Abstract
BACKGROUND: Most ventilators measure airway occlusion pressure (occlusion P0.1) by occluding the breathing circuit; however, some ventilators can predict P0.1 for each breath without occlusion. Nevertheless, few studies have verified the accuracy of continuous P0.1 measurement. The aim of this study was to evaluate the accuracy of continuous P0.1 measurement compared with that of occlusion methods for various ventilators using a lung simulator.
METHODS: A total of 42 breathing patterns were validated using a lung simulator in combination with 7 different inspiratory muscular pressures and 3 different rise rates to simulate normal and obstructed lungs. PB980 and Dräger V500 ventilators were used to obtain occlusion P0.1 measurements. The occlusion maneuver was performed on the ventilator, and a corresponding reference P0.1 was recorded from the ASL5000 breathing simulator simultaneously. Hamilton-C6, Hamilton-G5, and Servo-U ventilators were used to obtain sustained P0.1 measurements (continuous P0.1). The reference P0.1 measured with the simulator was analyzed by using a Bland-Altman plot.
RESULTS: The 2 lung mechanical models capable of measuring occlusion P0.1 yielded values equivalent to reference P0.1 (bias and precision values were 0.51 and 1.06, respectively, for the Dräger V500, and were 0.54 and 0.91, respectively, for the PB980). Continuous P0.1 for the Hamilton-C6 was underestimated in both the normal and obstructive models (bias and precision values were –2.13 and 1.91, respectively), whereas continuous P0.1 for the Servo-U was underestimated only in the obstructive model (bias and precision values were –0.86 and 1.76, respectively). Continuous P0.1 for the Hamilton-G5 was mostly similar to but less accurate than occlusion P0.1 (bias and precision values were 1.62 and 2.06, respectively).
CONCLUSIONS: The accuracy of continuous P0.1 measurements varies based on the characteristics of the ventilator and should be interpreted by considering the characteristics of each system. Moreover, measurements obtained with an occluded circuit could be desirable for determining the true P0.1.
- mechanical ventilation
- P0.1; respiratory drive
- bench test
- lung simulator
- ventilator circuit
- airway occlusion pressure
- work of breathing
Introduction
Proper monitoring of respiratory effort is important for preventing patient self-inflicted lung injury. Although several parameters have been proposed, airway occlusion pressure at 100 ms (P0.1) is widely used for respiratory monitoring considering the ease of measurement when using a ventilator.1–3 P0.1 , which was first reported in 1975,4 is a quantified measure of the strength of the negative pressure generated in the first 100 ms after the initiation of inspiratory effort.3 P0.1 should be measured with inspiratory flow interruption after 100 ms of triggered inspiration. P0.1 is used to monitor the early inspiratory pressure drop and strongly reflects the influence of the inspiratory effort by the respiratory center. P0.1 reportedly has a particularly good relationship with the respiratory work load and is useful, even in the presence of respiratory muscle weakness.5,6 P0.1 has also been studied as an indicator of weaning, and high P0.1 has reportedly been associated with weaning failure.7,8 When considering pediatrics, P0.1 was related to extubation failure.9
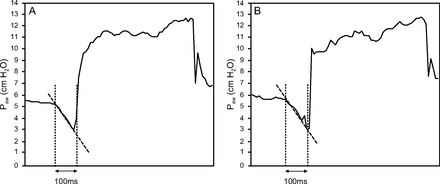
Although some ventilators can measure P0.1 at specified time intervals, evaluating P0.1 over time is challenging, owing to the need for bedside monitoring. Thus, a method for the continuous measurement of P0.1 was developed.10 For some ventilators, P0.1 can be continuously calculated based on the respiratory pressure waveform without requiring an expiratory hold (Fig. 1). This method makes it possible to measure P0.1 with every breath and is expected to possess the potential for mitigating lung injury or air hunger if clinical action should be undertaken based on a given P0.1 value or trend.11,12 Although few studies have attempted to verify the accuracy of continuous P0.1 measurement,13,14 its accuracy could be lower than P0.1 measurement with occlusion. However, few studies are available on the accuracy of continuous calculation of P0.1, which may vary based on the ventilators and methods used for calculation. In this study, we aimed to evaluate the accuracy of continuous P0.1 measurement compared with that of occlusion methods for various ventilator models when using a lung simulator.
Continuous P0.1 and occlusion P0.1 measurements. (A) Continuous P0.1: ventilation occurs at a time earlier than 100 ms. The slope of the decrease in airway pressure and the difference between the predicted pressure calculated at 100 ms and the airway pressure at the start of inspiration is considered as continuous P0.1. (B) Occlusion P0.1: ventilation does not occur until 100 ms. The difference between the airway pressure at 100 ms and the airway pressure at the start of inspiration is measured as occlusion P0.1. The vertical axis represents airway pressure; the horizontal axis represents time.
QUICK LOOK
Current Knowledge
Currently, airway-occlusion pressure 0.1 s after the start of inspiration against an occluded airway (P0.1), which is measured by occlusion, is needed to monitor inspiratory effort. Continuous P0.1 may be beneficial for monitoring because it can be measured without an occlusion procedure, although its accuracy has not been fully verified.
What This Paper Contributes to Our Knowledge
Continuous P0.1 measurements obtained by using a lung simulator exhibited accuracy differences based on the ventilators. Continuous P0.1 measurements also demonstrated differences in the accuracy for obstructive and normal lung patterns. Although the occlusion method is the most accurate technique to measure P0.1, when evaluating continuous P0.1, a degree of relevance exists, except for extreme values.
Methods
Study Design and Setting
This study was designed as a bench test to evaluate the accuracy of continuous P0.1 measurement. ASL5000 breathing simulator (software version 3.6) (IngMar Medical, Pittsburgh, Pennsylvania) was used to simulate spontaneous breathing. The study was conducted from February to November 2021 in the Division of Intensive Care, Department of Anesthesiology and Intensive Care Medicine at Jichi Medical University School of Medicine, Japan. Due to the nature of this study, the requirement for institutional review board approval was waived.
Ventilator Settings
The following ventilators were evaluated: Hamilton-G5 ventilator (Hamilton Medical AG, Rhäzüns, Switzerland), Hamilton-C6 ventilator (Hamilton Medical AG), Dräger V500 (Dräger, Lubeck, Germany), PB980 (Covidien, Carlsbad, California), and Servo-U (Getinge Group, Solna, Sweden). Assessments for each ventilator were performed in the pressure support mode by using the following parameters: PEEP of 5 cm H2O, pressure support levels of 5 cm H2O for the normal model and 10 cm H2O for the obstructive model, a pressure trigger of 1 cm H2O/min for the Hamilton-G5 ventilator and a flow inspiratory trigger of 2 L/min for the other ventilators, the fastest settings for inspiratory slope, and FIO2 of 0.21. This difference in the trigger mode was explained because the Hamilton-G5 could only measure P0.1 by using the pressure trigger.
The RT200 (Fisher & Paykel Healthcare, Auckland, New Zealand) was used as the ventilator circuit, which had a length of 150 cm. For heating and humidification, the MR850 system (Fisher & Paykel Healthcare) was used without filling the water by clamping the infusion line; the heater was switched off during the examination. When using the Hamilton-G5 ventilator, Hamilton-C6 ventilator, PB980, and Dräger V500, the original end-tidal carbon dioxide tension sensor attached to each ventilator was connected to the ventilator circuit to closely configure the running of the device in patient care for the purpose of dead space and circuit compliance.
Settings for the ASL5000
The lungs were evaluated in the obstructive and normal models with or without auto-PEEP. In the normal models, the resistance, compliance, and functional residual capacity were 10 cm H2O/L/s, 60 mL/cm H2O, and 0.5 L, respectively. Whereas, in the obstructive models, the inspiratory resistance, expiratory resistance, compliance, and functional residual capacity were 10 cm H2O/L/s, 30 cm H2O/L/s, 60 mL/cm H2O, and 0.8 L, respectively. The inspiratory hold was 0%, pause was 0%, and inspiratory release time was 20%. Seven levels of inspiratory muscular pressure, three patterns of inspiratory increase (%), which means that rise times and the breathing frequency were assessed by using a sinusoidal wave pattern, as shown in Tables 1 and 2. Expiratory muscular pressure was 0 cm H2O, whereas the expiratory rise time, expiratory hold, and expiratory release time were set to 0%.
Settings for the ASL5000: Normal Model
P0.1 Measurement
We evaluated 2 different P0.1 measurements: ventilator P0.1 and reference P0.1. The ventilator P0.1 was defined as the P0.1 displayed on each ventilator. Five ventilator P0.1 values were recorded, and the mean ventilator P0.1 value was calculated. For the Dräger V500 and PB980, the ventilator P0.1 was measured by using the occlusion method (occlusion P0.1). For the Servo-U, Hamilton-G5, and Hamilton-C6 ventilators, the ventilator P0.1 was recorded breath by breath without performing an occlusion maneuver (continuous P0.1). Hamilton-G5 and Hamilton-C6 ventilators measure continuous P0.1 breath by breath, whereas Servo-U displays an average of 8 breaths over time. The reference P0.1 was measured during end-expiratory occlusion as the drop in airway pressure until 100 ms on the ASL5000. Because the ASL5000 displays P0.1 as the actual pressure at 100 ms, the reference P0.1 was calculated to display P0.1 minus PEEP just before the start of inspiration. For the Servo-U, Hamilton-G5, and Hamilton-C6 ventilators, 5 end-expiratory occlusion maneuvers were performed to measure the mean reference P0.1 after the ventilator P0.1 measurement. After recording the data, the reference P0.1 was calculated in an offline analysis using the ASL5000 software. The ventilator P0.1 and reference P0.1 are expressed as positive values.
Statistical Analysis
All the statistical analyses were performed by using JMP 16 Pro (SAS Institute Inc., Cary, North Carolina). Data are presented as mean ± SD or as percentage. P values of <.05 were considered indicative of statistical significance. The relationships between the ventilator P0.1 and the reference P0.1 measurements were evaluated by using Bland-Altman plots. Bias was calculated as the difference between the measured ventilator P0.1 and reference P0.1 values. Precision was determined from the SD of the calculated bias. The bias and precision of the differences between the measured ventilator P0.1 and reference P0.1 values, 95% CIs, upper and lower limits of agreement, root mean square errors, and mean values of ventilator P0.1 were also calculated.
Results
Mean Values of Ventilator P0.1 and Reference P0.1
A total of 18 patterns were analyzed across the 5 ventilators. Three patterns in the obstructive model were excluded, owing to ineffective efforts by the Hamilton-G5 (phases 1, 8, and 15). Mean occlusion P0.1 and reference P0.1 were 4.3 ± 3.1 cm H2O and 3.8 ± 2.7 cm H2O, respectively, for the Dräger V500, and were 4.2 ± 2.9 cm H2O and 3.7 ± 2.4 cm H2O, respectively, for the PB980. Mean continuous P0.1 and reference P0.1 were 5.5 ± 3.9 cm H2O and 3.9 ± 2.6 cm H2O, respectively, for the Hamilton-G5, and were 1.3 ± 1 cm H2O and 3.4 ± 2.8 cm H2O, respectively, for the Hamilton-C6, and were 2.9 ± 2.3 cm H2O and 3.8 ± 2.6 cm H2O, respectively, for the Servo-U (Table 2).
Settings for the ASL5000: Obstructive Model
Correlation Between Ventilator P0.1 and Reference P0.1
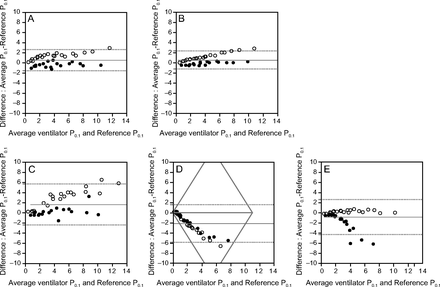
Bland-Altman analysis was performed to evaluate the correlation between ventilator P0.1 and reference P0.1, as shown in Figure 2 and Table 3. The bias and the precision values were 0.51 (95% CI 0.16–0.85) and 1.06, respectively, for the Dräger V500; 0.54 (95% CI 0.24–0.83) and 0.91, respectively, for the PB980; 1.62 (95% CI 0.95–2.28) and 2.06 for the Hamilton-G5; –2.13 (95% CI –2.74 to –1.51) and 1.91, respectively, for the Hamilton-C6; and –0.86 (95% CI –1.43 to –0.29) and 1.76, respectively, for the Servo-U. The root mean square error was 0.75 for Dräger V500, 0.64 for PB980, 1.45 for Hamilton-G5, 1.35 for Hamilton-C6, and 1.25 for Servo-U.
Bland-Altman plot comparing ventilator P0.1 and reference P0.1. The horizontal axis represents (ventilator P0.1 + reference P0.1)/2. The vertical axis represents ventilator P0.1 minus reference P0.1. The middle horizontal red line represents bias. The upper horizontal red dotted line represents the upper limits of agreement, and the lower horizontal red dotted line represents the lower limits of agreement. Black dots represent P0.1 in the obstructive model. White dots represent P0.1 in the normal model. A: Drager V500. B: PB980. C: Hamilton-G5. D: Hamilton-C6. E: Servo-U.
Accuracy of P0.1 Among the Ventilators
Discussion
The aim of the present study was to evaluate the accuracy of continuous P0.1 measurement compared with that of the occlusion methods for various ventilator models using a lung simulator. The current findings indicated that continuous P0.1 measurements were rather inaccurate when compared with those obtained when using the occlusion method, even for ventilators produced by the same manufacturer. The P0.1 calculated for the Hamilton-C6 ventilator was lower than the reference value, whereas that for the Hamilton-G5 ventilator tended to be equal to or slightly higher than the reference value. These findings highlight the need to account for these characteristics when using each ventilator for respiratory assessments.
An explanation for the low P0.1 measurements for the Hamilton-C6 ventilator could be the difference in constant flow. Constant flow refers to flow on the ventilator side rather than that at the flow sensor, which basically does not affect the patient unless the patient breathes. The Hamilton Medical ventilators used in the current study have a flow sensor at the Y-piece connector, which is more sensitive to triggers than those for other ventilators. However, when considering the steady flow of the Hamilton-C6 ventilator, once air is drawn in via inspiration, a pressure drop may not be recognized owing to the immediate correction due to its constant flow. Thus, when assessing respiratory drive when using the Hamilton-C6 ventilator, monitoring the change in esophageal pressure could be beneficial.
P0.1 measurements for the Hamilton-G5 ventilator were highly accurate in the obstructive model but tended to be overestimated in the normal model. The Hamilton-G5 ventilator calculates P0.1 only for the pressure trigger and not for the flow trigger. The Hamilton-G5 ventilator differs from the Hamilton-C6 ventilator because it requires compressed air. The advantage of the Hamilton-G5 ventilator, similar to the Hamilton-C6 ventilator, is its ability to monitor intra-esophageal pressure. Although a P0.1 < 4.0 is recommended to implement lung and diaphragm-protective mechanical ventilation,1 a ventilatory management strategy that monitors intra-esophageal pressure when P0.1 exceeds the normal value could be useful. In the normal model for the Servo-U ventilator, the values were generally equivalent to the reference P0.1. However, the values tended to be underestimated with obstructive models, which may be attributed to auto-PEEP. This result is similar to that reported in previous studies.14 The P0.1 values for the Dräger V500 and PB980 ventilators, measured by using the occlusion method, were nearly equal to the reference P0.1. Because the same results were obtained in previous studies,13,14 we believe that the occlusion method is preferable for ensuring reliable P0.1 measurements.
The results of our study indicate that continuous P0.1 is not perfectly accurate. However, the method itself is ideal. It is impossible to follow measurement continuity because, for measurement when using the occlusion method, the medical staff should operate at the bedside and only one point of respiration should be evaluated. Thus, despite its accuracy concerns, continuous P0.1 could be a potentially useful monitoring tool for inspiratory effort when the medical staff is unable to frequently visit the bedside, such as in the case of a patient with COVID-19. In addition, patient changes can be detected by evaluating the values over time. Except for extreme cases, the clinical use of this method is expected to improve the quality of medical care. However, the advanced algorithms of ventilators could make the continuous P0.1 evaluation method more challenging. Recently, new methods have been proposed to elicit the timing of patient inspiration and expiration more acutely based on the changes in the flow waveform.15 Further validation is warranted because the algorithm that currently displays continuous P0.1 is unable to match such techniques.
A meta-analysis reported in 2021 proposed the usefulness of P0.1 in predicting successful weaning; however, it also suggested that P0.1 is highly heterogeneous.16 This could be attributed to the accuracy of P0.1 measurement varying based on the ventilator, as demonstrated in the present study. The P0.1 value may also be affected by the pressure support and rise time, especially in patients with high airway resistance. Based on these concerns, it is preferable to validate the results in a clinical trial in the future.
This study had some limitations. First, this study was designed as a bench test and did not include subjects. Although the lung simulator was used to simulate the inspiratory effort of real patients by using a sinusoidal wave pattern, it remains unclear whether the same results can be obtained in clinical practice. Second, only 5 ventilators were evaluated in this study. Third, we did not humidify the circuit, and we did not reproduce water droplets in the circuit, which could cause vibrations that affect measurements. Fourth, we did not evaluate P0.1 with the end-tidal carbon dioxide sensor in the Servo-U because it was unavailable at our hospital. Fifth, whether the difference in the trigger mode, including a new trigger mode and the presence or absence of automatic tube compensation with or without nebulizers or heat and moisture exchangers, affects continuous P0.1, which we were unable to evaluate, is unclear in our study. Although the method of continuous P0.1 measurement is the same for the Hamilton-G5 and Hamilton-C6 ventilators, further verification is warranted because of the different characteristics of the ventilators, for example, constant flow.
Conclusions
The current results indicate that continuous P0.1 measurements tend to vary based on the constant flow and ventilators. Currently, the occlusion method is the most accurate P0.1 measurement method; hence, it should be the method of choice for precise evaluation. However, when evaluating continuous P0.1, a degree of relevance exists, except for extreme values. Based on the type of ventilator used, the characteristics of continuous P0.1 should be established for clinical use.
Acknowledgments
We thank Iwaki & Co, Tokyo, Japan, for assistance.
Footnotes
- Correspondence: Shinshu Katayama MD PhD, Division of Intensive Care, Department of Anesthesiology and Intensive Care Medicine, Jichi Medical University School of Medicine, 3311–1, Yakushiji, Shimotsuke, Tochigi 329–0498, Japan. E-mail: shinsyu_k{at}jichi.ac.jp
The study was performed at Jichi Medical University School of Medicine, Shimotsuke, Tochigi, Japan.
This study was funded by using internal department funds.
Dr Katayama has disclosed a relationship with Hamilton Medical. The other authors have disclosed no conflicts of interest.
A version of this paper was presented by Dr Katayama at the 44th Congress of the Japanese Society of Respiratory Care Medicine, held August 7, 2022, in Yokohama, Japan.
- Copyright © 2023 by Daedalus Enterprises