Abstract
INTRODUCTION: The application of expiratory positive airway pressure (EPAP) in patients with COPD during exercise may reduce dynamic hyperinflation, while, on the other hand, it can increase the resistive work of breathing. Therefore, we evaluated the effects of 2 intensities of EPAP during exercise on tolerance, dynamic hyperinflation, and dyspnea in subjects with moderate to very severe COPD.
METHODS: We performed a cross-sectional, experimental, 4-visit study. In visit 1, subjects performed symptom-limited cycling incremental cardiopulmonary exercise test (CPET). In visits 2–4, at least 48 h apart, in a randomized order, subjects performed constant CPET without EPAP, EPAP with 5 cm H2O (EPAP5), or EPAP with 10 cm H2O (EPAP10).
RESULTS: The study included 15 non-hypoxemic subjects ranging from moderate to very severe COPD (mean FEV1 = 35 ± 11% predicted). Increasing intensities of EPAP during constant CPET tended to cause progressive reduction in exercise tolerance (P = .11). Of note, 10 of 15 subjects demonstrated significantly shorter average exercise duration with EPAP10 compared to the test without EPAP (−151 ± 105 s, P = .03 or −41 ± 26%). Minute ventilation increment was constrained by EPAP, secondary to a limited increase in tidal volume (P = .01). Finally, dyspnea sensation and serial measurements of inspiratory capacity during exercise were similar when comparing the three interventions at isotime and at end-constant CPETs.
CONCLUSIONS: The application of EPAP5 or EPAP10 during exercise tended to cause a progressive reduction in exercise tolerance in subjects with COPD without improvement in dyspnea or dynamic hyperinflation at equivalent exercise duration.
- chronic obstructive pulmonary disease
- exercise
- positive-pressure respiration
- inspiratory capacity
- dyspnea
- respiratory therapy
Introduction
Dyspnea and exercise intolerance are among the most common symptoms experienced by patients with COPD, leading to poor quality of life.1,2 A growing body of evidence suggests that lung hyperinflation is a key factor related to dyspnea and exercise limitation in these patients,3 and is an important independent risk factor for mortality.4
Lung hyperinflation develops as a consequence of increased lung compliance (ie, reduced lung elastance), the effects of expiratory flow limitation, or a combination of both.3 Dynamic hyperinflation during exercise can be defined as the temporary and variable increase of end-expiratory lung volume above the resting value,5 which may cause functional respiratory muscle weakness, increased work of breathing and impaired cardiocirculatory function, collectively impairing performance.6,7,8 For these reasons, there is increasing interest in lung-deflating interventions aimed at improving symptoms and physical functioning in this population.
In COPD, dynamic hyperinflation can be reduced during exercise by interventions that either increase expiratory flow (bronchodilators or a mixture of helium and oxygen), increase the time available for expiration (supplemental oxygen or exercise rehabilitation), or both (lung volume reduction surgery).3,9 In this context, the application of pressure during expiration has long been believed to increase intraluminal airways pressure and shift the equal pressure toward central airways,10 which potentially can explain the improvement in lung mechanics and hyperinflation by pursed lip breathing.11,12 However, the clinical benefit of an external expiratory positive airway pressure (EPAP) device during exercise in patients with COPD remains to be determined.13 It has been effective in reducing dynamic hyperinflation14,15,16 and post-exercise dyspnea,17 as well as improving exercise capacity.14,18,19 On the other hand, EPAP can increase the work of breathing20 and decrease venous return,21 which could partially explain the deleterious effect on exercise endurance recently described.16,22
Cardiopulmonary exercise testing (CPET) has proven useful in establishing the link between exercise performance and mechanisms of limitation as well as in determining which interventions can improve this relationship. Changes in end-expiratory lung volume during exercise can be reliably estimated from repeated inspiratory capacity measurements.23,24 The majority of previous studies evaluating the effects of EPAP on clinical outcomes used self-paced tests, inspiratory capacity measurements before and after exercise, and one intensity of EPAP.14–18 A recent study19 evaluating 2 levels of EPAP (1 cm H2O and 10 cm H2O) on self-paced exercise performance showed that both intensities similarly improved average 6-min walk distance statistically but not clinically.25 Constant CPET is currently recognized as more responsive than incremental CPET and the 6-min walk test for demonstrating the benefits of a given intervention.26 To confirm the potential benefits for subjects with moderate to very severe COPD of this intervention during constant CPET on physiologic (inspiratory capacity) and clinical outcomes (dyspnea and exercise tolerance), we investigated the dose response to 2 different intensities of EPAP using an externally paced, high-intensity, constant work-rate (endurance) test.
QUICK LOOK
Current knowledge
Lung hyperinflation is a key mechanism related to dyspnea and exercise limitation in patients with COPD. The application of expiratory positive airway pressure (EPAP) during exercise in these patients seems to reduce dynamic hyperinflation with controversial results on exercise tolerance and dyspnea.
What this paper contributes to our knowledge
Using a high-intensity constant load test with serial measurements of inspiratory capacity during exercise, 2 intensities of expiratory positive airway pressure (5 and 10 cm H2O) caused a tendency to decrease exercise tolerance without significant benefits on dyspnea and lung hyperinflation. The majority of subjects (67%) showed a reduction in exercise tolerance (> 5%) with expiratory positive airway pressure 10 cm H2O. Expiratory positive airway pressure induced ventilatory and hemodynamic constraints seem to explain these findings.
Methods
Subjects
Subjects with a clinical and functional COPD diagnosis according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD)27 criteria, without any modification in their COPD treatment in the last 6 months, were consecutively recruited from our COPD out-patient tertiary care clinic. Subjects were excluded if they had an acute cardiac or pulmonary disorder during the study period or within the 8 weeks prior to study entry, absence of dynamic hyperinflation during incremental CPET, any other pulmonary, cardiac, orthopedic, or neurological disease that limited exercise tolerance, or if they were unable to comply with the study procedures.
Study Design
The Institutional Ethics Committee approved all aspects of this cross-sectional, experimental, 4-visit, proof-of-concept study (No. 1.094.384). Clinical and resting pulmonary function variables (spirometry, static lung volumes by whole-body plethysmography and diffusing capacity of the lung for carbon monoxide) were performed within, at most, 6 months before the first visit and obtained from medical records. During visit 1, subjects performed symptom-limited cycling incremental CPET. During visits 2–4, at least 48 h apart, they were randomized to perform constant CPET without EPAP, EPAP with 5 cm H2O (EPAP5), or EPAP with 10 cm H2O (EPAP10). Randomization was done by computer-generated random letters (simple method) considering the 6 possible sequences for a block of 18 subjects (http://www.randomization.com) and thereafter shuffled in opaque sealed envelopes.
Procedures
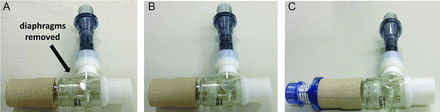
CPETs were performed on an electrically braked cycle ergometer (Corival, Lode, Groningen, the Netherlands) using a computer-based exercise system (Vmax Encore, CareFusion, Yorba Linda, CA). The following data were recorded as a mean of 20 s: oxygen uptake (V̇O2, mL/min), minute ventilation (V̇E, L/min), breathing frequency (f, breaths/min), and tidal volume (VT, L). Subjects rated their shortness of breath and leg effort using the 0–10 Borg scale28 immediately after inspiratory capacity measurements every 2 min and at peak exercise. Dynamic hyperinflation was defined as a reduction in inspiratory capacity from rest ≥ 150 mL or 4.5% of predicted.29 Oxyhemoglobin saturation (SpO2, %) was continuously measured by pulse oximetry (Takaoka Oxicap, São Paulo, Brazil). In incremental CPET, after a baseline of loadless pedaling for 2 min, the rate of power increment was individually selected (usually 5–10 W/min) to provide an exercise duration of 8–12 min. Constant CPET was performed with loaded pedaling at ∼75% of the peak work load achieved during incremental CPET. Symptoms and physiologic variables were compared at rest, maximal tolerable exercise, and at isotime, which is the longest exercise duration common to all constant CPET performed by a given individual. All constant CPET were performed using a nose clip and breathing through a silicone mouthpiece attached to a 2-way non-rebreathing T-shaped valve (2600 Medium, Hans Rudolph, Shawnee, KS), with an additional estimated dead space of 50 mL. During constant CPET without EPAP, all diaphragms of the valve were removed. The intensity of the EPAP offered with this device was verified with an external pressure transducer (MVD-300, Microhard System, Globalmed, Porto Alegre, Brazil) in the first 3 subjects included. Keeping all diaphragms of the T-shaped valve in place resulted in ∼5 cm H2O. The addition of the spring linear pressure resistor (Vital Signs, Totowa, NJ) adjusted at 5 cm H2O at the expiratory port of the valve resulted in a net expiratory pressure of ∼10 cm H2O. Thereafter, to obtain the EPAP level of 5 cm H2O, the diaphragms of the Hans Rudolph valve were used in their usual position. To obtain the EPAP of 10 cm H2O, the spring linear pressure resistor was connected to the outlet port of the Hans Rudolph valve and the resistor was adjusted to deliver a pressure of 5 cm H2O (Fig. 1). Shorter exercise duration was defined as any reduction in exercise tolerance during constant CPET with EPAP versus without EPAP.
Experimental device applied to obtain different EPAP intensities during exercise. A: Without EPAP, B: EPAP 5 cm H2O, and C: EPAP 10 cm H2O. EPAP = expiratory positive airway pressure.
Statistical Analysis
A sample size of 17 subjects was estimated, on the basis of a previous study, to detect an expected difference in inspiratory capacity variation from rest to peak exercise of 0.4 L (SD = 0.3) comparing tests with and without EPAP.15 In an interim analysis with the first 15 subjects, we decided to interrupt the study due to a clear tendency of exercise tolerance reduction associated with significant reduction in VT expansion and without difference in exercise dyspnea at isotime using EPAP.
Data are reported as mean ± SD or as median (range) according to distribution, unless otherwise stated. Generalized estimating equations were used to test for significant differences between interventions (without EPAP, EPAP5, EPAP10) at different time points (rest, isotime, and end-exercise), which is considered more sensitive than repeated measure analysis of variance to detect a given significant difference between and among groups and interventions.30 Pearson correlation analysis was performed between baseline cardiorespiratory variables (Tables 1 and 2) with exercise tolerance difference (EPAP10 − EPAP). Thereafter, significant correlations were assessed through multivariate linear regression analysis (stepwise method). A paired t test was used to compare exercise duration without EPAP versus EPAP10. All tests were 2-sided, and P < .05 was considered statistically significant. Statistical analyses was performed with SPSS statistics software (Version 22.0, Chicago, IL).
Baseline Characteristics of Subjects
Metabolic, Ventilatory, Cardiac, and Sensory Responses to Cycling Symptom-Limited Incremental Cardiopulmonary Exercise Testing
Results
The study included 15 non-hypoxemic subjects ranging from moderate to very severe COPD (2 subjects with GOLD stage II, 7 with stage III, and 6 with stage IV),27 with moderate to severe resting lung hyperinflation and reduction in diffusing capacity of the lung for carbon monoxide. Accordingly, they reported relevant dyspnea for activities of daily life (Table 1) and were receiving symptomatic treatment in accordance with current evidence-based guidelines.27 All were using a combination of long-acting ß2-agonists plus inhaled steroid and short-acting bronchodilators on an as-needed basis; 6 subjects were additionally being treated with a long-acting muscarinic receptor antagonist. No subjects participated in a pulmonary rehabilitation program or were engaged in regular exercise activities in the last 12 months before inclusion.
During incremental CPET, all subjects presented reduced peak aerobic capacity and dynamic hyperinflation, ranging from −0.97 L to −0.15 L (Table 2). Exercise tests were stopped most frequently due to severe leg discomfort/fatigue (n = 8), dyspnea (n = 5), or a combination of both (n = 2).
The application of different intensities of EPAP during constant CPET tended to cause a progressive reduction in exercise tolerance (Fig. 2). Of note, 10 of 15 subjects (67%) presented shorter exercise duration (ranging from −269 s to −12 s) with EPAP10 compared to the test without EPAP [−151 ± 105 s, P = .03 or −41 (−85 to −5%)]. Total lung capacity (TLC) and FVC (both expressed as percentage predicted) were the only baseline cardiorespiratory parameters significantly correlated with exercise tolerance change with EPAP, but only TLC remained as an independent variable in multivariate regression analyses (R2 = 0.34; unstandardized coefficient B = −2.91, P = .02).
Exercise tolerance with progressive intensities of EPAP. EPAP = expiratory positive airway pressure.
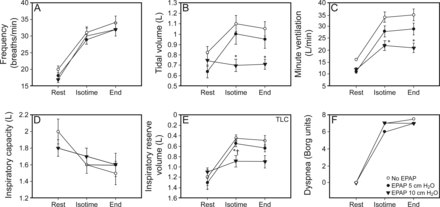
Minute ventilation increment during exercise was constrained by EPAP, secondary to a limited increase in VT (Table 3 and Table 4) (Fig. 3). Even without a significant impact on inspiratory capacity, the blunted VT response allowed the maintenance of a higher inspiratory reserve volume and lower end-inspiratory lung volume/TLC ratio at a standardized exercise time (isotime). Nevertheless, dyspnea sensations were similar along the exercise tests comparing the three interventions (Tables 3 and 4, Fig. 3). Interestingly, a significant reduction in inspiratory (VT/TI) and expiratory (VT/TE) flows were observed at isotime with crescent intensities of EPAP (Table 3), which was observed only regarding inspiratory flows at maximal tolerable exercise (Table 4).
Physiologic and Perceptual Responses at Isotime With Different Intensities of EPAP During Constant Load Cardiopulmonary Exercise Tests
Physiologic and Perceptual Responses From Rest to Maximal Tolerable Exercise With Different Intensities of EPAP During Constant Load Cardiopulmonary Exercise Tests
Breathing pattern, operational lung volumes, and dyspnea sensation without and with different EPAP intensities during exercise. *P < .05 vs without EPAP. † P < .05 vs EPAP 5 cm H2O. EPAP = expiratory positive airway pressure.
Finally, the application of EPAP during constant high-intensity exercise tests caused a significant dose-response reduction in oxygen pulse at submaximal exercise (Table 3).
Discussion
The main findings of our study were that, in stable subjects with moderate to very severe COPD, the application of our experimental device during exercise showed a clear tendency to impair exercise tolerance, without clear benefits in terms of dynamic hyperinflation and exercise dyspnea. These are probably be related to the induction of ventilatory (decreased V̇E, decreased VT, and decreased expiratory and, more markedly, inspiratory flows) and hemodynamic (decreased V̇O2 and O2 pulse) constraints.
The supposed advantage of applying pressure support only during expiration, ie, to attenuate dynamic airway compression and reduce lung hyperinflation (and elastic work of breathing),10–12 seemed to be counterbalanced by increases in expiratory muscle effort (and resistive working of breathing) during exercise.20,31 Moreover, because we continuously monitored ventilatory and metabolic parameters during exercise, we used a device (Fig. 1) that allowed measurements of those parameters by a computer-based system while simultaneously applying the pressure in the expiratory outlet. With increased flows during exercise, the Hans Rudolph T valve may offer differential pressure through the inspiratory diaphragm as described in the manufacturer data sheet (http://www.rudolphkc.com/pdf/691151%200713%20K.pdf, accessed March 27, 2017). This can be particularly relevant to our results, especially considering how the whole experimental device (pneumotachograph + T valve + spring pressure resistor; Fig. 1) was assembled for the different interventions.
During tests without EPAP, the expiratory diaphragm of the T valve was removed to allow a free pathway for expiration and inspiration. Accordingly, both EPAP interventions (5 cm H2O and 10 cm H2O), beyond the increment in expiratory resistance, probably applied some level of inspiratory resistance, which could substantially increase the work of breathing (especially considering the high exercise f)8 and contribute to limit VT increase. The dramatic reduction in inspiratory flow (even higher than the expiratory flow) and the absence of increase in breathing frequency with EPAP5 and EPAP10, which would occur if VT limitation were secondary to respiratory muscle weakness or fatigue, support this concept. This may also contribute to the contradictory effects of EPAP application on exercise tolerance found in the literature. Studies that used devices with an inspiratory diaphragm16,22 (like our study) impaired exercise performance, in contrast to those where no inspiratory resistance was applied (control intervention with normal breathing).14,18 Finally, higher TLC was associated with lower exercise duration with EPAP. Any increased inspiratory resistance would be particularly detrimental to those with more hyperinflation. In contrast to healthy subjects, the combined recoil pressure of the lungs and chest wall is inwardly directed in hyperinflation, resulting in an inspiratory threshold load.32
The application of the 2 intensities of EPAP, considering that the inspiratory loads were the same for both interventions, allowed us to observe, that EPAP per se contributed significantly to constrain ventilation (Fig. 3) with a clear tendency to impair exercise tolerance (Fig. 2). While a dose-response reduction in operational lung volumes during exercise was observed with EPAP (ie, increased inspiratory reserve volume and decreased end-inspiratory lung volume/TLC; Table 3), the effect on inspiratory capacity (the true room for VT expansion) was modest and non-statistically significant. The reduction in exercise lung volumes appear, in fact, to be secondary to a significant constraint to VT and, without a compensatory increase in breathing frequency, lead to VE remaining lower than expected during exercise as previously described.20 This can explain a similar dyspnea observed in the present and previous study17 or even worse dyspnea22 during exercise, regardless of a significant improvement in important indexes of lung hyperinflation.33 It means that constraints to VT increases continued to happen with exercise progression.
In addition, a significant reduction in V̇O2 was observed accompanied by a significant reduction in oxygen pulse, a surrogate for systolic volume,6 indicating hemodynamic response impairment. This is possibly related to decreased venous return caused by excessive expiratory muscle recruitment,21 leading to reduced lung perfusion to ventilation ratio and cardiac output.1 Furthermore, augmented sympathetic vasomotor outflow during exercise was observed with increasing expiratory resistance.34 Added to the central hemodynamic and ventilatory constraint, all these mechanisms may contribute to impair exercise capacity.
Although the exercise tolerance reduction with EPAP was not statistically significant in the present sample (n = 15), we decided to interrupt the study since there was a clear tendency to exercise performance impairment (the opposite of what was expected) associated with similar exercise dyspnea at isotime (our two main clinical outcomes). Furthermore, two-thirds of the current sample demonstrated a reduction in exercise performance, presenting a statistically and clinically important35 average reduction in exercise tolerance. Moreover, there was a clear dose-response effect of higher EPAP associated with lower exercise tolerance. Finally, external dead space (VD) loading has the potential to stress the ventilatory control system such that a greater V̇E is required to keep the same arterial carbon dioxide pressure at any given metabolic rate.36
This issue is particularly crucial to patients primarily limited by ventilation,37 with aggravating conditions such as high VD/VT even in mild disease compared to healthy controls,38 which worsens in tandem with COPD severity.1 Although our experimental device caused a small but potentially clinically relevant increase in VD, we consider its influence on the main results to be hardly significant because the amount of VD increase was similar during all interventions.
Conclusions
The delivery of EPAP5 or EPAP10 during exercise tended to cause a progressive reduction in exercise tolerance in subjects with COPD, without significant improvement in exercise dyspnea and dynamic hyperinflation at isotime. Ventilatory and hemodynamic constraints seem to underlie these findings.
Footnotes
- Correspondence: Danilo C Berton, Rua Ramiro Barcelos, 2350, Room 2050, Postal Code 90035–003, Porto Alegre, Brazil. E-mail: dberton{at}hcpa.edu.br.
The authors received support from Fundo de Incentivo à Pesquisa do Hospital de Clinicas de Porto Alegre (FIPE-HCPA) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CAPES-Brazil).
A version of this report was presented by Mr Gass at the Brazilian Pulmonology Society Congress in Rio de Janeiro, Brazil, in October 2016.
- Copyright © 2017 by Daedalus Enterprises