilation.9 Given the constraints of the pandemic, and the necessity of obtaining actionable results as quickly as possible, we settled on a convenience sample size of around 100 subjects. Post hoc calculations showed that this sample size was able to detect a difference of 30% in intubation rate between HFNC and NIV with a power of 80% and 2-sided alpha of 0.05. Eligible subjects were randomized with a computer-generated random number table (www.randomizer.org) in to either group A (HFNC) or group B (NIV) according to a computer-generated random number table. Allocation concealment was done with sealed-envelope technique. The ICU doctor informed the subjects about group allocation, obtained consent, noted the baseline data, and initiated the intervention.
The subject and the clinical management team were not blinded to the allocated intervention. However, an independent investigator unaware of the group allocation noted the outcome variables after 48 h of randomization and thereafter from the subjects’ database and files.
Intervention
HFNC arm: Subjects received HFNC through large-bore binasal prongs with a high-flow heated humidifier device (Optiflow, Fisher & Paykel Healthcare, Auckland, New Zealand). The initial gas flow was set at 50 L/min and 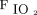 of 1.0. The flow and
of 1.0. The flow and 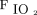 were subsequently adjusted between 30–60 L/min and 0.5–1.0, respectively, to maintain
were subsequently adjusted between 30–60 L/min and 0.5–1.0, respectively, to maintain 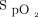 of 94% or more.
of 94% or more.
NIV arm: Subjects allocated to NIV arm were applied to NIV with either mask/helmet device connected to an ICU ventilator with the setting of pressure support (PS) of 10–20 cm H2O adjusted with the aim of obtaining an expired tidal volume of 7–10 mL per kilogram of predicted body weight and PEEP 5–10 cm H2O and 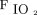 0.5–1.0 titrated to target
0.5–1.0 titrated to target 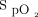 > 94%.
> 94%.
Other clinical management: Clinical management of all subjects including fluid therapy, monitoring of vitals, baseline blood investigations, chest radiograph, and point-of-care ultrasound was as per standard institute protocol. All subjects received supportive drug therapy as per current institutional protocol. Awake prone positioning was encouraged to subjects and allowed at the discretion of attending ICU physician.
Study Outcomes
The primary outcome was early intubation rate, proportion of subjects requiring invasive mechanical ventilation at 48 h of ICU admission. Invasive mechanical ventilation was considered as rescue therapy and considered as failure of HFNC or NIV. Invasive mechanical ventilation was initiated if  < 90% or frequency > 40 breaths/min for > 10 min in subjects already on HFNC/NIV or if they developed hemodynamic instability or deterioration of neurologic status. Secondary outcomes were late intubation rate (proportion of subjects requiring invasive mechanical ventilation at day 7 of ICU admission), early improvement in oxygenation (
< 90% or frequency > 40 breaths/min for > 10 min in subjects already on HFNC/NIV or if they developed hemodynamic instability or deterioration of neurologic status. Secondary outcomes were late intubation rate (proportion of subjects requiring invasive mechanical ventilation at day 7 of ICU admission), early improvement in oxygenation ( , frequency, and
, frequency, and 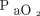 /
/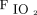 ratio in arterial blood gas at 2 h and 24 h), proportion of patients requiring awake prone positioning within 48 h, and in-hospital mortality. We also decided to report in-hospital intubation rate.
ratio in arterial blood gas at 2 h and 24 h), proportion of patients requiring awake prone positioning within 48 h, and in-hospital mortality. We also decided to report in-hospital intubation rate.
Statistical Analysis
Data analysis was carried out by using statistical software Stata 13.0 (StataCorp, College Station, Texas). Data were presented as median and interquartile range (IQR) for continuous variables and as absolute numbers or percentages for categorical variables. Unrelated data were compared by Mann-Whitney U test or chi-square test as applicable. Risk ratio and 95% CI were estimated by generalized linear modeling of binomial family. Correlated variables were compared by paired sample t test or Wilcoxon matched-pairs test. A 2-sided P value < .05 was considered as significant. Probability of death during hospital stay was evaluated by Kaplan-Meier survival analysis, and hazard ratio (HR) with 95% CI was reported. Baseline imbalance between the 2 study groups was adjusted individually by binary logistic regression model, and adjusted odds ratio for individual unbalanced parameters was reported.
Results
During the study period from August to December 2020, 391 COVID-positive patients were admitted in our ICU, out of which 145 patients were assessed for eligibility. Thirty six patients were ineligible for the study as they either met the criteria for intubation or declined to participate. Out of the 109 subjects who underwent randomization, 55 were assigned to the HFNC group and 54 to the NIV group. Subjects’ flow in this study is depicted in Figure 1.
Flow chart. HFNC = high-flow nasal cannula, NIV = noninvasive ventilation.
Baseline Characteristics
Baseline characteristics were similar in both the groups (Table 1), and median (IQR) 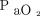 /
/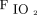 ratio at the time of randomization was 105.3 (92.0–139.3) and 111.2 (89.8–145.0) in the 2 groups, respectively, (P = .92). However, there was a statistically significant difference in the arterial pH between the 2 groups (7.42 [7.34–7.48] in the HFNC and 7.36 [7.28–7.43] in the NIV group [P = .005]). The number of patients with chronic kidney disease (CKD) was higher in the NIV group (P = .02).
ratio at the time of randomization was 105.3 (92.0–139.3) and 111.2 (89.8–145.0) in the 2 groups, respectively, (P = .92). However, there was a statistically significant difference in the arterial pH between the 2 groups (7.42 [7.34–7.48] in the HFNC and 7.36 [7.28–7.43] in the NIV group [P = .005]). The number of patients with chronic kidney disease (CKD) was higher in the NIV group (P = .02).
Characteristics of Subjects at Baseline
Outcome
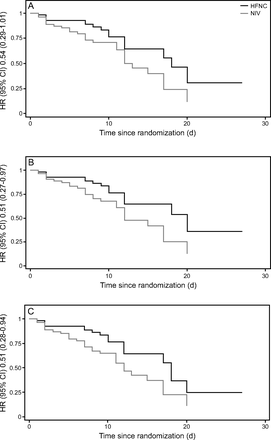
There was no statistically significant difference in intubation rate at 48 h between NIV (18 of 54 subjects) and HFNC (11 of 55 subjects) (relative risk 0.6, 95% CI 0.31–1.15, P = .12). However, at 7 d, intubation rate was lower in the HFNC group (15 of 55 subjects, 27.27%) compared to the NIV group (25 of 54 subjects, 46.29%) (relative risk 0.59, 95% CI 0.35–0.99, P = .045). Risk of late intubation remained lower with HFNC even after adjustment for incidence of CKD and arterial pH (adjusted odds ratio 0.40, 95% CI 0.17–0.93, P = .03). HFNC was associated with a reduced risk of intubation during the first 30 d of hospital stay, (HR 0.51, 95% CI 0.27–0.97, P = .04, Harrell C = 0.59, [Figure 2]).
Kaplan-Meier survival analysis showing A: death, B: intubation, and C: composite outcome (death and/or intubation) up to day 28 since randomization.
Various markers of oxygenation such as 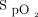 ,
, 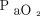 /
/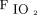 ratio, and breathing frequency at 2 h and 24 h did not show any significant difference between the 2 groups (Table 2). Hospital mortality was similar between HFNC (29.1%) and NIV (46.2%) group (relative risk 0.6, 95% CI 0.38–1.04, P = .06). Kaplan-Meier survival analysis revealed a possible reduction in probability of in-hospital mortality with HFNC (HR 0.54, 95% CI 0.29–1.01, P = .05, Harrell C = 0.60) and composite outcome of death or intubation (HR 0.51, 95% CI 0.28–0.94, P = .03, Harrell C = 0.60) during the first 30 d of hospital stay. All 3 survival plots are depicted in Figure 2.
ratio, and breathing frequency at 2 h and 24 h did not show any significant difference between the 2 groups (Table 2). Hospital mortality was similar between HFNC (29.1%) and NIV (46.2%) group (relative risk 0.6, 95% CI 0.38–1.04, P = .06). Kaplan-Meier survival analysis revealed a possible reduction in probability of in-hospital mortality with HFNC (HR 0.54, 95% CI 0.29–1.01, P = .05, Harrell C = 0.60) and composite outcome of death or intubation (HR 0.51, 95% CI 0.28–0.94, P = .03, Harrell C = 0.60) during the first 30 d of hospital stay. All 3 survival plots are depicted in Figure 2.
Primary and Secondary Outcomes
The proportion of subjects doing awake prone position could not be compared, as the data were unreliable. Subjects in the NIV group were noncompliant due to practical difficulty with NIV interface, whereas almost all subjects in HFNC group complied to awake prone session.
Discussion
In this single-center randomized controlled trial in 109 subjects with severe COVID-19 pneumonia, we observed that intubation rate at 48 h and early improvement in oxygenation status by 2h and 24 h were not significantly different between the subjects in either the HFNC or NIV group. We further observed a reduction in the intubation rate at day 7 and a trend toward reduced in-hospital mortality with the use of HFNC. However, the study was neither designed nor sufficiently powered to report a significant impact of HFNC on mortality.
In a systematic review in subjects with acute respiratory failure, which included 2 studies comparing HFNC versus NIV, Zhao et al10 found that HFNC did not affect the rate of intubation and early improvement in oxygenation status compared to NIV. The FLORALI trial5 showed that the intubation rate at day 28 did not significantly vary between HFNC and NIV in acute hypoxemic respiratory failure. However, a subgroup analysis of subjects with  /
/ ratio < 200 mm Hg demonstrated a significant reduction in the intubation rate in the HFNC group (33% vs 58%, P = .009).5 In a multicenter retrospective study conducted in France involving 379 subjects with COVID-19, Demoule et al11 observed a lower 28-d intubation rate in subjects with HFNC. The differences in results across the studies are possibly due to differences in the timing of the intubation defined and the severity of the hypoxia. In a multicenter observational study in subjects with COVID-19 in China, Duan et al12 found that time from initiation of NIV or HFNC to intubation was 8.4 d, whereas those who did not get intubated could be weaned off the noninvasive respiratory support within 7.1 d. These data along with results from the current study suggest HFNC does not lead to early improvement in oxygenation by 48 h but may reduce overall intubation rate by day 7. However, we chose early intubation by 48 h over intubation by day 7 or throughout the index admission, as we thought early identification of improvement/deterioration of subjects during the peaks of pandemic would be vital. Moreover, other factors like hospital-acquired pneumonia and sepsis may contribute to requirement of intubation subsequently.
ratio < 200 mm Hg demonstrated a significant reduction in the intubation rate in the HFNC group (33% vs 58%, P = .009).5 In a multicenter retrospective study conducted in France involving 379 subjects with COVID-19, Demoule et al11 observed a lower 28-d intubation rate in subjects with HFNC. The differences in results across the studies are possibly due to differences in the timing of the intubation defined and the severity of the hypoxia. In a multicenter observational study in subjects with COVID-19 in China, Duan et al12 found that time from initiation of NIV or HFNC to intubation was 8.4 d, whereas those who did not get intubated could be weaned off the noninvasive respiratory support within 7.1 d. These data along with results from the current study suggest HFNC does not lead to early improvement in oxygenation by 48 h but may reduce overall intubation rate by day 7. However, we chose early intubation by 48 h over intubation by day 7 or throughout the index admission, as we thought early identification of improvement/deterioration of subjects during the peaks of pandemic would be vital. Moreover, other factors like hospital-acquired pneumonia and sepsis may contribute to requirement of intubation subsequently.
The intubation rate observed in the current study is similar in HFNC group and higher in NIV group compared to previous published series in COVID-19 subjects by Franco et al.13 In the series by Demoule et al,11 intubation rate reported was much higher in both HFNC and NIV subjects. However, they reported intubation rate at d 28 compared to the current study, and it is not known how many of them were intubated late after the first week of admission. It is interesting to note that the subjects in the current study were more hypoxemic 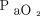 /
/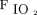 (ratio 105–111), reflecting a higher threshold for intubation. The baseline
(ratio 105–111), reflecting a higher threshold for intubation. The baseline 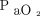 /
/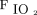 was 150–160 mm Hg in the FLORALI trial5 and 130 mm Hg in the study by Demoule et al.11 Although avoiding or delaying intubation in such hypoxemic subjects may be debatable, the encouraging results favor such approach in carefully selected subjects with COVID-19. This may be more relevant in the resource-poor countries or during peaks of pandemic even in resourceful countries when adequate experienced intensive care nursing staff is limited to cater for sick mechanically ventilated patients.14
was 150–160 mm Hg in the FLORALI trial5 and 130 mm Hg in the study by Demoule et al.11 Although avoiding or delaying intubation in such hypoxemic subjects may be debatable, the encouraging results favor such approach in carefully selected subjects with COVID-19. This may be more relevant in the resource-poor countries or during peaks of pandemic even in resourceful countries when adequate experienced intensive care nursing staff is limited to cater for sick mechanically ventilated patients.14
We observed a trend toward reduced mortality with the use of HFNC. In previous studies in subjects with COVID-19 by Demoule et al11 and Franco et al,13 no differences in mortality were observed with the use of HFNC over other oxygen therapy devices. However, both studies were observational and not aimed at finding mortality differences. The FLORALI investigators5 observed a higher mortality rate in NIV subjects compared to HFNC group, which was attributed to a possible increase in the ventilator-induced lung injury (VILI) in the NIV group due to excess of tidal volume being delivered. High transpulmonary pressure swings leading to increased tidal volume during spontaneous breathing in NIV are considered a major mechanism of patient self-inflicted lung injury (P-SILI) in COVID-19 pneumonia.15 In the current study, the role of possible VILI/P-SILI as the underlying cause of an increase in late intubation rate and trend toward increased in-hospital mortality in NIV group cannot be ruled out. Although NIV was initiated with PS 10–20 cm H2O, most of the subjects required PS ≤ 5 cm H2O, and tidal volume of 7–10 mL/kg was targeted over 6–8 mL/kg to account for leak around the mask. Subjects on NIV usually felt claustrophobic and frequently complained of dry mouth, leading to repeated detachments of the oxygenation interface. Moreover, most of the subjects on NIV were not compliant to awake prone positioning, as it was difficult with NIV interface, whereas most of the subjects on HFNC were compliant to prone position. Increased subject compliance and ease with which awake proning could be facilitated in HFNC group could have influenced the outcome in favor of the latter. However, it was not possible to present accurate data on the percentage of subjects who followed prone and daily duration of prone as even the subjects on HFNC frequently changed positions.
We understand that there are multiple limitations of this study. This is a single-center trial, and blinding of primary caregiver was not possible due to obvious reasons. We could not report the proportion of subjects performing awake prone sessions. Although all the subjects were encouraged for awake prone sessions, frequent self-changing of positions by subjects in HFNC group and noncompliance in NIV group did not allow proper data keeping. We calculated sample size on the basis of 30% reduction in endotracheal intubation rate, but it was not achieved; hence, our study was actually underpowered to detect such actual difference in the primary outcome. But the study has its own strengths. There was no participant dropout, and all subjects underwent full follow-up till discharge or death. Moreover, allocation concealment was sealed; outcome assessors were blinded, and predefined intubation criteria were followed. Although we observed difference in the prevalence of CKD and the arterial pH between the groups, it was likely due to chance, and we did not identify any other explanation such as breach of allocation concealment at randomization.
Conclusions
We were not able to demonstrate a statistically significant improvement of oxygenation parameters nor of the intubation rate at 48 h between NIV and HFNC. These findings should be further tested in a larger randomized, controlled trial.
Acknowledgments
We would like to acknowledge the contribution of all health care providers for their dedicated work in COVID ICU of the institute.
Footnotes
- Correspondence: Dalim Kumar Baidya MD EDIC, 5th Floor, Teaching Block, All India Institute of Medical Sciences, New Delhi-110029, India. E-mail: dalimkumar.ab8{at}gmail.com
The authors declare no conflicts of interest.
- Copyright © 2021 by Daedalus Enterprises