Abstract
With a rising incidence of obesity in the United States, anesthesiologists are faced with a larger volume of obese patients coming to the operating room as well as obese patients with ever-larger body mass indices (BMIs). While there are many cardiovascular and endocrine issues that clinicians must take into account when caring for the obese patient, one of the most prominent concerns of the anesthesiologist in the perioperative setting should be the status of the lung. Because the pathophysiology of reduced lung volumes in the obese patient differs from that of the ARDS patient, the best approach to keeping the obese patient's lung open and adequately ventilated during mechanical ventilation is unique. Although strong evidence and research are lacking regarding how to best ventilate the obese surgical patient, we aim with this review to provide an assessment of the small amount of research that has been conducted and the pathophysiology we believe influences the apparent results. We will provide a basic overview of the anatomy and pathophysiology of the obese respiratory system and review studies concerning pre-, intra-, and postoperative respiratory care. Our focus in this review centers on the best approach to keeping the lung recruited through the prevention of compression atelectasis and the maintaining of physiological lung volumes. We recommend the use of PEEP via noninvasive ventilation (NIV) before induction and endotracheal intubation, the use of both PEEP and periodic recruitment maneuvers during mechanical ventilation, and the use of PEEP via NIV after extubation. It is our hope that by studying the underlying mechanisms that make ventilating obese patients so difficult, future research can be better tailored to address this increasingly important challenge to the field of anesthesia.
Introduction
Providing mechanical ventilation to severely obese patients during surgery can involve technically complex challenges, including maintaining a patent airway, properly ventilating the lungs, and successfully liberating the patient from the ventilator. Our review focuses on this second challenge, properly ventilating the lungs of the obese patient during surgery, as well as the pre- and postoperative use of noninvasive ventilation (NIV) in the obese patient. Obesity poses a major stress upon the respiratory system in the form of thoracic and abdominal fat. The crushing weight of adipose tissue reduces lung volumes when the obese patient is sedated and paralyzed and, without adequate pressure to oppose this force, leads to a decrease in lung compliance and difficulty in adequately maintaining gas exchange. In the following sections, we will further describe the physiological and anatomical changes of the respiratory system in obese patients; discuss the current research on the respiratory management of obese patients in perioperative settings (ie, during induction, intraoperatively, and from the time of extubation through the postanesthesia care unit discharge); and provide our evaluation of this research and establish guidelines for the perioperative management of the obese patient.
Obesity: Epidemiology and Morbidity
The World Health Organization defines obesity as a body mass index (BMI) > 30 kg/m2, with class I obesity BMI being between 30 and 34.99 kg/m2, class II obesity BMI between 35 and 39.99 kg/m2, and class III obesity BMI > 40 kg/m2.1 The National Institutes of Health defines morbid obesity, or “clinically severe obesity,” as a BMI > 40 kg/m2, or > 35 kg/m2 with comorbidities, such as coronary heart disease, other atherosclerotic diseases, type 2 diabetes, and sleep apnea.2 Obesity is a major public health problem in the United States. The prevalence of obesity in the United States is 34.9% in adults, 8.1% in infants and toddlers, and 16.9% in children 2–19 y of age.3 Although the adverse comorbidities commonly associated with obesity are well known, the predictive value of BMI for outcomes in surgical or critically ill patients is not well understood, with some arguing for a paradoxical “protective” effect.4 Shearer pointed out that BMI might be a much less useful predictor of outcome than measurements of waist circumference, a potential surrogate for quantifying central or abdominal obesity.5 Recently, Schumann et al6 conducted a prospective statistical survey of a large clinical registry of bariatric surgery outcomes and found that both metabolic syndrome and increased BMI were significantly associated with postoperative pulmonary complications. Such complications included any one or more of the following: pneumonia, atelectasis, pleural effusion, pneumothorax, ARDS, and respiratory failure.6
Physiological Characteristics of the Respiratory System During Spontaneous Breathing in Morbidly Obese Patients
Central obesity represents a significant problem for the respiratory system, causing a number of physiological changes. During spontaneous breathing, obese individuals have reduced lung volumes, especially functional residual capacity and expiratory reserve volume.7,8 The massive load of the obese abdomen and the distribution of adipose tissue in the thoracic region reduce lung volume and impair the stability of the airways.9,10 The mass of the abdomen against the diaphragm also hinders the normal range of diaphragmatic excursion. Obese patients exhibit significantly more atelectasis during spontaneous breathing than nonobese patients do,11 although this might be overlooked outside of the context of surgery.
When the alveoli and airways are exposed to these forces, small airways collapse. When the small airways collapse, air is trapped, preventing normal exhalation. This expiratory flow limitation leads to dynamic hyperinflation.12 Dynamic hyperinflation is caused by lung regions that are unable to deflate to normal volumes, because of the obstructed flow caused by small airways collapse. This differs from true hyperinflation like that seen in emphysema where expiratory flow limitation leads to higher than physiological lung volumes. In the obese patient, it is the reduction in physiological lung volumes that leads to this “dynamic” hyperinflation. That is, the force of body mass collapsing lung tissue also collapses airways, resulting in air trapping and auto-PEEP, but not generalized hyperinflation. Auto-PEEP is defined as the end-expiratory elastic recoil pressure caused by incomplete expiration in the presence of expiratory flow limitation.13,14 Expiratory flow limitation worsens the elastic properties of the lung due to heterogeneous ventilation of lung units.15 Expiratory flow limitation also leads to an increased work of breathing,16 because every breath requires additional force to overcome the auto-PEEP, open small airways, and generate air flow.
Morbid or severely obese patients can suffer from obesity-hypoventilation syndrome, formerly referred to as Pickwickian syndrome, which is defined by a combination of BMI > 30 kg/m2 and awake hypercapnia without other diagnosed causes of hypoventilation.17 Obstructive sleep apnea (OSA) is another common breathing disorder among the obese that, in addition to obesity-hypoventilation syndrome, must be taken into account before surgery.18,19
Physiological Characteristics of the Respiratory System During Mechanical Ventilation in Morbidly Obese Patients
During anesthesia and mechanical ventilation, atelectasis can develop into a significant problem for obese patients. Expiratory flow limitation in mechanically ventilated obese patients can often be resolved relatively easily and thus is often not a major clinical concern.11,20
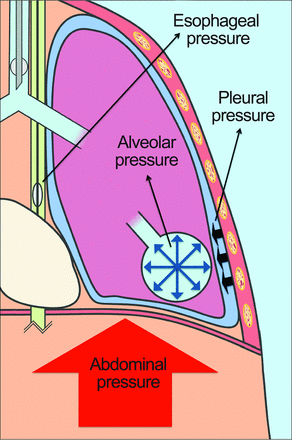
The major contributing factors to pulmonary atelectasis are paralysis, sedation, and supine positioning.20,21 The functional residual capacity of anesthetized patients decreases in part due to a decrease in respiratory muscle tone, resulting in a new lung-chest wall equilibrium at a lower overall volume of the respiratory system.22 There is also a cranial displacement of the diaphragm that occurs during general anesthesia and in the supine position that contributes to atelectasis formation especially in obese patients, because the abdominal content that is displaced is of tremendous weight (Fig. 1).20
In supine obese people, the weight of the abdomen pushes against the diaphragm, causing a cranial displacement of the muscle. This increased pressure inside the pleural cavity causes atelectasis and hypoxemia, worsening the elastic properties of the respiratory system (elastance – cm H2O of pressure applied to the airways to change the volume of the respiratory system by 1 L). The reduction of aerated lung tissue at end-expiration reduces the functional residual capacity. The application of adequate levels of PEEP can prevent lung collapse. However, inadequate PEEP results in cyclic opening and closing of dependent alveoli, leading to ventilation-induced lung damage.
Also, during paralysis, the muscle tone of the diaphragm is lost, because abdominal pressure is transmitted mostly to the gravity-dependent region of the lung, and the nondependent regions of the lungs are preferentially ventilated, leading to ventilation-perfusion mismatch. This occurs even with applied PEEP.23 Thus, in obese patients, the duration of paralysis should be limited as much as possible, given the extra strain on the diaphragm caused by a large abdominal mass.
Prevention of atelectasis both during anesthesia and immediately after surgery is imperative for the integrity of the lung. Atelectasis impairs gas exchange and increases physiological shunt, ventilation-perfusion mismatch, and work of breathing.24 Lung mechanics are also impaired in the atelectatic lung, because the lung is less compliant at lower volumes.22
In normal-weight anesthetized subjects, Rothen et al25 showed by chest CT imaging that atelectasis is enhanced by the use of 1.0 FIO2 during preoperative induction compared with the use of 0.3 FIO2 as a result of absorption atelectasis. Lower oxygen concentrations might reduce the formation of atelectasis but also shorten the safe apnea period during intubation.26 As a result, we recommend that preinduction oxygenation be performed with 100% oxygen but also with the application of 10 cm H2O CPAP to prevent atelectasis and to prolong the safe apnea interval.
Body Positioning: Supine, Sitting, and Prone Positions
In the supine obese patient, the cranial displacement of the diaphragm and the gravitational effect of the abdominal contents upon the diaphragm and the thoracic cavity reduce lung volumes.12,27,28 The use of paralytics during general anesthesia and the subsequent decrease in diaphragmatic muscle tone further enhances the atelectasis induced by the abdominal contents.
Valenza et al29 found that morbidly obese subjects (BMI = 42 ± 5 kg/m2) undergoing laparoscopic gastric banding have a lower lung elastance and a greater end-expiratory lung volume (EELV) when in the beach chair position compared with the supine position. Lemyze et al28 observed in critically ill mechanically ventilated obese subjects (BMI = 48.4 [95% CI 45–51.2] kg/m2) that the sitting position can significantly reverse EFL and leads to a significant drop in auto-PEEP compared with supine positioning. Dixon et al30 observed that obese subjects preoxygenated in a 25° head-up position (BMI = 44.9 kg/m2) took longer to desaturate to 92% than subjects preoxygenated in the supine position (BMI = 47.3 kg/m2). The described positioning techniques might be difficult to implement in the operating room but nonetheless have important physiological implications. The positioning techniques used by Dixon et al30 create gravitational conditions – with respect to the abdominal contents, the mediastinal contents, and the fat of the thoracic region – that are less favorable to the formation of atelectasis. In a study by Pelosi et, al31 it was found that prone positioning compared with supine positioning leads to an increase in functional residual capacity, an increase in lung compliance, and improved oxygenation in mechanically ventilated obese subjects undergoing elective surgery in the prone position (BMI = 34.6 ± 4.8 kg/m2). This increase in lung volume observed in the prone position might be most indicative of the influence upon the lungs of the mediastinal contents during supine positioning. The improvement in oxygenation is also a result of a more uniform distribution of pulmonary perfusion in the prone position that has been seen in studies with healthy nonobese volunteers.32,33
PEEP
PEEP can be applied during all modes of ventilation to prevent lung collapse. With applied positive pressure to the airways during the lowest pressure point of the tidal breathing cycle, airways and alveoli are kept open. However, PEEP needs to be individualized for each patient. Five cm H2O PEEP in a patient with 2 cm H2O end-expiratory pleural pressure should provide sufficient end-expiratory pressure to maintain alveolar patency and a positive end-expiratory pleural pressure. However, 5 cm H2O PEEP in a patient with 15 cm H2O end-expiratory transpulmonary pressure will be grossly insufficient to prevent alveolar collapse and atelectasis at end-expiration. Alternatively, applying too high a level of PEEP could potentially overdistend the lung. In short, appropriate selection of PEEP can stabilize lung volume in supine, sedated, and paralyzed mechanically ventilated patients.
It has been shown that the application of PEEP in supine mechanically ventilated morbidly obese patients is beneficial for reversing EFL and auto-PEEP. Lemyze et al28 found in obese subjects that applying PEEP set at the level of auto-PEEP significantly reduces auto-PEEP and EFL. In a study by Koutsoukou et al34 it was also found that the application of PEEP abolishes EFL, decreases auto-PEEP, and improves EELV in obese subjects. Koutsoukou et al34 claim that this increase in lung volume can be attributed to alveolar recruitment. Although it is certainly possible that some alveoli are recruited by the application of PEEP alone, it is worth noting that in this study, oxygenation did not improve with applied PEEP. This lack of improved oxygenation could suggest that the atelectatic alveoli were not recruited and that the increase in lung volume was primarily in nonatelectatic areas, thus overdistending previously aerated parts of the lung.
Futier et al35 also conducted a study in which PEEP alone, without recruitment maneuvers, was applied in mechanically ventilated obese and nonobese subjects (obese: BMI = 45 ± 9 kg/m2; nonobese: BMI = 24 ± 3 kg/m2). The authors found that the application of 5 cm H2O and then 10 cm H2O of PEEP in obese patients improved EELV, but not oxygenation. The authors argue, however, that because both EELV and compliance were increased and because there was no clinically important change in dead-space fraction with PEEP, nonatelectatic portions of the lung were not overdistended and that recruitment of atelectatic lung occurred. We agree with the authors that compliance must increase with an increase in EELV to claim that recruitment of alveoli is occurring. However, we nonetheless believe that without a measured improvement in oxygenation, significant ambiguity exists regarding whether clinically important recruitment of atelectatic alveoli has occurred. What is much less ambiguous, however, is that recruitment maneuvers used in conjunction with appropriately applied PEEP have the potential to both recruit atelectatic portions of the lung and keep them open.
Recruitment Maneuvers
Atelectasis is not a homogenous condition. There are both atelectatic portions of lung and open, nonatelectatic portions of lung. Thus, a recruitment maneuver is a necessary intervention for patients with pulmonary atelectasis, because it in theory rehomogenizes lung tissue. A recruitment maneuver is the temporary application of an end-expiratory pressure that is significantly greater than pleural pressure. The driving pressure is typically delivered over several seconds to allow for the opening of so-called “slow-fill” alveolar units. The applied pressure gradient needs to be high enough to expand collapsed alveoli that have opening pressures higher than the normal ventilating peak pressures. It is the peak end-inspiratory pressure, not the PEEP, that recruits atelectatic alveoli. The 2 major variables to consider when performing recruitment maneuvers are (1) the level of pressure applied and (2) the time over which such a pressure is applied. Current evidence suggests that these can include an increase from 0 cm H2O PEEP to around 40 cm H2O PEEP during CPAP held for 15–40 s36 to more gradual, stepwise increments of PEEP during pressure control-continuous mandatory ventilation (PC-CMV).37
A useful bedside technique to determine whether the lung is prone to collapse is the measurement of transpulmonary pressure. Transpulmonary pressure is a measurement of the difference between alveolar pressure and pleural pressure. Esophageal manometry is one technique used during mechanical ventilation as a surrogate for measuring pleural pressure. In obese patients, it is especially important to understand the degree of pressure the chest wall exerts on the lungs in the form of pleural pressure. Measuring esophageal pressure as a surrogate for pleural pressure in the obese patient is useful for determining the level of PEEP needed to reach a positive transpulmonary pressure. If transpulmonary pressure falls below atmospheric pressure at end exhalation, lung units are at risk for collapse. With repeated cycles of alveolar collapse and reopening, shearing injury can result in ventilator-induced lung injury.38,39
At the same time, by using esophageal manometry, end-inspiratory transpulmonary pressure can be investigated in comparison with plateau pressure. In the obese patient, it is unclear whether certain plateau pressures are actually injurious. The measurement of transpulmonary pressure is better able to tell us that a seemingly high plateau pressure corresponds to a noninjurious transpulmonary pressure. But because no definitive studies have been performed on this topic, we recommend maintaining plateau pressures <28 cm H2O in all patients.39
First, it should be noted that there are 2 major categories of interventions in studies investigating intraoperative recruitment maneuvers in mechanically ventilated obese patients: recruitment maneuvers followed by no PEEP and recruitment maneuvers followed by PEEP. None of the studies in Table 1 show a significant improvement caused by recruitment maneuvers performed without subsequent applied PEEP.36,40,41 Although a recruitment maneuver without subsequent applied PEEP will temporarily recruit atelectatic portions of the obese lung, the subsequent return to zero end-expiratory pressure (or any inadequate PEEP level) results in a return to applied end-expiratory pressure that is lower than the closing pressures of the recruited alveoli, leading to the reformation of atelectasis, or “de-recruitment.” The results of these studies36,40,41 suggest that there is a need in obese patients to maintain a positive end-expiratory transpulmonary pressure after a recruitment maneuver by the application of adequate PEEP. The high peak airway pressures resulting from the high levels of PEEP used during a recruitment maneuver open alveoli, but adequate PEEP must be applied following recruitment so that the alveoli stay open.
Studies Using Intraoperative Recruitment Maneuvers
One of the best studies on the use of recruitment maneuvers and PEEP in mechanically ventilated obese subjects was conducted in 2009 by Reinius et al40 In this physiological study, morbidly obese subjects (45 ± 4 kg/m2) were randomized to receive PEEP of 10 cm H2O; a recruitment maneuver and subsequent zero end-expiratory pressure; or a recruitment maneuver and subsequent PEEP of 10 cm H2O. The recruitment maneuver consisted of a 10-s inspiratory hold of 55 cm H2O on PC-CMV. CT scans were performed in 30 study participants (1) before induction, (2) 5 min after induction and intubation, (3) 5 min after and (4) 20 min after the study procedure (ie, a recruitment maneuver or the start of PEEP). A significant increase in atelectasis was seen after the induction of anesthesia compared with baseline. The authors found that the beneficial effects of the recruitment maneuver (eg, reduced atelectasis and improved oxygenation) were only sustained in the group that received PEEP after recruitment. In the group that returned to zero end-expiratory pressure after recruitment, atelectasis reappeared within 20 min after the recruitment maneuver. Also, the group that received PEEP alone saw no improvements in atelectasis or oxygenation, differing from the conclusion made by Futier et al35 that PEEP alone can reverse atelectasis.
The role of pneumoperitoneum in the results of the studies in Table 1 should also be noted. In a study by Almarakbi et al,36 60 subjects receiving laparoscopic banding were randomized into 4 groups: PEEP of 10 cm H2O (Group P), recruitment maneuver consisting of inspiratory pressure of 40 cm H2O for 15 s once (Group R), Group R recruitment followed by PEEP 10 cm H2O (Group RP), or Group RP recruitment/PEEP procedure but with the recruitment maneuver repeated every 10 min (Group RRP). Each group underwent its respective procedure after the induction of pneumoperitneum. The average BMI of the subjects in these 4 groups was relatively low (Group P: 33 ± 2 kg/m2; Group R: 33 ± 1 kg/m2; Group RP: 34 ± 1 kg/m2; Group RRP: 33 ± 1 kg/m2) compared with other similar studies (in fact, many of the studies in Table 1 have a BMI requirement of at least 35 kg/m242,43 or 40 kg/m2.37,40,44) The increased intraabdominal pressure caused by pneumoperitoneum, and not solely the BMI of the subjects in the Almarakbi et al36 study, potentially contributed to the need for recruitment maneuvers and PEEP to optimally improve lung function, because an increase in intra-abdominal pressure leads to a decrease in lung volume and an increased need for PEEP.45 To further support this possibility that pneumoperitoneum contributes significantly to pleural pressure, Futier et al42 show that a recruitment maneuver followed by PEEP improves EELV, respiratory mechanics, and oxygenation in both obese and healthy weight individuals during surgery involving pneumoperitoneum.
More recently, Defresne et al43 studied morbidly obese subjects undergoing laparoscopic gastric bypass surgery. The control group (n = 25; BMI 40.9 (95% CI, 35–50) kg/m2) received volume control-continuous mandatory ventilation (VC-CMV) with 10 cm H2O of PEEP and 6 mL/kg predicted body weight of VT during surgery. The study group (n = 25; BMI 41.3 (95% CI, 36–46) kg/m2) received VC-CMV with 10 cm H2O of PEEP, 6 mL/kg predicted body weight of VT, and 2 recruitment maneuvers: one after the induction of pneumoperitoneum and another after exsufflation. The recruitment maneuver consisted of a 40-s inspiratory hold at 40 cm H2O CPAP. The investigators recorded functional residual capacity, FVC, FEV1, mean SpO2, percentage of time spent with SpO2 < 90%,and apnea-hypopnea index both during the preoperative assessment and on surgical day 1. They found a statistically similar, small decrease in functional residual capacity in both groups pre- to postsurgery. There were no significant changes in other spirometric data or differences in other spirometric data between the 2 groups.43 The results of this study run contrary to the findings of Futier et al42 in which morbidly obese subjects (study: BMI 46 ± 9 kg/m2; control: BMI 45 ± 5 kg/m2) and healthy weight individuals either received 10 cm H2O of PEEP 10 min after pneumoperitoneum until the end of surgery or the same protocol with a recruitment maneuver (CPAP of 40 cm H2O for 40 s) before the start of PEEP. Subjects in the recruitment maneuver plus PEEP group, both obese and nonobese, had improved EELV, with the obese subcategory having a significantly greater improvement in EELV than the nonobese group had.
What differs between the Defresne et al43 study and the Futier et al42 study is the timing of recruitment maneuvers with respect to measurements of lung function. In the Futier et al42 study, EELV was measured during surgery and at the very end of surgery, in conjunction with the interventions performed, whereas Defresne et al43 did not measure the effects of the intraoperative interventions interoperatively. It is equally possible that the study group de-recruited after the cessation of PEEP or that the control group regained near-normal lung volumes once extubated and spontaneously breathing.
A multi-center clinical trial is currently ongoing – the PROBESE trial (ClinicalTrials.gov identifier NCT02148692) – investigating the effects of intraoperative high PEEP (≥12 cm H2O) with recruitment maneuvers versus low PEEP (4 cm H2O) without recruitment maneuvers in surgical obese patients.
Recently, Pirrone et al46 conducted a study in which mechanically ventilated morbidly obese ICU subjects (BMI 50.7 ± 16.0 kg/m2) underwent recruitment maneuvers and PEEP titration using both esophageal manometry and a best decremental PEEP trial. Both methods for PEEP titration were used in each study subject. The authors found that both techniques identified comparable optimal PEEP levels (20.7 ± 4.0 vs 21.3 ± 3.8 cm H2O, P = .40, manometry vs best decremental). These optimal PEEP levels were higher than the average PEEP levels set in the study subjects by their clinicians in the ICU (11.6 ± 2.9 cm H2O). The higher PEEP levels were associated with increased EELV, oxygenation, and decreased lung elastance. These results suggest that in severely obese patients, the use of esophageal manometry along with lung recruitment or a decremental PEEP trial after lung recruitment without esophageal manometry can both identify optimal PEEP.
Strategies to Keep the Lung Open Peri-Induction
The application of PEEP before induction and after intubation is an important method for preventing atelectasis. The presence of reduced lung volumes in the obese could be an important factor in reduced safe apnea time that clinicians encounter with these patients. Additionally, absorption atelectasis, secondary to the high concentration of oxygen applied during induction, is another cause of atelectatsis. Although maintaining a safe apnea period is imperative for intubation, especially during what can be difficult intubations in obese patients, lowering the FIO2 during preoxygenation is an approach some investigators have pursued to prevent absorption atelectasis.25 However, given the evidence that recruitment maneuvers and PEEP can effectively recruit atelectatic lung during surgery, we cannot recommend that clinicians take the risks associated with a lower FIO2 during induction, especially in obese patients, simply to prevent reversible atelectasis.
In 2004, Coussa et al47 found that using 10 cm H2O CPAP in morbidly obese subjects before induction of anesthesia and 10 cm H2O PEEP immediately after intubation led to reduced atelectasis compared with the simple administration of oxygen. This study addressed the problem of atelectasis at its onset, rather than after a period of time had passed during which atelectasis could form.
Futier et al48 in 2011 conducted a similar study with morbidly obese subjects (BMI 46 ± 6 kg/m2) in which a recruitment maneuver was applied to one study group immediately following intubation after having received preintubation NIV with 10 cm H2O of PEEP. Another group received preintubation NIV with 10 cm H2O of PEEP followed by no recruitment maneuver, and the control group only received standard preoxygenation. Both study groups had higher EELV and oxygenation following intubation. The NIV-alone group had higher EELV compared with the control group, while the NIV-plus recruitment maneuver group had significantly improved EELV and oxygenation compared with both the NIV-alone and control groups.
Most recently, Harbut et al49 investigated the effects of 5 cm H2O PEEP plus pressure support ventilation of 5 cm H2O versus simple oxygen therapy during 2 min of preoxygenation with 80% oxygen in morbidly obese subjects (study: BMI = 43 ± 6.3 kg/m2; control: BMI = 44.1 ± 6 kg/m2) undergoing laparoscopic gastric bypass surgery. Oxygenation was significantly improved in the study group immediately after intubation. Lung volumes were not measured.
In summary, these studies on mechanical ventilation techniques around the time of induction of anesthesia in obese patients show that relatively modest adjustments to standard clinical practice can effectively help keep the obese lung open. However, it should be clarified that after induction and intubation have occurred, CPAP should be replaced with positive-pressure ventilation.
Ventilator Modes and Tidal Volume
There has been debate about which ventilator setting is the best to use in obese patients. When tolerated, pressure support ventilation (PSV) might be the most beneficial for an obese patient, because patients receiving PSV will need to maintain some muscular effort (ie, with the diaphragm and accessory respiratory muscles) to trigger each ventilator-delivered breath. The maintaining of muscular tone could in turn facilitate better weaning from mechanical ventilation and prevent posterior-basilar atelectasis.
In a prospective randomized control trial of 36 obese subjects, Cadi et al50 compared VC-CMV with PC-CMV, using tidal volumes of 8 mL/kg of ideal body weight in both groups. The authors found that obese subjects ventilated during surgery with PC-CMV had better oxygenation than those ventilated with VC-CMV. The authors believe that a better ventilation/perfusion ratio was achieved with PC-CMV due to better alveolar recruitment. Other authors51,52 have pointed out that subsequent studies found no advantage to PC-CMV over VC-CMV in obese surgical subjects.53,54 Most recently, Dion et al55 compared VC-CMV, PC-CMV, and pressure controlled, volume-guaranteed ventilation (PCV-VG) in a prospective cross-over cohort trial of 20 subjects (BMI 49.3 ± 9.3 kg/m2). Subjects received each mode of ventilation for 20 min, with the sequence of the 3 modes randomized. No difference in oxygenation was observed between VC-CMV, PC-CMV, or PCV-VG. The only difference observed was a lower peak inspiratory pressure for subjects on PC-CMV and PCV-VG. Zoremba et al56 compared PC-CMV with PSV in moderately obese subjects (BMI 32 ± 2 kg/m2) undergoing minor surgery, finding that PSV was associated with better oxygenation intra- and postoperatively and better lung function postoperatively. Given the dearth of conclusive evidence, we suggest clinicians use PSV when possible but otherwise adopt a controlled mode of ventilation most consistent with their standard of practice.
There is considerable debate concerning the appropriate tidal volume that should be set for mechanically ventilated patients,57,58 with most studies and authors indicating that lower tidal volumes (6–8 mL/kg predicted body weight) are safer than higher tidal volumes (10–12 mL/kg predicted body weight).59 Obese patients undergoing anesthesia can have drastically reduced lung volumes compared with nonobese patients. This is attributed to the influence of the chest wall upon the lungs and due to the weight of the abdominal contents and is not due to an ARDS-like stiffening of the lung. Silva et al51 argue that low tidal volumes should be used when ventilating obese patients due to the reduced lung volumes caused by the thoracic fat and mediastinal load. Although we agree with Silva et al51 and others that, in obese patients, lower tidal volumes are safest, we come to our conclusion by a different rationale. The lower lung volumes in obesity caused by the influence of the chest wall have a vastly different pathophysiology than the lower lung volumes associated with ARDS, and thus the caution applied to the ARDS lung with regard to recruitment maneuvers and high PEEP is not warranted in the obese. The reduced lung volumes in the obese caused by the influence of the chest wall are reason to increase pressures, but in the form of PEEP and recruitment maneuvers, to relieve the lungs from the load of the chest. Increasing tidal volume, however, is a less effective and safe way of accomplishing this than a recruitment maneuver followed by PEEP. Not only does a large tidal volume induce lung injury by overdistention, but a large tidal volume in the absence of PEEP also results in cyclical alveolar collapse at end-expiration, causing atelectrauma, because positive end-expiratory transpulmonary pressure is not maintained.
Monitoring of the Mechanically Ventilated Obese Patient
In general, monitoring of the obese patient is no different than monitoring of other patients who are mechanically ventilated. All patients should have a tidal volume between 4 and 8 mL/kg ideal body weight, a plateau pressure < 28 cm H2O, a driving pressure ≤ 15 cm H2O and an appropriate PEEP. Thus, all of these variables should be monitored with every patient/ventilator assessment. In addition, auto-PEEP, compliance, and airways resistance should be monitored regularly with the frequency dependent on the patient's overall condition. Auto-PEEP can be monitored using the ventilator's automated system, as in other patients suspected of having auto-PEEP.
The need for the placement of an esophageal balloon is limited to those patients in whom concerns regarding plateau pressure, tidal volume, or driving pressure exist. Many of these patients require PEEP levels of 20 cm H2O, in which case plateau pressure may exceed 28 cm H2O. The only way to be sure that this increase in pressure is acceptable is the measurement of transpulmonary pressure, which requires an esophageal catheter. However, in the obese patient under controlled ventilation, plateau pressure may exceed end-inspiratory transpulmonary pressure by a large margin, making it safe to accept plateau pressures of 35–40 cm H2O, because transpulmonary pressure commonly remains < 20 cm H2O. However, it is not necessary to place an esophageal balloon to determine optimal PEEP. As indicated earlier, a decremental PEEP trial following a recruitment maneuver determines the same optimal PEEP level as setting PEEP to a positive end-expiratory transpulmonary pressure of +1 to +2 cm H2O.
Respiratory Care in the Postanesthesia Care Unit and Postextubation
Postoperative pulmonary considerations in the postanesthesia care unit are similar to those of the intraoperative phase: keeping the lung open and preventing atelectasis should be the primary concerns of the anesthesiologist (see Table 2). It has been shown in healthy60 and obese patients11 that there is considerable atelectasis and reduction in lung volumes postextubation. This makes the postoperative care of the obese patient crucial in preventing postsurgical pulmonary complications. The use of NIV in obese patients is recommended once the patient is awake, following commands, and breathing spontaneously. In fact, the majority of these patients use nocturnal NIV, because of their underlying sleep apnea, primarily over 12 cm H2O.61
Studies Using Noninvasive Positive-Pressure Ventilation (NIV) Postextubation
It should also be restated that OSA/obesity-hypoventilation syndrome is a highly common condition in this patient population. The prevalence of OSA in obese subjects presenting for bariatric surgery was recently reported to be as high as 73%,62 with a previous similar study reporting 78% overall prevalence and increasing rates with increasing BMI.63 Additionally, the prevalence of obesity-hypoventilation syndrome has also been found to increase with increasing BMI, with one study finding a prevalence of OHS as high as 30.4% in subjects with a BMI ≥ 40 kg/m2.64 It was recently shown by Kaw et al65 that subjects with either obesity-hypoventilation syndrome alone or obesity-hypoventilation syndrome plus OSA are more likely to develop postoperative respiratory failure, postoperative heart failure, and prolonged intubation time compared with patients with OSA alone. As a result, it is imperative that after extubation obese patients be immediately transitioned to the NIV strategy used to treat their underlying OSA/obesity-hypoventilation syndrome.
Both Gaszynski et al66 and Pessoa et al67 have shown that noninvasive ventilation improves oxygenation in postoperative obese subjects. More recently, Wong et al68 compared the use of the Boussignac CPAP mask with the standard air-entrainment mask and found improved PaO2/FIO2 but no difference in postoperative %FEV1 and %FVC.
Most interestingly, Neligan et al69 in 2009 compared starting CPAP with the Boussignac system immediately postextubation versus 30 min postextubation with standard of care mask in obese subjects. After 30 min, the Boussignac group was switched to CPAP on the standard of care mask. They found better lung function and volumes (FEV1, FVC, and PERF) in the immediate postextubation group compared with the group that started CPAP later. This study further articulates the importance of keeping an open lung as often as clinically possible: These authors observed a clinically important change in lung function, most likely attributable to the development of atelectasis within the 30-min postoperative period.
There have been studies investigating the beneficial effects of postoperative incentive spirometry in nonobese70 and obese subjects.71 Zoremba et al71 found that obese subjects who undertook postoperative incentive spirometry had better pulse oximetry values than controls had upon first mobilization, and that these subjects recovered lung function significantly faster during their time in the postanesthesia care unit.
Staehr et al72 investigated the impact of FIO2 in postoperative obese subjects on surgical site infection, as well as pulmonary complications, including atelectasis as determined by chest radiographs and computed tomography. They found no significant differences with respect to surgical site infection or pulmonary function between subjects who received 0.8 versus 0.3 FIO2.
Most recently, Corley et al73 conducted a randomized control trial in which high-flow nasal cannula therapy was used in subjects with BMI ≥ 30 kg/m2 immediately following extubation after cardiac surgery. The primary end point was reduction of atelectasis, and there was no difference in atelectasis between the high-flow nasal cannula group and the standard of care group. It is important to note that in obese subjects with hypoxemia, whether they are intubated or not, PEEP is necessary at relatively high levels to keep open lung parenchyma. High-flow nasal cannula is only capable with delivering minimal PEEP levels (2–5 cm H2O) even in the best of settings with adult patients. At this time, more evaluation of the use of high-flow nasal cannula therapy is needed, and we would not recommend this approach as an alternative to CPAP for obese patients postextubation.
Summary
Obese patients undergoing anesthesia and surgery risk developing atelectasis, expiratory flow limitation, auto-PEEP, increased work of breathing, and decreased oxygenation. During the periopertative period, attention must be paid to avoiding these complications (see Table 3). Because most obese patients cannot be kept in the sitting position during surgery, PEEP needs to be applied during the perioperative period. Preinduction NIV with ≥10 cm H2O PEEP should be standard practice. Following intubation, 10–15 cm H2O PEEP should be applied depending on the surgical procedure, and the lung should be recruited to a peak pressure of 40 cm H2O after each procedure that has the likelihood of inducing more atelectasis: postinduction; any time the ventilator circuit is disrupted; and any time a marked change in position occurs. During invasive mechanical ventilation, tidal volume should be maintained between 6 to 8 mL/kg predicted body weight and either VC-CMV or PC-CMV can be used. If the patient is breathing spontaneously, PSV with 10–15 cm H2O PEEP is ideal. In those patients who are difficult to oxygenate, a recruitment maneuver followed by a decremental PEEP trial should be used to identify the optimal PEEP level. Postextubation, all morbidly obese patients should be immediately transitioned to NIV with at least 10 cm H2O PEEP or the previously prescribed NIV level, continuing for 8 to 48 h, depending on the patient's status. When possible, the head of the bed should always be maintained in at least a 30° head-up position.
Ten Recommendations for the Safe Management of the Obese Perioperative Patient
Footnotes
- Correspondence: Lorenzo Berra MD, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital. Phone: 617-643-7733, E-mail: lberra{at}mgh.harvard.edu.
Mr Fisher has disclosed a relationship with Hollister.
Dr Kacmarek has disclosed relationships with Covidien and Venner Medical.
Dr Berra has disclosed a relationship with Endoclear and Venner Medical.
The other authors have declared no conflicts of interest.
- Copyright © 2016 by Daedalus Enterprises