Abstract
Patient-ventilator synchrony is a complex issue affected by ventilator performance, patient characteristics, and the patient-ventilator interface. The history of patient-ventilator interaction includes avoidance of pharmacalogic paralysis, the development of spontaneous breathing systems, microprocessor technology to maximize interaction, and closed-loop control. While most clinicians agree that patient-ventilator synchrony is desirable, there remain no cause-and-effect data that asynchrony is associated with poor outcome.
Introduction
Early adventures in mechanical ventilation were closely intertwined with resuscitation following drowning, and the development of surgical techniques.1 Under those conditions, considerations of patient-ventilator interaction were clearly unnecessary. In fact, the first mention of such concerns with mechanical ventilators appears only in the classification of mechanical ventilators by Mushin and colleagues. Mushin et al provided the framework for classification of ventilators, which created the basis for understanding patient-ventilator interaction. In their initial classification, ventilators were classified based on the ability to be patient-cycled (now called triggered). At the time of the initial writing in 1959, patient triggering meant pressure-triggered. A ventilator unable to respond to patient effort was termed a “controller,” and a ventilator capable of being triggered was termed an “assistor.” This terminology may explain some of the confusion regarding classification schemes present to this day. Mushin and colleagues made no value judgments based on patient triggering, nor did they consider patient-ventilator interaction to any extent. They did suggest that inspiratory flow should be 40–80 L/min and that expiratory resistance be low, but that recommendation appears to be out of concern for preventing air-trapping, not for improving patient-ventilator interaction.1 Refinements of the Mushin system by Chatburn will be used to identify the phases of mechanical ventilation wherein patient and ventilator interact to create either synchrony or asynchrony.2
Historical Considerations
Patient-Ventilator Interaction During Continuous Positive-Pressure Breathing
In the late 1930s, Poulton and Oxon3 and Barach and colleagues,4 working separately, described the use of continuous positive-pressure breathing in the treatment of asthma and cardiac asthma (cardiogenic pulmonary edema). Known today as continuous positive airway pressure (CPAP), continuous positive-pressure breathing does lend itself to some inspection of patient-ventilator interaction. Both authors made astute observations on patient-device interaction and were perhaps the first investigators to associate device operation with patient comfort. Note that neither group provided ventilation. The devices used to provide continuous positive-pressure breathing consisted of a high-flow low-pressure blower and a spring loaded valve to control end-expiratory pressure. In many ways these devices are not that different than CPAP devices used today.
Poulton and Oxon constructed the “pulmonary plus pressure machine” from a vacuum cleaner and spring-loaded pressure valve. They noted that a high flow was necessary to aid in patient comfort. They were also among the first to note the importance of heating inspired gases, although humidity was not necessarily considered. Their recommendations for patient comfort included placing a hot water bottle in the bag compartment of the vacuum cleaner or to position the device such that “it sucked in air from a radiator or electric fire.” The former was probably more comfortable than the latter. Poulton and Oxon further suggested that if a household vacuum cleaner were to be used, that it be run for several minutes prior to use to “clear out any dust.”3 Barach and colleagues noted that limiting the fluctuation in inspired and expired pressure to < 2–3 cm H2O improved patient comfort.4 This finding probably spurred a long line of investigations into the work of breathing (WOB) with CPAP systems, demand flow systems, and modern ventilators.5–10 Both groups described improvements in patient comfort, manifest by reductions in dyspnea and respiratory rate. Neither, however, attributed the comfort to patient-device interaction.
Negative-Pressure Ventilation
The polio epidemics of the 1940s and 1950s ushered in the use of negative-pressure ventilation with the iron lung.11 As the respiratory failure from polio is caused by respiratory paralysis, the issue of patient-ventilator interaction wasn't considered. On several occasions I spoke with Jack Emerson about the issue of patient reactions to ventilation in the iron lung. In a discussion regarding how to know that ventilation was adequate, Emerson described the technique of “taking their breath away” (personal communication). Essentially the patient was asked to speak, and ventilation was considered adequate if speech fell silent, signaling the movement of gas into the lungs.
Positive-Pressure Ventilation
Negative-pressure ventilation dominated the early 1900s, until Lassen reported the benefits of manual positive-pressure ventilation in the treatment of poliomyelitis.12 The era of positive-pressure ventilation as a life-saving technique had begun.
A series of papers in the late 1950s and early 1960s on the treatment of flail chest and pulmonary contusion represent what might be considered the antithesis of patient-ventilator synchrony.13–16 In fact, the synchrony was perfect, because the patients were rendered apneic. The landmark paper by Avery and co-workers, “Critically crushed chests: a new method of treatment with continuous mechanical hyperventilation to produce alkalotic apnea and internal pneumatic stabilization,” concentrated on treatment of the chest wall.13 Pulmonary contusion was not recognized as an important pathologic feature.
Pulmonary dysfunction following massive chest trauma and flail chest was felt to be the result of the paradoxical movement of the chest wall and internally the presence of “pendelluft.”13–16 The instability of the chest wall was thought to result in ineffective breathing and the movement of gases between the lung segments, preventing adequate alveolar ventilation. Windsor and Dwyer described the treatment of a number of crushed chest injuries, and their own words here detail the therapy. In the case of a 44-year-old man who was involved in a head-on motor-vehicle accident and was struck in the chest by the steering column, they noted:
At that time he was lying supine. He was semiconscious, cyanosed, and extremely dyspnoeic. He had gross, generalized, surgical emphysema with violent, piston-like, paradoxical movement of the right upper anterior chest and sternal regions. Control of the paradox had been attempted by the use of a hook attached to an overhead frame and inserted into an upper right anterior rib. Radiographs of the chest showed a right hemopneumothorax, which had been treated by a needle in the right pleural cavity. Bronchial obstruction due to secretions was marked…. The pulse rate was 100 per minute and the respirations 32 per minute. Morphia, 96 mg, pethidine, 50 mg, pethilorfan, 100 mg, and paraldehyde, 10 mL, had been given in an effort to control pain and restlessness. It appeared to be absolutely necessary to control the paradoxical respiration and seemed to be possible only by abolishing the patient's spontaneous respiration with a muscle relaxant and instituting intermittent positive-pressure respiration.17
Other treatments include placing towel clips around the ribs and using piano wires to suspend the clips from overhead traction, to “splint” the rib cage. A hallmark of this treatment was deep sedation and paralysis, arbitrarily imposed for 2–3 weeks. Amazingly, these treatments were frequently successful. This treatment option is probably the genesis for “weaning” from mechanical ventilation. After 2 weeks of paralysis, patients were awakened, undoubtedly suffered from respiratory muscle atrophy, and the slow withdrawal of ventilator support required. During this time, patient-ventilator interaction was avoided and synchrony was not a concern.
Patient Triggering
The concept of patient-ventilator interaction and triggering appears to have been first championed by Harrison. He described “patient-triggered augmented ventilation” in a review published in the South African Medical Journal in 1962. Harrison noted:
Recent advances in the design, sensitivity, and response time of triggering mechanisms in some mechanical ventilators now available, make patient-triggered augmented ventilation the method of choice in many cases requiring mechanical ventilation today.18
He went on to say that the use of patient-triggered ventilation in flail chest would be unwise, as the negative pressure created would be contraindicated. This concern was based on the thought that negative pressure would result in deformation of the chest wall, negatively impacting gas exchange and rib healing. However, Harrison recognized early the importance of triggering and inspiratory flow on patient comfort and the WOB in patient-triggered approaches. He reported that:
For the patients with respiratory distress characterized by tachypnoea, to match the patient's respiratory efforts it is essential to have a ventilator that has a rapid response time and is capable of delivering very high flow rates of gas. (For this we used the Bird Mk. 8 ventilator.)18
The Return of Spontaneous Breathing
In 1973, Downs and colleagues introduced intermittent mandatory ventilation (IMV) as a method to facilitate weaning in adults.19–21 This seemingly common-sense approach of sharing the respiratory work between the patient and the ventilator resulted in widespread use of the technique and adoption of the method into mechanical ventilators. This latter development probably represents one of the most common causes of patient-ventilator asynchrony.
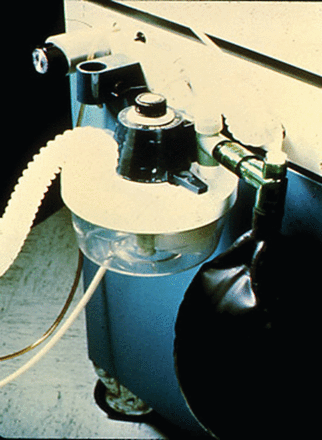
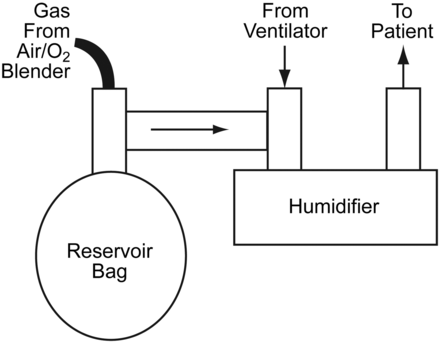
In its infancy, IMV was an add-on to the ventilator, using an H-valve with one-way valves and a separate air-oxygen source for continuous flow (Fig. 1).22 These systems were easily placed in the circuit, either pre-humidified in the inspiratory limb or, if dry, placed prior to the humidifier. Systems were considered “open” or “closed” based on the circuit configuration. (Figs. 2 and 3) The most common closed system was an H-valve and elastic reservoir bag connected to the inlet of the humidifier. During ventilator inspiration, the one-way valve closed and the reservoir bag filled with gas. During the expiratory phase a continuous flow of gas was available for spontaneous breathing. While this method avoided the problems associated with triggering and cycling the ventilator, other factors impacted the WOB, including the flow provided, the volume of the reservoir, and the characteristics of the expiratory valve.22,23 The presence of continuous flow during the expiratory phase confounded tidal-volume monitoring, and if the flow was set high enough could augment delivered tidal volume. High flows also resulted in high oxygen usage and waste, as well as rapid evaporation of water in the humidifier. These problems and inconveniences resulted in the development of demand-valve regulators and eventually the introduction of synchronized intermittent mandatory ventilation (SIMV).
An early intermittent mandatory ventilation system that included an H-valve and an integral one-way valve to provide continuous flow for spontaneous breathing.
Schematic of a closed intermittent-mandatory-ventilation system. VT = tidal volume. (Adapted from Reference 22.)
Schematic of an open intermittent mandatory ventilation system. VT = tidal volume. (Adapted from Reference 22.)
IMV Versus SIMV
SIMV was introduced as an integral part of the mechanical ventilator, with the promise of reduced gas usage, accurate expired tidal-volume measurement, and prevention of breath stacking. In what serves as a classic tale of device approval and testing, Shapiro et al evaluated the intermittent demand valve of the Puritan Bennett MA-1.24 The evaluation consisted of a group of anesthesiologists breathing on the ventilator, with and without the intermittent-demand-valve controller, via a mouthpiece. All the research subjects preferred the intermittent-demand-valve controller. That was the sum total of research prior to the introduction of SIMV. Subsequent animal trials by Heenan et al found no important differences between IMV and SIMV.25 Peak inspiratory pressure was slightly higher during SIMV, but the difference was < 1.5 cm H2O. Aoki et al described SIMV that could be adapted to even pneumatic ventilators and suggested an advantage,26 but I found no evidence of commercialization of that device.
It is important to note, that in the hands of Downs, Kirby, and Klein, the inventors of IMV, a set ventilator rate of 10 breaths/min would have been considered high.19 The introduction of SIMV complicated ventilators and ventilation. The work to trigger the spontaneous breath, the flow available to the patient, and the cycling of the breath were all areas of contention and differences between devices. These initial observations eventually gave rise to terms such as response time, rise time, and flow termination.
Continuous Versus Demand Flow
Several researchers evaluated the WOB during continuous-flow IMV and demand flow IMV during the late 1970s and 1980s. Gherini and colleagues compared spontaneous breathing with PEEP and CPAP in normal volunteers, following the lead of Barach, and in an eloquent paper demonstrated that minimizing inspiratory-expiratory pressure differences increased functional residual capacity and reduced WOB.27 Schlobohm et al also found that during spontaneous breathing, CPAP improved PaO2 and functional residual capacity, compared to expiratory positive airway pressure.28 The same group astutely noted the negative effects of zero expiratory positive airway pressure prior to extubation on functional residual capacity, PaO2, and WOB in patients.29 That exquisite, seminal work on the importance of low-level PEEP in all mechanically ventilated patients, unfortunately, appears to have been missed by many.
Gibney et al compared WOB between the continuous-flow Emerson system and the demand-flow system of the Bear 1 and the Puritan Bennett MA-2. With 10 healthy physician volunteers they demonstrated that the WOB with the demand systems was roughly twice that of the continuous-flow systems.30 Christopher et al used a lung model to study the peak negative pressure to initiate inspiratory flow and the expiratory resistance of 2 continuous-flow and 4 demand-valve systems. They compared the IMV Emerson and the Puritan Bennett MA-1 with an H-valve system to the Puritan Bennett MA-1 with intermittent demand valve, the Bear 1, the Monaghan 225, and the Servo 900B, and found wide differences in the negative pressure required to trigger the breath: the Servo 900B and Puritan Bennett MA-1 required 6 cm H2O, compared to only 2 cm H2O with the continuous-flow systems. They also noted the importance of expiratory resistance on spontaneous breathing and demonstrated large differences between the ventilators and between disposable and non-disposable ventilator exhalation valves.31 Katz et al measured the WOB of several new ventilators, including microprocessor-controlled devices. They found substantial differences in operation and were the first to demonstrate that the new microprocessor-controlled ventilators often overshoot the PEEP level and deliver a small amount of pressure support. They also noted that ventilators differed in the pressure required to initiate flow (now known as the pre-trigger phase) and the flow and pressure following breath-detection (now known as the post-trigger phase).32 These studies were repeated with new devices in dozens of papers over the last 3 decades. The main implication is that ventilator performance can positively or negatively influence WOB and patient-ventilator synchrony.
Work of Breathing and Synchrony During Mechanical Ventilation
In 1986 Marini and colleagues published a classic paper on WOB during mechanical ventilation.33 This was followed by studies in both normal volunteers and patients, which demonstrated that appropriate sensitivity and inspiratory flow were key components in reducing WOB.34,35 A follow-up study of SIMV demonstrated that patients were unable to adapt to changes in breath type (mandatory vs spontaneous) on a breath-to-breath basis.36 These observations are among the earliest contributions to our understanding of patient-ventilator interaction during delivery of mandatory breaths.
Ventilator Operation as it Relates to Synchrony
Ventilator operation was described by Mushin and colleagues, who used a system consisting of 4 phases:1 the change from expiratory phase to inspiratory phase (triggering); the inspiratory phase (limit and control); the change from inspiratory phase to expiratory phase (cycling); and the expiratory phase (PEEP). This system was updated by Chatburn to eliminate redundancy of terms (Mushin used cycling to mean both the start and end of inspiration), and I will use Chatburn's terminology for the next discussion.2
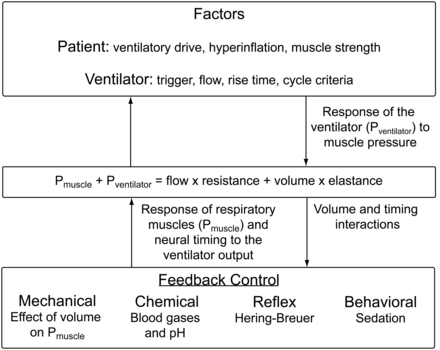
Given the multiple interactions between the patient's innate control of breathing and ventilator operation, it is not surprising that patient-ventilator synchrony is more often the exception than the rule. Figure 4 depicts the equation of motion for the respiratory system and the multiple patient responses to ventilator output, which are manifest by changes in muscle pressure and timing, and the response of the ventilator to the patient, manifest by changes in airway pressure. This diagram leads to some important issues in ventilator operation. It is not simply that the patient initiates a breath and then is passively ventilated. The number of inputs controlling ventilatory drive, some which have nothing to do with patient needs or the ventilator, impact patient-ventilator interaction. Good examples of this are agitation in the patient with alcohol withdrawal, and tachypnea in the patient with encephalopathy. Neither problem is remedied by the ventilator settings or mode. The ventilator responds to patient effort based on its control, trigger, limit, and cycle variables, through manipulation of airway pressure. The patient also responds to the ventilator output, through manipulation of muscle pressure. These complementary and sometimes competing pressures are further influenced by timing and volume. Patient-ventilator interaction is much more complex than simple observation suggests.
Relationship of the equation of motion, mechanical ventilator outputs, patient factors, and respiratory muscle output. This diagram helps explain the complexity of patient-ventilator interaction. Pmuscle = pressure from the muscles. Pventilator = pressure from the ventilator.
Another important point regards the data from lung-model studies. While a lung model can simulate numerous breathing patterns and compliance and resistance conditions, it cannot respond based on the relief of dyspnea. The lung model makes no modifications based on neural, behavioral, or chemical feedback. A perfect example is rise time during pressure support. In a lung model, the fastest rise time (ie, the most rapid rise to peak pressure) usually results in the lowest measured WOB, but this is not always true in patients, where a moderate rise time often results in the best patient-ventilator synchrony. Work and synchrony do not always go hand in hand.37
Triggering
Patient-ventilator synchrony may be affected by the trigger variable. Triggering, which once could only be based on measurements pressure, volume, or flow, can now be based on other signals, including motion, waveform triggering, transdiaphragmatic pressure, and electromyography.38 These signals will be discussed in Sassoon's paper from this conference.39
The Post-Trigger Phase
The post-trigger phase reflects the ventilator's response once patient effort has been detected.39 Ventilator operation during the post-trigger phase is influenced by the target (pressure or volume) and the limit variables, the ventilator's driving system, the flow setting and flow pattern (in volume-control ventilation), and the rise time (in pressure-control ventilation).
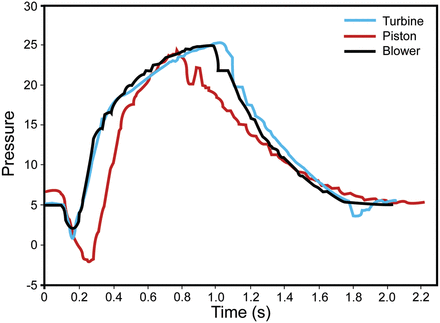
The driving system and control of the driving system are important with regard to reaching the required flow or pressure. Blower-based and turbine-based ventilators have advantages over piston ventilators. In critical care ventilators, the use of compressed gas as the power for ventilation and proportional solenoids and other flow-control devices are likewise efficient. Figure 5 compares pressure/time curves from blower, turbine, and piston ventilators. With simulated spontaneous breathing with a test lung, 4 ventilators (3 blower and 1 micro-piston) were set at a PEEP of 5 cm H2O and pressure support of 20 cm H2O. The blower ventilators typically provide a low constant flow, so they are already in motion when triggering is detected. The piston is motionless prior to triggering and must overcome inertia to reach the required positive pressure, so the piston ventilator's response time is slower than that of the blower ventilators.
Pressure/time curves from 3 ventilators (1 turbine, 1 piston, and 1 blower) during simulated triggering, with PEEP 5 cm H2O and pressure support 20 cm H2O. The piston-based ventilator lags behind the others in reaching the set pressure.
Cycling
Under ideal circumstances the end of ventilator inspiration corresponds with the end of the patient's neural inspiration. Recent evidence, however, suggests that this is rarely the case.40 Like triggering, cycling is most commonly based on time or measurement of airway pressure, volume, or flow. Cycling can be based on a single variable or on a hierarchy of signals, to increase safety. As an example, while pressure-support breaths are typically flow-cycled, there are also time and pressure cycling criteria for every pressure-support breath. The pressure variable avoids overdistention and allows cycling when patient expiratory effort begins prior to the end of mechanical inspiratory time. The time-cycle variable assists in patients with leaks and during noninvasive ventilation.
Automated determination of optimal cycling during pressure support was described by Yamada and Du and commercialized in the Newport ventilators. While this system appears to have advantages, there are few clinical studies to support automated determination of cycle criteria, compared to either a fixed or clinician-adjustable cycle criteria.41–43
The impact of each of these phases of ventilator operation are covered in detail in subsequent conference papers.
Summary
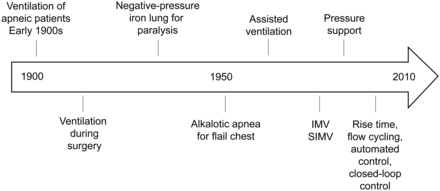
Patient-ventilator synchrony is a complex issue, affected by ventilator performance, patient characteristics, and the patient-ventilator interface. The history of patient-ventilator interaction includes paralysis, the encouragement of spontaneous-breathing microprocessor technology to maximize interaction, and closed-loop control (Fig. 6). While most clinicians agree that patient-ventilator synchrony is desirable, there remain no cause-and-effect data that asynchrony is associated with poor outcome.
Timeline of patient-ventilator interaction. IMV = intermittent mandatory ventilation. SIMV = synchronized IMV.
Discussion
Epstein:
Having surveyed 140 years of mechanical ventilation, as you look to the future, what do you see as the greatest challenge for making further improvements in patient-ventilator interaction?
Branson:
I think the greatest challenge has to do with the sensing. NAVA [neurally adjusted ventilatory assist] is a good example of a technique that seems to work very well, but the invasive placement of the catheter is a problem: it doesn't work if it's in the wrong spot. If somebody were to invent NAVA using transdiaphragmatic monitoring, I would think we would all use it tomorrow, because it wouldn't matter if the patient has auto-PEEP or if there are leaks in the system. Unless there is another way to capture the patient's own respiratory demand and get that signal quickly to the ventilator and have the ventilator respond, I'm not sure what else should be done. We want that great physiologic signal, but if it's too difficult to get to or unreliable because the sensor moves, I don't know if it's that big of an advantage.
Kacmarek:
Just to be provocative, as we begin this conference and look at all the patients whom we mechanically ventilate, and look at the traditional approaches to ventilation—assist-control, pressure support—what we'll hear from a number of the presenters is that we can set the ventilator to ensure reasonable synchrony with traditional modes. In what percentage of patients that you interact with do you believe that these newer advanced approaches to ensuring synchrony are really necessary?
Branson:
There's an assumption that you have patient-ventilator synchrony. I think that for years people have put patients on assist-control at a rate of 20 breaths/min, but didn't realize that the patient was making 30 inspiratory efforts a minute. So sometimes you have the issue of “just because you don't see it doesn't mean it's not there,” and that may be the biggest issue about patient-ventilatory synchrony.
Neil [MacIntyre] introduces the idea that pressure support is more comfortable for patients, so what do people do? They put the COPD patient on pressure support and when the patient gets tachypneic they turn up the pressure support. When the pressure support goes up, the ventilator says the rate is 16 breaths a minute, and they pat themselves on the back and go down for coffee—but the patient is still breathing 32 times a minute. It's just that the cycle synchrony and the hyperinflation prevent the synchrony from really being there.
I think, within the context of that question, Bob, there is the question of do we really recognize asynchrony when it's there? And if it is there, is there something we can do about it with the simple techniques, or is the physiology so challenging that it requires something special to get around the patient's pathophysiology?
Kacmarek:
So is it an educational issue or is it a technology issue?
Branson:
I think the issue is clearly a bedside-clinician knowledge issue. The question becomes, is it something that we can educate people about, or is it simply whether or not people are going to accept these changes? It's 2010 and less than half the hospitals in America use low-tidal-volume ventilation, 10 years after a study showed that it reduces mortality. We all tend to do what we've always done, and we don't change, but sometimes technology can overcome people's resistance to improve.
Hess:
Rich, if you look into your crystal ball, would it be possible or advantageous to have the ventilator prompt the user that there is poor patient-ventilator interaction, that there are failed triggers or some other issue with the interaction between the patient and the ventilator, and then perhaps even close the loop and perhaps do some changes, such as to raise the PEEP level if there are failed triggers due to auto-PEEP?
Branson:
There are at least half a dozen papers on automated detection in patient-ventilator asynchrony. I'm all for closing the loop and continuing to work in that arena. The problem is that asynchrony is so multifactorial. If you have missed triggers, it might be because the PEEP is insufficient or it might be because the patient has weak respiratory muscles. Without some measure of respiratory drive or direct measurement of respiratory muscle strength, I'm not sure we have all the data to make those kinds of closed-loop decisions.
The manufacturers are always asking, “What should we do?” and I say, “Make our job easier. Make us smarter.” If it misses the triggers, then how does it know that they're there? If it knew that they were there, then it would trigger! The waveforms, perhaps, can tell you about missed triggers, but maybe we need a little window that pops up and says, for instance, “10 missed triggers in the past 60 seconds.” An asynchrony index of more than 10% is a problem in terms of vital outcomes, so maybe just knowing and getting somebody attracted to the bedside to do something about it would be helpful.
Kallet:
For the sake of being provocative, I would make the case that we don't always want to treat asynchrony. There are situations when we don't always want to perfectly match a patient's spontaneous effort. I've seen enough patients who are in extreme respiratory distress with spontaneous rates in the 50s. One of my primary focuses over the years has been trying to better synch up the patient and the ventilator, but if we do it so well, then we get the ventilator trying to breathe 50 or 60 times a minute. There are very real risks of hemodynamic compromise and barotrauma in that situation! So there are certain circumstances when we have to chemically treat the patient. There's a limit to our good intentions to promote synchrony, because there are certainly clinical situations where we really shouldn't. I think that's something that sometimes clinicians miss.
Branson:
I agree. Not all patient-ventilator synchrony has anything to do with the ventilator. Some head-injury patients breathe at a certain respiratory rate regardless of the ventilation mode, the breath type, the triggering, or the cycling.
Kacmarek:
But Rich, how do we know we're right and the patient's wrong? Maybe we should let the patient breathe 40–50 times a minute. That's what the patient's brain is telling them to do, and I don't think we have really good evidence to say our taking over and sedating and paralyzing that patient is better than letting the patient assume a ventilatory pattern with appropriate assistance and good synchrony that their neurocontrol says is the best for them.
Younes:*
I fully agree with Bob. We have used PAV [proportional assist ventilation] extensively, and we've seen many patients like that, whose own rate is 40–45 breaths a minute with great asynchrony when on pressure support. Then when you put them on PAV, they become synchronized, the respiratory rate goes down to, say, 35 breaths a minute and they are fully synchronous and just go on like that for a long time.
I have weaned many patients who were deemed unweanable because every time you drop the pressure support the ventilator rate goes up from 20 to 40. They say the patient is unweanable. Then you put them on a synchronous mode and they are very comfortable and you can wean them.
We just have to accept the fact that in some cases the respiratory rate is 40 or more and it's not going to hurt them, because this is what they want and are comfortable with. In fact we've found in many cases that when you extubate that patient who has a high undistressed rate, often their respiratory rate goes down. We concluded that the endotracheal tube itself is a stimulus to the fast respiratory rate.
I have a historical comment to add: when I was developing PAV in the late '80s and '90s, we put lots of patients on it and found that the average VT is 7mL/kg. Now, at the time, the standard of care was 15mL/kg, so I went to Bryan Kirk, who was one of the early pioneers of mechanical ventilation, and I asked him, “Why is it that you put people on 15mL/kg?” He said, “If you want to sleep at night, you have to put them on that,” and the reason for it is that half the patients want more than 7mL/kg, and if you set the VT to the average VT (7mL/kg) for everybody, half the patients are going to be in distress and wanting more. I'm not really sure that the idea of putting a limit of 900mL on the ventilator is a good idea; it will force you to paralyze some patients who need higher volumes and in whom these volumes are not harmful.
Branson:
I don't know that I would set a limit at 900, but I do think 2.2 is a little high. My comment about that—and it really doesn't affect ICU ventilators so much—but if I make a home-care ventilator (and even home-care ventilators go to 2.2, by the way, and some of the transport ventilators go to 3 L) that only has to go to say 1.2 or 1.3 L, I can improve the battery life, minimize the blower size, and reduce the power requirement.
There are a lot of technical advantages to having a more physiologic range of ventilator operation, as opposed to what we currently get from ventilator manufacturers. Ask a manufacturer why they are doing 2.2, they'll say it's because if we don't do 2.2, our competitor will say, “They can only do 1.5, so there must be something wrong with their machine.” And that's the stupidity of it all. I think it would make a difference to ventilator design and improvements in ventilator design if we dealt with a more normal range of physiologic tidal volumes.
Younes:
You lamented the fact that there was no method to know what the patient's muscles are doing in terms of ventilator response rate in response to NAVA. What about PAV? Is that not how PAV works?
Branson:
In my experience with PAV on the Puritan Bennett ventilator, I'm more concerned with how do I know the patient's ventilatory drive and respiratory muscle strength. The perfect example is P0.1 [airway-occlusion pressure 0.1 s after the start of inspiratory flow]. In a patient with normal respiratory muscles and a strong ventilatory drive, P0.1 is a good marker of demand. In a patient with COPD with hyperinflation and respiratory muscle fatigue, the brain signal may be just as strong or stronger, but the muscle strength only creates a pressure of –2 cm H2O.
So how do I determine which patients have high ventilatory drive or high demand when I measure the muscle strength, versus which ones have a high demand but not the muscle strength to show me that? My understanding about PAV is that it's kind of 50/50. Without setting it separately you have 50% flow-assist and 50% volume-assist.
In my ICU we get a lot of obese or trauma patients with a stiff chest wall, in whom the 50% volume-assist is insufficient and the 50% flow-assist is excessive. I've seen patients on pressure support of 10 or 12 cm H2O and with a rate of 20–22 breaths/min go on PAV and immediately we saw accessory respiratory muscle use and patient discomfort. I think the issue is not so much that PAV monitors the elastance and resistance and tries to match the patient, but actually having a good noninvasive signal from the respiratory muscle to let me know if the patient has fatigue.
Footnotes
- Correspondence: Richard D Branson MSc RRT FAARC, Division of Trauma and Critical Care, Department of Surgery, University of Cincinnati Medical Center, 231 Albert Sabin Way, ML 0558, Cincinnati OH 45267-0558. E-mail: richard.branson{at}uc.edu.
Mr Branson has disclosed relationships with Covidien, Ikaria, Hamilton, CareFusion, and General Electric.
Mr Branson presented a version of this paper at the 46th Respiratory Care Journal Conference, “Patient-Ventilator Interaction,” held March 19-21, 2010, in Cancún, Quintana Roo, Mexico.
↵* Magdy Younes MD FRCP(C) PhD, Department of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
- Copyright © 2011 by Daedalus Enterprises Inc.