Abstract
The nasal cannula has been a commonly used patient interface to provide supplemental oxygen since its introduction in the 1940s. Traditionally, it has been categorized as a low-flow device and capable of delivering a 0.4 FIO2 with flows up to 6 L/min to adults with normal minute ventilation. However, there is considerable performance variability among patients and design, which results in an exponential decline in delivered FIO2 as breathing frequencies increase. The nasal cannula has also been successfully adapted for use in perinatal and pediatric respiratory care; flows are reduced, in the range of 0.25–1 L/min, due to smaller minute volumes. A decade or so ago, high-flow nasal cannula (HFNC) oxygen therapy was introduced, accompanied by heated humidification systems to prevent the associated drying of upper airway mucosa and to increase patient comfort. Therapeutic flows for adults were in the 15–40 L/min range; FIO2 could be independently adjusted with air/O2 blending. The HFNC has also found additional clinical application in perinatal care, as delivery systems with flows > 2 L/min could create a distending pressure similar to nasal CPAP. There is a small but growing body of information from clinical trials that supports use of HFNC as an alternative oxygen interface for adults who present with moderate hypoxemia that persists after receiving oxygen by reservoir-bag masks or similar therapy. Clinical observations report greater patient acceptance and comfort versus oxygen masks. HFNC therapy has also been considered valuable in perinatal care in treating the respiratory distress syndrome or supporting patients after extubation similar to nasal CPAP. At present, research-based evidence for the role of HFNC for its perinatal applications remains unclear. This review will identify proposed mechanisms for therapeutic effectiveness, current delivery equipment, guidelines for rational patient application, and direction for further research.
Introduction
Since the introduction of the nasal cannula in the mid-1900s, it has become the most commonly used appliance to provide patients with supplemental oxygen in hospital or ambulatory care. More recently there has been renewed interest and research on its application with gas flows substantially higher than those traditionally recommended. Currently there are no established guidelines and/or decision-making pathways to guide use of the high-flow nasal cannula (HFNC) therapy for adults or infants. A lucid approach to apply specific oxygen delivery devices or systems can be complex. Initially the treatment setting, scenario, presenting signs and symptoms, and patient history are reviewed. Oxygen therapy delivery devices are then considered, based on how their performance characteristics best match their ability to either support or correct the patient's pathophysiologic conditions. Decisions may be modified if other comorbid conditions are found to be present, as well as to deal with issues such as convenience, cost constraints, and patient comfort. The purpose of this review is to summarize physical features, advantages, limitations, and current literature on clinical application of HFNC. The goal will be to assist clinicians in identifying important aspects as to the efficacy of these systems and to guide decisions for their use with adult, perinatal, and pediatric patients.
History
The clinical application of supplemental oxygen has been credited to Thomas Beddoes and colleagues James Watt and Sir Humphry Davies, who worked in England during the late 1700s. Initial patient interfaces were modified anesthetic masks or mouthpieces; oxygen was manufactured on-site and made available at local apothecaries. By the early 1900s small diameter (8–10 French) rubber catheters were used to stream oxygen directly into the nasopharynx. That technique was clinically applied to victims of World War I gas poisonings, and by the 1920s used in pediatric care.1 In the United States, Alvan Barach developed a Y-tube to split oxygen flow into a double nasal catheter in order to halve the effect of high flow on nasal mucosa.2 By 1925 the British developed a less invasive single and dual oxygen prongs that allowed oxygen to be directed just inside naris or nares. In the mid-1930s, rubber-tipped metal nasal prongs were available. They could be positioned in the nose by using eyeglass frames or held on the forehead by laryngologist head-mirror straps. An early version of the modern plastic over-the-ear nasal cannula appeared by the 1940s, in the form of a blind-ended tube with smaller side tubes to direct flow into the nose. It did not take long for clinicians to recognize cannula limitations in terms of the discomfort and low level of oxygen supplementation to the patient's minute ventilation (V̇E). In response, accessory masks, reservoir bags, or sponge-plugs fitted into the nostril were configured. However, they also recognized that the nasal cannula advantages, compared to masks, was in allowing patients to eat, drink, and speak more easily, and also to avoid the claustrophobic sensation of a face mask.1,3
Clinical Evaluation of Adult Low-Flow Nasal Cannulas
The early clinical literature on oxygen therapy began recommending specific devices based on some objective criteria, especially the ability of equipment to achieve specific ranges of oxygen concentration. That information served clinicians as a general guide in care of patients with varying levels of dyspnea and/or hypoxemia. Selection was also tempered by consideration of comfort or patient acceptance. The first textbook on inhalation therapy suggested that ≈30% oxygen could be obtained with a nasal cannula in adult patients with flow of 4 L/min. It also advocated (unheated) water-bottle humidification for both nasal cannula and catheter. Barach noted considerable variation in the literature as to the deliverable concentration, depending both on oxygen flow and level of ventilation.4 Initial estimation of the oxygen concentration delivery performance was based on crude calculation or speculation.
There has been a considerable research effort to objectively measure the cannula's oxygen performance. A standard technique measured gas samples by aspirating nasopharyngeal samples. Gas analysis was performed by paramagnetic O2 analyzers: the Scholander device or gas chromatographs.3 In 1963 Kory et al studied small groups (10–15) of normal subjects. They suggested that in common clinical situations, cannulas could be expected to deliver FIO2 in the range of 0.35–0.45 with flows of 8–10 L/min (Table 1); they also subjectively assessed patient comfort by having subjects rank the cannula with other devices. Subjects found the cannula more comfortable than face masks, face tents, and catheters, but not as comfortable as an oxygen tent. They also evaluated the effect of closed versus open mouth with flow of 6 L/min and found negligible difference with either cannula or catheter. However, these researchers commented that the nasopharyngeal sampling technique was likely influenced by oxygen streaming into the sampling catheter; this would result in higher concentrations. Kory's group estimated that nasopharyngeal samples were approximately 5–10% higher than what would be expected at the alveolar level.3
Oxygen Concentrations Delivered by Nasal Cannula
These data became part of early inhalation therapy textbooks in terms of performance and recommended upper limit of flow for comfort.5 With the advent of arterial blood gas (ABG) analysis, a number of studies were published that identified mean PaO2 values and ranges of response variations for normal subjects breathing at various levels of oxygen flow; open and closed mouth breathing was also measured.6 A landmark study by Cherniack and Hakimpour measured response to nasal cannulas (over a range of oxygen flows) in patients with a variety of pulmonary diseases and severity of hypoxemia.7 Pulmonary function data, including diffusing capacity and the ratio of dead space to tidal volume, allowed the authors to correlate varying levels of oxygen responsiveness to other pathophysiologic indices. Those authors strongly recommended that oxygen administration by cannula should be titrated based on ABGs. They also found that oxygen-induced hypoventilation and worsening hypercarbia were rarely seen in patients with COPD who also presented with elevated baseline PaCO2.7 In 1976 Julian Leigh continuously measured inspired and exhaled oxygen and carbon dioxide levels and breath-by-breath changes with a pneumotachograph. Using an oxygen-carbon dioxide diagram developed by Rahn and Fenn, he extrapolated an intercept on the oxygen axis that represented an average delivered FIO2. He recorded changes in FIO2 based on the device being able to match the subject's inspiratory flow, and used that information to distinguish between low-flow variable performance systems. Although Leigh did not specifically measure nasal cannula performance, he touted the ability of large bulk flows through high air flow oxygen entrainment used by the “ventimask,” which could better match patient inspiratory flow for more stable oxygen concentrations.8
Researchers continued to measure FIO2 levels produced in the trachea at different nasal cannula flows with more accurate and rapid response devices, such as the mass spectrometer. In 1976 Gibson et al connected a spectrometer to a cricothyroid catheter to measured intra-tracheal FIO2 at increasing levels of V̇E and peak inspiratory flow, to simulate dyspneic breathing patterns.9 A similar approach was used by Markovitz and coauthors as they compared direct gas samples from transtracheal catheter and oral catheters with exhaled gas, using the Rahn and Fenn method.10 There was good correlation between direct sampling techniques and gas extrapolation; they documented nasal cannula performance (97% oxygen concentrator source gas) at 1, 3, and 5 L/min while subjects breathed at normal breathing frequency and pattern. In contrast to many undocumented textbook estimates of cannula performance, at low flows they found the delivered FIO2 increased 0.025 (2.5%) per each 1 L/min increase in flow. With nasal cannula flow of 5 L/min, tracheal sampling recorded FIO2 of 0.318 ± 0.005, and with the exhaled technique 0.327 ± 0012 (Fig. 1).10
FIO2 delivered by nasal cannula measured at mouthpiece, using exhaled gas technique. The 97% oxygen reflects delivery from an oxygen concentrator. (From Reference 10.)
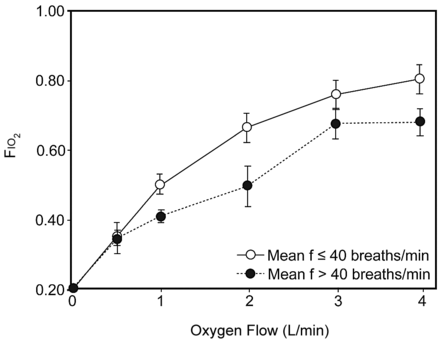
Schacter and colleagues compared hypopharyngeal sampled FIO2 to those obtained via catheters inserted through “sealed” permanent tracheostomies (Table 2). Mean FIO2 for hypopharynx or intratracheal samples at 4 L/min were 0.27 ± 1.0 and 0.26 ± 1.2, respectively. They also demonstrated considerable inter-subject variations as well as found substantial secondary air dilution with tachypnea. On 4 L/min with a breathing frequency of 40 breaths/min, mean FIO2 decreased to ≈0.245.11 Wettstein and colleagues demonstrated similar effect from tachypnea (doubling of normal breathing frequency) using hypopharyngeal sampling in normal subjects (Fig. 2).12
FIO2 Measured Via Hypopharyngeal and Intratracheal Sampling
Mean ± SD FIO2 for pharyngeal sampling of nasal cannula breathing with a breathing frequency (f) 2 times normal for adults. (From Reference 12.)
Using Leigh's technique, Bazuaye et al estimated cannula performance and also measured oxygen response with both normal subjects and patients, using an ear oximeter.13 That technique found concentrations slightly higher than the cricothyroid catheter data; it also recorded substantial inter-subject variation. At 1 L/min, normal subjects had a mean FIO2 of 0.267, with a coefficient of variation of 10%. With 2 L/min, mean FIO2 was 0.301 and the coefficient of variation was 8.6%. Of interest was a much wider variation in patients. At 2 L/min, FIO2 varied from 0.237–0.349. That group also stated that “the variability with nasal cannulas during an exacerbation is likely to be appreciably greater than that found here.”13
The aforementioned research makes clear several points: measurement of nasal cannula FIO2 is not clinically practical; there is performance variability even among normal subjects; textbook predictions are overly optimistic and not based on research data; and delivered FIO2 decreases considerably during conditions associated with dyspnea. Because of these 4 factors, the noninvasive pulse oximeter has gained nearly universal acceptance to allow titration of oxygen flow to the cannula. Periodic arterial blood gas analysis would be indicated to also assess hypercapnia and co-oximetric hemoglobin saturation.14–16
Clinical Evaluation of Low-Flow Perinatal and Pediatric Nasal Cannulas
Early approaches to provide enriched oxygen to infants was done using an incubator with supplemental oxygen delivery capability (eg, Isolette) and oxygen tents/hoods. Unregulated levels led to complications of retinopathy of prematurity and wide FIO2 variations when enclosures were opened for access to infants.17,18 The nasal catheter and cannula did become established as standard appliances. Technical issues included use of special low-flow flow meters to accurately measure flows < 3 L/min. Beginning in the late 1970s, interest in low-flow nasal cannula (LFNC) administration increased for application to newborns with resolving respiratory distress syndrome (RDS) and bronchopulmonary dysplasia.19,20
In 1989 Vain and colleagues reviewed 10 neonates with gestational ages ranging from 25–39 weeks, and documented the potential for infants to receive FIO2 > 0.4 when flow exceeded 0.5–0.75 L/min.21 Of additional interest was early promotion of flow meters driven by oxygen-air blenders to provide less than 100% O2 (FIO2 of 0.4, 0.6, and 0.8) to the cannula. They found that independent adjustment of flow and FIO2 allowed better control and less dramatic changes when reducing oxygen levels during weaning.21 Kuluz and colleagues reported mean hypopharyngeal FIO2 of > 0.4 when infants (age range 7–17 months) received oxygen flows exceeding 1 L/min. Some infants received FIO2 > 0.8 with flows as low as 2 L/min (Fig. 3). Both the range and mean delivered FIO2 were reduced at oxygen flows > 1 L/min if the infant's breathing frequency exceeded 40 breaths/min.22 Current American Association for Respiratory Care clinical practice guidelines make no statement with regard to delivered FIO2 levels, yet note how they can be affected by both changes in V̇E and the patient's inspiratory flow. However, the clinical practice guidelines do caution clinicians with regard to accuracy of low-flow flow meters and the potential for inadvertent CPAP, and therefore recommend maximum flow levels of 2 L/min.23
Infant cannula FIO2 versus oxygen flow. f = breathing frequency. (From Reference 22.)
Physical and Anatomical Aspects Affecting Oxygen Delivery and Efficacy of the Nasal Cannula
A number of the previously mentioned studies illustrated important points and problems for both researchers and clinicians attempting to predict FIO2 delivered via nasal cannulas.3,7,8 To objectively evaluate performance and potential efficacy, a number of variables must be either controlled for in research or considered when evaluating cannula systems in a real clinical setting (Table 3). Since the LFNC is an open system and by design is not intended to provide the entire inspired volume the patient inhales, room-air dilution (inboard leak) will alter the system's gas and the FIO2. With higher system flow, resident airway gas may be altered by incoming system gas during the expiratory phase.
Variables in Evaluating Cannula Systems
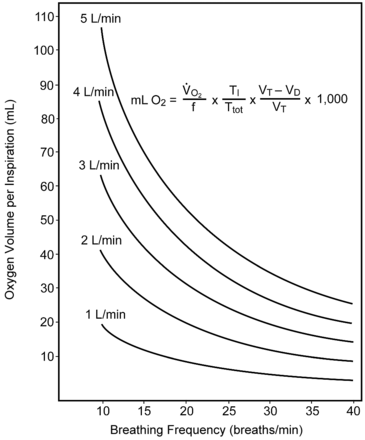
The relationships of gas flow, oxygen concentration from the flow meter, and patient inspiratory flow pattern were discussed by Shigeoka and Bonekat in an editorial, and are illustrated in Figure 4; their equation identifies the nonlinear effect of those variables on delivered FIO2.24 For that graphic the ratio of inspiratory time to total respiratory cycle time was set at 0.35, and the ratio of dead space to tidal volume was fixed at 0.4. The flow pattern during spontaneous breathing varies significantly and changes with inequality of inspiratory-expiratory ratio and degree of dyspnea. Although the inspiratory waveform during resting breathing does not conform to a half-sine wave, it closely approximates that pattern when spontaneous V̇E increases. Equations predicting peak flow levels have been developed that may be useful in estimating inspiratory flow demand.25
Physical variables affecting FIO2 of nasal cannula with increasing breathing frequency (f), at flows from 1–5 L/min. Graphic and equation identify multiple factors that cause an exponential decline in volume of oxygen that can be inspired with increasing f. Tidal volume (VT) and dead space (VD) were held constant. V̇O2 = oxygen uptake. TI = inspiratory time. Ttot = total respiratory cycle time. (From Reference 24.)
The anatomy and geometry of the human nasal cavity, nasopharynx, and oropharynx should also be considered. A combination of oronasal breathing occurs in awake adults during speech and in response to exercise. Air flow profiles have been investigated in the past with in vitro models made using model casts from adult human cadavers. Hydrodynamic analysis of flow dynamics can be estimated based on visualizing smoke or radioactive tracers, small implanted Pitot tubes, or laser Doppler velocimetry. More recently mathematical models have been developed based on 3-dimensional computed tomography of the nose and computational fluid dynamic techniques. Results from these calculations appear to agree with human experimental data.26 The cross-sectional area of the nasal cavity can also be measured using acoustic rhinometry. In this technique an acoustic pulse is followed as it traverses each nasal cavity during an inspiratory cycle.27 Peak nasal inspiratory flow can be measured in human subjects using a peak flow meter with a mask. The following is a summary of pertinent findings that may relate to clinical aspects of HFNCs.
Initial high-velocity inspiratory air flow passes through the lower half of the nasal cavity; this is primarily a respiratory function. The olfactory groove in the upper portion of the nasal cavity receives lower velocity gas, yet flow is enhanced with sniffing.
The anterior nasal cavity's functional nasal valve is the narrowest point of the nasal passage and major determinant of flow; this area is the flow limiting segment. The minimum cross-sectional area in adults is approximately 3 cm from the anterior naris.
The posterior cavity is wider and circular; expiratory flow is directed along the turbinates.
Nasal cavity volume and area have a linear relationship to flow; the nasal cavity does not directly follow the Poiseuille equation.
Dynamic changes in nasal resistance may be affected by collapse of soft tissues as well as downstream resistance of the lower airways.
Differences in the newborn's nasal airway anatomy should also be considered.
The nose is the preferred route, but is supplemented by oral breathing during sleep; the term “obligatory nasal breathers” has traditionally been used.28
While feeding, a neonate's breathing is facilitated by the posterior tongue pressing upward on the soft palate to block the oral airway, which is referred to as the “veloglossal sphincter.” This contributes to mouth breathing, requiring more coordination and creating higher resistance than only nasal breathing.29
Primary oronasal breathing occurs normally with crying and returns to uninterrupted nasal or some combination of oronasal. When the nose is occluded, approximately 40–50% of normal term infants will quickly switch to oral breathing in 3–9 seconds; others require up to 30 seconds.28
There is a high level of resistance with oral breathing in preterm infants, which may limit effective breathing and result in obstructed efforts. Ability to adjust to sustained oral ventilation if nasal resistance or occlusion occurs is an important adaptive phenomenon in neonates and older infants.30
A premature infant's skin is especially susceptible to injury from nasal mask prongs from nasal CPAP (NCPAP), or HFNC devices. Damage may occur at the nares, intranasal septum, the septum's anterior tip (columella), or philtrum. Both correct sizing and stabilization to prevent abrasion from device or tubing traction can minimize nasal lesions.
In summary, the anatomy and nature of the nasal and nasopharyngeal airway of preterm, neonate, and infant babies suggests a complex pattern of resistance level and nose versus mouth breathing. There are also complex physical relationships in the adult airway, which result in changing dynamics on inspiration and exhalation. These factors may make bench or in vitro studies using resuscitation-type manikins difficult to apply to humans.
Proposed Benefits of High-Flow Oxygen Via Cannula in Adult and Neonatal Care
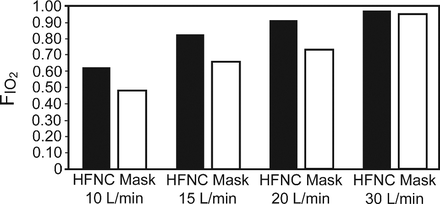
To improve the efficiency of the standard adult nasal cannula use at low flows, 2 approaches have been used to increase the volume of the inhaled oxygen bolus and reduce air dilution. The first technique was to attach storage reservoirs to the cannula. That approach was describe by Barach in 1960.31 Contemporary storage cannulas on those themes exist today but are largely relegated to ambulatory therapy.32,33 The alternative approach was to increase levels of gas flow to the cannula. Since the mid-1960s respiratory care textbooks have cautioned directing flow > 6 L/min with the cannula34,35; such recommendations continue.15 Reasons given include general patient discomfort, frontal sinus pain, as well as physical irritation and drying of nasal mucosa. The separate technical issue of humidification is a complicating factor. In current practice, oxygen at lower flows is either not humidified at all or is humidified with unheated “bubbler” humidifiers that do not meet recommended levels for the upper airway.36 In 2002 the first abstracts appeared describing use of HFNC for adults, with flows of up to 40 L/min.37,38 In a bench study, normal subjects breathed with a breathing frequency of 20 breaths/min through an adult airway model. Tiep and Barnett observed ultrasonic fog to evaluate the function of the nasal cavity and upper airway as an oxygen reservoir. They also measured peak FIO2 with the Vapotherm HFNC system and compared it to a non-rebreather reservoir mask (NRB). They found the HFNC capable of delivering FIO2 of 0.62, 0.82, 0.9, and 0.92 with flows of 10, 15, 20, and 30 L/min, respectively (Fig. 5).37 Under such in vitro conditions, the cannula achieved higher FIO2 levels than a NRB bag mask at comparable oxygen flows. The authors postulated that the higher FIO2 levels were achieved due to high flow's limited inboard air dilution, and the nasopharynx and oropharynx acted as an (internal) anatomic reservoir that could increase the volume of inhaled oxygen for the subsequent inhalation.37 Using a cardiopulmonary-resuscitation-type manikin-airway and dual chambered test lung simulator, Malinowski and Lamberti found that an FIO2 of 0.83 could be achieved with a breathing frequency of 40 breaths/min, tidal volume of 0.5 L, and 25 L/min flow to the cannula.38
Bench evaluation comparing FIO2 delivered by high-flow nasal cannula (HFNC) versus reservoir mask. Peak FIO2 levels measured at beginning of inspiration are plotted for Vapotherm HFNC versus non-rebreather O2 mask at 4 flows. The system combined an airway model with human subjects ventilation at a breathing frequency of 20 breaths/min. (From Reference 37.)
In 2004, a clinical study compared high-flow 40% oxygen delivered by Vapotherm humidifier to low-flow oxygen via mouthpiece during rest and exercise, using a non-randomized comparison crossover study. Ten COPD patients with exercise limitation were studied as they exercised on a cycle ergometer.39 The authors found statistical improvement in PaO2 and SpO2 with the Vapotherm system with non-statistical improvement in duration of exercise and dyspnea index. They attributed these results to reduced room air entrainment, increased anatomical reservoir, and dead space washout effect. The authors suggested that the high-flow humidified gas might also be applied in clinical care as an intermediate device between traditional oxygen appliances and noninvasive ventilation (NIV).39 In 2004, a bench-study compared the humidification capability of 2 high-flow cannula systems; both achieved minimum recommended humidification levels, but the study did not evaluate oxygen delivery performance.40 In 2005, the performance of the Salter cannula and humidifier was studied in 10 normal subjects breathing at various breathing frequencies as well as during mouth closed versus open breathing.12 When 6–15 L/min flows were used, pharyngeal sampling demonstrated mean FIO2 ranging from 0.54–0.75 during normal resting nose breathing. FIO2 dropped slightly with increased breathing frequency but increased with mouth breathing.12
In contrast to the ability of higher flows to improve delivered oxygen concentrations to adults, the emphasis in perinatal care has been on higher flows creating CPAP-like distending pressures. Soon after the elucidation of the pathophysiology of infant RDS, Gregory et al described use of CPAP with intubated neonates to counteract atelectasis caused by surfactant deficiency.41 In the early 1990s, CPAP-like distending pressure was demonstrated when premature neonates received higher flows using standard nasal cannula prongs. Locke and colleagues found that nasal cannulas (0.2 and 0.3 cm outer diameter) could generate up to 9.8 cm H2O at gas flows of 2 L/min. This positive pressure effect was felt to improve both oxygenation and thoraco-abdominal synchrony. They suggested careful prong size selection and cautioned against its indiscriminate use.42 In the mid and late 1970s studies were published that noted an additional therapeutic effect of NCPAP. Besides its treatment for RDS, NCPAP was found to affect respiratory control in newborns, as it decreased the number of central apneas (also known as apnea of prematurity) and subsequent bradycardia and oxygen desaturation; HFNC has been used as an alternative delivery device.43,44
HFNC therapy continues to be evaluated as an alternative nasal interface for newborns with RDS or as transition support immediately following extubation and mechanical ventilation.
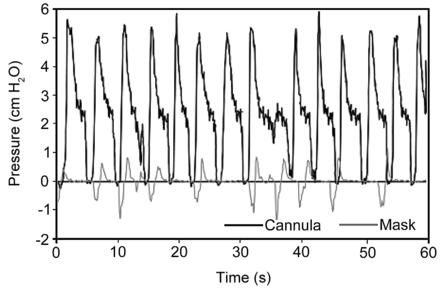
The ability of HFNCs to also provide low level distending pressure to the upper airway of adults was documented a few years ago.45,46 In both studies, pressure measurement was performed by inserting catheters within the nasopharynx of normal subjects. In the Groves study, with HFNC flow of 40 L/min and mouth closed, expiratory pressures ranged from 3.2–5.2 cm H2O.45 A representative pressure versus time scalar from the Parke et al study is shown in Figure 6. When the HFNC delivered 35 L/min, mean nasopharyngeal pressure (during the entire respiratory period) was found to be 2.7 cm H2O. Of note is that the pressure waveform dropped to zero during inspiration. This documented a difference of the HFNC from an ideal CPAP delivery system.46 However, the HFNC's upper airway distending pressure was proposed to be transmitted to lower airways and alveoli to potentially benefit patients with either low pulmonary ventilation-perfusion regions causing hypoxemia and/or mechanical defect resulting from low functional residual capacity (Table 4).
Upper airway pressure versus time scalar of a subject using a high-flow nasal cannula. A nasopharyngeal cannula measured a mean pressure of approximately 2.7 cm H2O (bold line). The cannula was set at 35 L/min and the subject breathed with mouth closed. The gray line shows the pressure with an aerosol type face mask. (From Reference 46, with permission.)
Summary of Proposed Effects That Determine Therapeutic Effects of High-Flow Nasal Cannula
In the past 5 years there has been increasing interest in the HFNC as an alternative oxygen delivery interface versus more established adult delivery devices such as NRB or masks, which use air-entrainment as part of large-volume aerosol nebulizer systems. In perinatal and pediatric care, besides oxygen delivery, the HFNC potential as an alternative NCPAP device has generated substantial research as to its application. A review of literature on the evaluation of the efficacy of both therapeutic approaches follows below.
Equipment for Delivery of High-Flow Nasal Oxygen
HFNC for Adults
A delivery system to provide HFNC oxygen requires 3 components: a patient interface, a gas delivery device(s) to control flow and FIO2, and a humidifier. An adult nasal cannula with standard dimension prongs can accommodate high inlet flow of at least 60 L/min. Several manufacturers provide cannulas of similar style and appearance, which are designated for high-flow applications (Fig. 7). The Fisher & Paykel OptiFlow cannulas use a different design. The nasal prongs are held in place on the upper lip with an elastic over-ear head band. There is a larger diameter flex tubing proximal to the prongs and an around-the-neck elastic that connects to support the weight of the connecting tubing (see Fig. 7D). They are available in large, medium, and small sizes, with oval shaped prong orifices measuring approximately 6.5 mm outer diameter and 4 mm outer diameter, respectively (see Fig. 7E).
Examples of adult high-flow nasal cannulas. A: Salter Labs 1600HF high-flow adult cannula. (Courtesy of Salter Labs.) B: Comfort Flo. (Courtesy of Teleflex Medical.) C: Aquinox high-flow cannula. (Courtesy of Smiths Medical.) D: OptiFlow adult cannulas. (Courtesy of Fisher & Paykel.) E: OptiFlow high-flow adult cannula on an adult patient.
Medical gas for LFNCs is commonly provided by a (0–5 or 0–16 L/min) calibrated oxygen flow meter. To accommodate higher flows, commercially available calibrated high-flow (0–70 L/min) oxygen flow meters can be applied. To allow independent adjustment of FIO2 < 1.0, separate high-flow air and O2 flow meters can be connected via a “Y-piece” adapter. To allow more convenient application, high-flow air/O2 proportioner valve blenders or high-flow “Venturi” air mixing valves can be used. With either approach, an oxygen analyzer is needed to confirm accurate air/O2 mixing.
A key element for clinical use of nasal oxygen at high flow is effective humidification. Supplemental water vapor is not commonly provided for low flows, ≤ 2–3 L/min. There has been a tradition of humidification for flows > 5–6 L/min. Unheated bubble-type humidifiers have been used in spite of both lack of evidence of overall efficacy and decreasing efficiency of humidification as flows increased.15,16,47 Minimum water content for low-flow cannula use has not been defined. When patients breathe dry oxygen from a nasal cannula, additional water vapor is entrained with room air from their environment. This would vary significantly depending on both temperature and relative humidity. The ASTM International (formerly the American Society for Testing and Materials) recommends a minimum absolute humidity of 10 mg H2O/L for gas that does not bypass the upper airway. This would approximate a relative humidity of 50% at 22°C, which reflects normal environmental conditions.48
In early 2000, the first 2 commercial adult HFNCs appeared, which were promoted to deliver flows > 10 L/min with accompanying humidifiers. Salter Labs produced a high-flow (unheated) bubble humidifier (Fig. 8). It resembles a low-flow bubbler design. The diffuser's design is touted to allow reduced resistance; standard narrow bore tubing is used en route to the cannula, and meets minimum humidity standards when used at flows up to 15 L/min. That maximum flow capability is on the lower end of other commercial adult HFNC systems. About the same time, Vapotherm developed the 2000i High-Flow Therapy system, which consisted of a designated cannula with a heated cartridge-type humidifier using membrane technology for water vapor transfer (see Fig. 8B). Flow capacity with adequate humidification was advertised up to 40 L/min; the system requires a separate air/O2 blender and flow meter. A 2004 bench study reviewed both systems for the capability to provide humidification over the manufacturers' recommended flows for clinical use.38 The Salter device's relative humidity levels at ambient temperature (21–23°C) ranged from 76.9 ± 5.8% and 78.7 ± 4.6% at flows of 10 and 15 L/min, respectively. The Vapotherm system achieved close to 100% relative humidity with the heated system operating at 36.5°C.38 The Vapotherm 2000i was recalled in 2005 due to concerns of bacterial contamination, but was reinstated after manufacturing controls and recommendations for use of only sterile water, single-patient use, and disinfection procedures.49
Humidification systems for high-flow cannulas. A: High-flow bubble humidifier. (Courtesy of Salter Labs.) B: Vapotherm 2000i heated humidifier with continuous feed system and high-flow air/O2 blender (BL S2001-HF) with click-style flow meter (FM40). (Courtesy of Vapotherm.) C: Fisher & Paykel 850 heated humidifier (Courtesy of Fisher & Paykel) and Maxtec MaxVenturi combination O2/air entrainment device, oxygen analyzer, and flow meter with single-limb heated-wire circuit. D: Comfort Flo humidification system. (Courtesy of Teleflex Medical.) E: Aquinox high-flow humidification system. (Courtesy of Smiths Medical.) F: Pari Hydrate humidification system for high-flow nasal cannula. (Courtesy of Pari Respiratory Equipment.) G: Vapotherm Precision Flow humidification system. (Courtesy of Vapotherm.)
The Fisher & Paykel OptiFlow HFNC became commercially available in 2006 (see Fig. 8C). The system uses a heated humidifier with hot-plate and single-use water chamber, similar to those for application for noninvasive or invasive mechanical ventilation. A separate or combined air/O2 blender or air-oxygen entrainment device and flow meter provide oxygen mixtures with flow ≥ 40 L/min. An oxygen analyzer is also required. Humidified gas mixtures exit the humidifier through large bore corrugated tubing that connects to the cannula with a 15 mm outer diameter adapter. A heated-wire circuit is used to minimize condensation to prevent liquid water from potentially obstructing the HFNC. Flow continues through an 18 cm length of 10 mm outer diameter flex tubing and finally to the cannula. In the past few years other manufacturers have developed similar systems. The Teleflex Comfort Flo humidification system was made available in 2006. It incorporates a cartridge-humidifier and is designed to accommodate continuous gas flow up to 40 L/min (see Fig. 7D). The Smiths Medical Aquinox high-flow humidification system allows humidification up to 35 L/min (see Fig. 8E). Both of the latter 2 devices require adjunct oxygen/air blenders and flow meters. The Pari Hydrate humidifier was approved in 2007 for clinical use, including HFNC applications (see Fig. 8F).
In 2008 Vapotherm released its Precision Flow high-flow humidification system (see Fig. 8G). It is similar to the 2000i, as it uses a cartridge-like humidifier. This model differs from the Vapotherm 2000i and the other commercial systems as the air/O2 blender and oxygen analyzer are integrated within the humidifier module.
Because of the need to provide independent control of oxygen concentration and a heated humidifier, the acquisition cost of an HFNC system is substantially higher than for a typical LFNC system. Purchase cost for a commercial HFNC system (stand, blender/flow meter/analyzer, humidifier, heated-wire circuit, and cannula) is approximately $2,600. A less expensive alternative system could be assembled if air and O2 high-flow (0–70 L/min) flow meters were substituted for the air/O2 blender-flow meter and already available oxygen analyzer, and humidifier were used. In addition to free-standing humidified high-flow blenders, there are some current mechanical ventilators that can also serve as sources for continuous flow of humidified blended gas for HFNCs.
HFNC Equipment for Newborn and Infants
As was mentioned previously, the clinical application of nasal cannula oxygen with flow > 1 L/min applied to premature babies, newborns, and pediatric patients had an earlier and slightly different evolution than with adults. A number of patient interfaces were initially developed for NCPAP, including head-hood or facial chamber, sealing face or nasal mask, and long nasopharyngeal tube. Currently NCPAP is most commonly applied using short bi-nasal pharyngeal prongs, with humidified gas source and circuit. A recent Cochrane Collaboration analysis literature review has suggested that short bi-nasal prongs are more effective than the nasopharyngeal tubes. They noted that the literature is not clear on the optimal pressure generating source.50 Of key importance with NCPAP nasal prongs is correct sizing, as they are designed to seal within the nares to maintain constant pressure. Attachment and fixation of the prongs is commonly accomplished by head cap. Caps permit prongs to be placed so that they either avoid touching or gently rest on the columella and minimize abrasion from movement of the child or traction from tubing. NCPAP nasal injuries from prongs and masks are common and have been documented in the past. They can range from hyperemia to necrosis and result in permanent damage.51,52 The most important independent risk factor for complications is duration of NCPAP use.53
As was noted previously, an NCPAP-like effect has been recognized when using (non-sealing) nasal cannulas at higher flows.42,54 Equipment includes nasal cannulas with a number of sizing options. Figure 9 identifies 2 examples of commercially available perinatal nasal cannulas for high-flow application. Manufacturers typically identify the outer diameter of prong, and septum width; maximum patient flow is usually recommended. For both premature and newborns, prong diameter is approximately 2.4 mm outer diameter; septum width is 2.5 mm for premature and 3.5 mm for neonates. Infant and pediatric prong diameters would be approximately 2.4–3.7 mm outer diameter, and septum widths vary from 4.5 to 8 mm. Methods to create distending pressure include underwater column (also known as bubble CPAP) or flow generator with inspiratory-expiratory switching valve. The humidification systems used include those previously identified for HFNC for adults. An example of a Fisher &Paykel system is shown in Figure 10A. Blended air/oxygen mixtures are provided by flow meters capable of accurately indicating actual flow in low ranges (≈1–5 L/min). Heated-wire circuits are commonly used to reduce the potential for condensate formation and liquid water from reaching the nasal prongs. Because of their distending pressure effect, it is important that systems have some method to prevent high pressure from either unexpected increases in downstream resistance or inadvertent increase in delivered flow (see Fig. 10B). The Vapotherm Precision Flow device uses sensors to detect downstream obstruction, which causes an audio alarm and visual alert.
Examples of perinatal high-flow cannulas. A: Fisher & Paykel premature, neonatal, and intermediate infant cannulas. (Courtesy of Fisher & Paykel.) B: Neotech RAM cannula. (Courtesy of Neotech.)
A: Example of a perinatal high-flow nasal cannula system. B: Detail showing pressure-relief valve.
Randomized or Quasi-Randomized Trials of HFNC for Adult Patients
The major objective of published evaluations of adult HFNCs has targeted its ability to provide enriched oxygen to hypoxemic patients. The reports have included bench study simulations and patient investigations, many published only in abstract form.
Several peer-review published summaries have also included abstracts in their analysis of evidence.55,56 There are also several published papers that were conducted with normal healthy adult volunteers to specifically quantify the capability to generate positive airway pressure.45,46; one was in the form of a case study.57
All 3 studies measured pressure via pressure transducer fitted to nasopharyngeal catheters. Groves and Tobin varied HFNC flow from 10–60 L/min and measured pressures when the mouth was closed and open. With closed mouth, mean pharyngeal pressures rose linearly from 3.7, 7.2, and 8.7 cm H2O at flows of 20, 40, and 60 L/min, respectively. With mouth open, pressures fell to 1.4, 2.2, and 2.7 cm H2O at the same flow levels.45 The Parke group maintained a constant 35 L/min flow and found mean pressure to be 2.7 cm H2O with mouth closed and 1.2 cm H2O when mouth open.46
In the recent past there have been an increasing number of clinical studies that have potential to guide clinicians in use of HFNC systems. A brief summary of 7 selected studies will follow and note key findings and limitations. The following criteria were used for their selection:
Studies not published in peer-reviews journals or abstracts were excluded.
In vitro bench studies (mostly with manikins) were excluded.
Normal human and animal studies were excluded.
Studies that did not have a measurable outcome related to oxygen therapy were excluded.
Studies that did not provide adequate technical details of the oxygen delivery systems used (eg, brand/model of devices, FIO2 settings, or gas flow levels) were excluded.
A chronological review of the selected studies with discussion will follow; Table 5 provides a summary.39,58–63 In 2004 Chatila and colleagues investigated Vapotherm humidified high-flow (25 L/min) blended oxygen during rest and exercise with COPD patients.39 The authors set up a crossover comparison with low flow oxygen. Their hypothesis was that the high flow and humidification of the Vapotherm device would provide improved oxygenation during exercise and reduce discomfort of nasal dryness. Although the humidifier was used, nasal cannulas were not worn by patients; gas was supplied to a mouthpiece as part of a pneumotachograph. Although a small study and not randomized to sequence of high or low flow, the authors did document statistical significance in PaO2 and SpO2 during 12 min cycle ergometer exercise, as well as duration of exercise with the high-flow Vapotherm. There was non-statistical improvement in breathing frequency, breathing frequency/tidal volume ratio, and inspiratory time/total breathing time ratio. Differences were seen even though the FIO2 for low-flow oxygen was adjusted to the equivalent 0.4 FIO2 of the high-flow system, which was set at 20 L/min. The study found no differences in V̇E, tidal volume, or work of breathing. It served only to suggest that high flow is probably better than low flow during periods when patients require high inspiratory flow. One limitation to applying these data was that oxygen delivery was not directly provided by nasal interface.39
Summary of Published Clinical Studies on Use of HFNC in Adults
In 2008 Price and coauthors performed a retrospective 2-year audit of 72 patients who received care in an Australian hospital's postoperative surgical high-dependence unit. Fifty-five of the subjects had a diagnosis of “type I” hypoxemic respiratory failure.58 The audit was done as the Vapotherm humidified HFNC had been newly added as an oxygen delivery system. The purpose of the study was to evaluate performance and identify problems and general level of acceptance by both patients and staff. HFNC O2 therapy was initiated after baseline ABGs and other vital signs were recorded. The average pre-trial baseline PaO2 was 61 ± 13 mm Hg, and SaO2 89.7 ± 5%. One hour after HFNC therapy (flow was 20–35 L/min), the average PaO2 increased to 93 ± 43.7 mm Hg and SaO2 to 94.55 ± 3.6%. However, precise performance analysis was limited, as the flow and FIO2 of the HFNC systems were individually titrated to the patient, the latter ranging from 0.6 to 1.0 with an average of 0.81 ± 0.21. The authors also noted a modest reduction in average breathing frequency, from 26.27 ± 7.1 to 22.57 ± 6.13 breaths/min. There were no changes noted in PaCO2 or pH. Ten of the 72 patients later required intubation and mechanical ventilation, but this could not be compared to historical data. Patient satisfaction with HFNC was rated at 90% positive, but not based on comparison to other oxygen therapy or analysis of dissatisfaction. The paper concluded that the HFNC could provide oxygen that was adaptable to post-surgical patients and was well accepted by staff.58
In 2010 Tiruvoipati and colleagues performed a prospective randomized crossover trial of 50 patients who required high-flow supplemental oxygen following extubation.59 The objective was to compare efficacy of the HFNC to high-flow oxygen delivered by mask in terms of gas exchange; secondary effects on vital signs, comfort, and patient tolerance were also studied. The Fisher & Paykel OptiFlow cannula was randomized to high-flow face mask therapy (not identified). During a 30-min post-extubation stabilization period, FIO2 was titrated to achieve desired oxygen saturations. Blind randomization identified patients for either HFNC or face mask system for 30 min, at the end of which vital signs were obtained. Patients then were switched to the alternate system and data collection repeated. Both oxygen delivery devices provided 30 L/min total flow, and FIO2 matched those required during the initial stabilization period. Comfort was evaluated by nurse interview, using a visual analog scale. Tolerance, or exit criteria, was based on observation of worsening respiratory, cardiovascular, and central nervous system signs. Results identified no statistically significant differences in gas exchange or vital signs. There was a statistically significant difference in patient tolerance in favor of the HFNC. Although also not statistically significant, there was a positive trend toward patient comfort for the HFNC group.59
Also published in 2010 was a prospective sequential comparison trial by Roca et al, using HFNCs with 20 patients identified as having hypoxemic respiratory failure refractory to conventional O2 therapy.60 These patients were selected if they did not increase their SpO2 > 96% after receiving standard oxygen therapy, which consisted of a combination LFNC and O2 via face mask (estimated FIO2 = 0.5 and total flow 15 L/min). After 30 min, ABGs, vital signs, and subjective evaluation of the face mask system's comfort were evaluated. The patients were then switched to HFNC, using a Fisher & Paykel humidifier and OptiFlow cannula, and the patient response was reassessed. The face mask's estimated 0.5 FIO2 was maintained by adjusting HFNC air/O2 flow meters, but total flow was set at 20 L/min and could be increased to 30 L/min if needed. Statistical significance was noted in increased oxygen levels and reduction in breathing frequency. PaO2 with mask increased from a mean value of 77 to 127 mm Hg with HFNC. Breathing frequency while mask breathing dropped from a mean of 28 to 21 breaths/min after switching to the HFNC; PaCO2 and pH were unchanged. Subjective evaluations for comfort (dyspnea, mouth dryness, and overall comfort) also favored the HFNC. Of interest were that some patients experienced cervical-thoracic discomfort with HFNC, which abated after reducing total flow.60
In 2011 Parke and coauthors published a prospective randomized comparison study of 60 patients admitted to a cardiothoracic and vascular ICU with mild to moderate hypoxemic respiratory failure.61 The patients presented with a wide range of baseline room-air PaO2 levels, which ranged from 56–92 mm Hg. Dyspnea was identified by tachypnea (breathing frequency ≥ 25 breaths/min) and/or other clinically observable signs. Study enrollment was based on hypoxemia and dyspnea, which persisted after treatment with either LFNC (≥ 4 L/min) or oxygen face mask (≥ 6 L/min). Patients were randomly placed on either OptiFlow HFNC or oxygen by air-entrainment diluter with an aerosol-type face mask; similar Fisher & Paykel heated humidifiers were used for both systems. There was no crossover in therapy. The outcomes evaluated were fewer treatment failures within 24 hours of treatment (patients required more aggressive treatment, consisting of NIV) and improved patient tolerance/comfort. Technical issues to note were that, as patients were placed on the HFNC system, flow level began at 35 L/min and was increased to potentially 60 L/min; FIO2 was also independently titrated to achieve an SpO2 of ≥ 95%. With the face mask system, total system flows were not reported (either calculated or measured). There was potential for the total flow to decrease as FIO2 was increased at the air-entrainment diluter. This is especially critical with an open-design face mask, as mask outlet holes allow secondary room entrainment if the patient's inspiratory flow demand is not met. This spiral of dyspnea and hypoxia causing increasing tachypnea can be accelerated as the diluter is adjusted to higher FIO2, which further decreases the system's total flow. Results of the comparison did identify statistically significantly fewer treatment failures in the HFNC group. Desaturation events were less common in the HFNC group (0.79/patient), compared to those receiving face mask oxygen (1.86/patient). It was not possible to analyze if the reason for this related to improved O2 delivery characteristics, mild distending pressures of the HFNC system, or problems with the mask being removed for communicating, eating/drinking, or taking oral medications. The potential of the HFNC system to develop positive airway pressure was not evaluated as a mechanism for improved oxygenation. The lessons provided by this research may be more valuable than only demonstrating that a certain oxygen delivery system was more capable than another. This study illustrated that the challenges of clinical research when matching a device to a patient problem is based both on hard science, critical thinking, and the art of responding to often subtle clinical clues. In addition, the authors noted that some clinicians altered their usual clinical practice by placing 5 patients on HFNC instead of NIV after perceived treatment failure of the face mask. This study provided insight that the more expensive and technically complex HFNC systems may be selected at an intermediate level between more modest devices (LFNC or entrainment masks) and NIV. The authors noted the limitation and difficulty of being able to perform detailed (real-time) analysis of factors that prompted oxygen desaturation episodes and treatment failure. Clinicians involved in research projects are required to have an understanding of technical specifications and limits of an oxygen delivery apparatus but must also balance patient factors such as changing breathing frequency, inspiratory patterns, dynamics of cardiopulmonary disease states, and ethics of placing patient safety before research.61
Sztrymf and coauthors performed a non-randomized prospective evaluation of HFNC applied to patients with acute hypoxemic respiratory failure in an ICU setting.62 Thirty-eight patients received OptiFlow HFNC oxygen if they failed to achieve an SpO2 of ≥ 92% with conventional oxygen therapy of NRB with ≈14 L/min oxygen flow. HFNC was applied with an FIO2 of 0.88 ± 0.016 and 49 ± 9 L/min flow; FIO2 was not measured but estimated with the NRB. The etiology of respiratory failure included a wide range of medical problems, with lung infection (70%) the most common etiology and community-acquired pneumonia being the largest group (39%). Objective evaluation after HFNC was initiated included: ABGs and vital signs. Patient tolerance for HFNC was evaluated by a visual numeric scale but not compared to NRB therapy. This study carefully followed patients after they received HFNC. Most notable were observed patterns of breathing frequency and thoraco-abdominal asynchrony. There was fairly rapid improvement in those 2 clinical signs, which also correlated with patients' reported dyspnea score (Fig. 11). Within 1 hour of HFNC therapy, changes in key clinical signs, as well as PaO2 and PaO2/FIO2 reached statistical significance. In addition, the authors' observations provide insight in using physical exam findings and ABG data to identify and predict treatment failure of HFNC. Nine HFNC patients subsequently required intubation and invasive mechanical ventilation; the majority required intervention within 4 hours. The authors identify the importance of findings of sustained tachypnea and continued thoraco-abdominal asynchrony, as well as blunted oxygenation response post HFNC therapy as significant predictors for HFNC failure.62
A: Evolution of clinically observed signs after 38 patients received high-flow nasal cannula oxygen. Mean FIO2 from OptiFlow cannula was 0.88 ± 0.16 with flow of 49 ± 9 L/min. Results are expressed as mean ± SD. The SpO2 box plots show the medians and interquartile ranges, and the open circles show the maximum values. * P < .05 vs before value. † P < .005 vs before value. (From Reference 62, with permission.)
The same group followed their previous ICU-patient evaluation with a similar prospective observational study to evaluate patients with hypoxemic respiratory failure in an emergency department (ED) setting.63 After initial assessment to gauge inclusion criteria, the OptiFlow cannula was applied to patients if they remained hypoxemic (SpO2) with clinical signs of dyspnea after receiving standard therapy with NRB with 10–15 L/min oxygen flow. Of 178 patients who initially received oxygen therapy upon ED admission, 17 patients met inclusion criteria for HFNC therapy; respiratory infection was the most common diagnosis. HFNC was applied with FIO2 between 0.7 and 1.0, with flows of 30–40 L/min. Response to HFNC was monitored at 15, 30, and 60 min after its initiation. Similar to the aforementioned studies, when compared to patient data collected during NRB therapy, the authors found statistically significant reductions in breathing frequency, and increase in PaO2. Eight out of 9 patients were able to complete Borg and visual analog scale scoring, which identified preference for the HFNC. They concluded that HFNC therapy systems could feasibly be used in the ED setting and could be considered as a first line option for selected patients who represented more severe hypoxemic failure after simpler oxygen devices failed to adequately oxygenate.63
The aforementioned observations and controlled studies provide several common findings on clinical application of HFNCs in adult care.
The HFNC offers potential for improved oxygen delivery for a majority of patients with moderate hypoxemic respiratory failure.
The HFNC can be adapted for use in a variety of clinical settings (exercise lab, ED, and ICU).
The physical exam findings of reduction in breathing frequency can provide a reliable guide to predict improved oxygenation, as well as treatment failures, in addition to SpO2 monitoring.
To date, evaluations of HFNC therapy have been most frequently conducted as retrospective audits or prospective comparison studies. The most common alternative device was the NRB, with inlet flows of 10–15 L/min. The oxygen delivery performance of that mask fell short of the HFNC in matching inspiratory flow demand in adults with hypoxemic respiratory failure who had respiratory frequency > 25 breaths/min. Flow to the NRB may be limited by clinicians who fail to advance flows higher than the traditionally accepted levels or the modest size of the bag's reservoir (≈0.6 L). Although limited, there is some evidence that the HFNC may better match adult patients' high inspiratory flow demand to then interrupt the vicious circle of hypoxemia-dyspnea-tachypnea. The mechanism is likely some combination of higher bulk flow preventing secondary room air entrainment, and using anatomic airways as a reservoir during end exhalation. There is no direct evidence to evaluate the proposed mechanism of the HFNC rinsing dead space gas from the upper airways to improve ventilation. It can be inferred from one observational study in which PaCO2 levels remained constant while breathing frequency decreased by ≈4 breaths/min following HFNC.58 There is currently no direct evidence to specifically identify impact of the low-level CPAP by adult HFNCs to treat low ventilation-perfusion regions of lung with distending pressure. In 2 studies, a small number of patients were identified as having either congestive heart failure or cardiogenic pulmonary edema.64,65 Based on current evidence it would seem prudent to consider mask CPAP the first line therapy for those patient groups if they can be reliably identified by history, physical exam, and/or echocardiography. Although pressurized facial masks have drawbacks, their threshold pressure levels are more predictable and measurable than with the HFNC. The other feature of HFNC that is more difficult to objectively evaluate is patient tolerance and comfort. The NRB is typically not used with a heated humidification system, and in some circumstances no humidifier is used at all. A number of studies quantitated some visual analog scale, feelings of freedom from mask-induced claustrophobia, improved ability to speak, et cetera. However, there were no clear comparisons of how warm, humidified gas impacted tolerance, comfort, or changes in nasal resistance with HFNC therapy.
Observational and Randomized or Quasi-Randomized Trials for Perinatal Patients
Although receiving initial acceptance since introduction a few years ago, there have been only a few randomized controlled studies to objectively assess efficacy and to guide patient selection. Study of HFNC for premature and term newborns shares a similar history. Although utilization has increased and generated substantial literature, there have been few published randomized controlled trials. Most studies define “high flow” for premature or term infants as > 1 or 2 L/min. As noted, outcomes of clinical analysis of adult HFNC therapy primarily focused on its ability to provide supplemental oxygen. Although oxygen supplementation is a goal, the primary focus for perinatal care has been on HFNC's ability to provide “distending pressure to the lungs.” Many observational studies have compared it to traditional NCPAP systems, which, by design, seal in the nares. The Cochrane Collaboration published an analysis of literature of NCPAP administration devices in 2008.50 The goals for the clinical application for either HFNC or NCPAP in premature or term newborn care include 3 main clinical rationales: prophylaxis or treatment of RDS soon after delivery as an alternative to intubation and mechanical ventilation; as post-extubation therapy; and as treatment for apnea of prematurity.
A 2009 “state of the art” review analyzed 9 papers on use of HFNC in preterm infants published up until 2008.66 That review included 5 prospective and 2 observational studies, of HFNC with premature and term babies.67–71 The objective of 4 prospective studies was either to quantify CPAP levels of HFNCs or to note direct comparison to NCPAP in prophylaxis and/or treatment of RDS.68–71 In that group, common findings were as follows.
There was similar effectiveness of HFNC and NCPAP, with a near linear relationship between distending pressure and HFNC flow level.68–70
Difficulty was noted in either predicting distending pressure based on flow or monitoring pressure level with an HFNC. HFNC systems do not typically have built-in pressure alarms.68–71
It was documented that larger babies required higher flows to create comparable distending pressure.71
One of the observational studies only compared effectiveness for treatment of apnea of prematurity, and found no difference with NCPAP.71 Sreenan's group used a formula (below) to estimate HFNC flow to infants using a constant-flow device and a Salter Labs infant-size standard nasal cannula, which produced a distending pressure of 4.5 cm H2O.70
The aforementioned 2009 “state of the art” summary66 and the 2011 Cochrane Database73 review identified and commented on 4 randomized controlled trials on use of HFNC with preterm infants.74–77 Each will be briefly discussed and also summarized in Table 6.
Randomized Controlled Trials of HFNC with Preterm Infants
Nair and colleagues compared HFNC to NCPAP in 67 infants randomly assigned to either therapy.74 The results were published in abstract form, but the authors provided “additional unpublished data” for the Cochrane analysis. Mean flow with the HFNC was 5–6 L/min; mean NCPAP pressure was 5–6 cm H2O. The primary measured outcome was treatment failure that resulted in intubation. Results were comparable (P = .96); 4/33 failed with HFNC, and 4/34 failed CPAP. Secondary outcomes comparing complications (nasal trauma, pneumothorax, sepsis), duration for respiratory support, and length of hospital stay showed no difference.74
Campbell et al compared 40 premature infants who, following extubation, were randomized to either HFNC (mean flow of 1.8 L/min) or NCPAP (mean pressure of 5–6 cm H2O).75 Of note was that gas from the HFNC was humidified but not heated. The primary outcome was incidence of reintubation within 7 days. The NCPAP generator used was a Viasys Infant Flow system, which uses a fluidic switching (flip-flop) circuit to change flow from inspiration to exhalation. Criteria for intubation included severe apnea and/or acute respiratory acidemia with pH ≤ 7.25, or need for FIO2 ≥ 0.6. Although a small study, there was a statistically significant (P = .003) increased requirement for reintubation in the HFNC group (12/20) versus those on NCPAP (3/20). The relative risk of HFNC was 2.1, with a 95% CI of 1.3–3.0. There were no differences in secondary outcomes or complications.75
Woodhead and colleagues performed a random crossover study of 30 neonates who were extubated and then placed on either a heated-humidified Vapotherm system or an unheated-unhumidified high-flow system; the nasal cannulas were not varied.76 The infants breathed on one system for 24 hours and then were switched to the alternate system. Primary outcome was treatment failure requiring reintubation. Secondary outcome evaluations were changes in appearance of nasal mucosa and physical examination score of increased respiratory effort. The humidified Vapotherm group had no treatment failures. In the unhumidified cannula group, 2/15 required reintubation within 24 hours after evaluation revealed hypercapnia and atelectasis that failed to respond to increasing cannula flow. Five failed in the unhumidified group in the second 24 hours; 2 because of apnea events, 2 from hypercapnia and tachypnea, and 1 due to increasing FIO2 requirement. All 5 improved after being switched back to heated-humidified Vapotherm therapy.76
Miller and Dowd conducted a randomized controlled study comparing 2 different brands of humidifier: Fisher & Paykel and Vapotherm. Both systems were set to deliver 6 L/min to HFNCs supplied by the respective companies.77 Thirty-nine infants with an average gestational age of 28.2 weeks were studied; all previously had required intubation for respiratory failure. The primary outcome was rate of extubation failure, defined as reintubation within 72 hours of receiving either HFNC system. Criteria were set based on level of hypercarbia, acidosis, or increased requirement for FIO2 ≥ 0.7. Acquisition cost analysis was also noted. All infants were followed for 1 week for secondary effects, including development of chronic lung disease. Results showed no statistical difference in need for reintubation within 72 hours (P = .69) using either brand of humidifier or cannula. There were also no differences in adverse events or other complications between either system.77
The Cochrane Collaboration reviews found 4 methodologically sound randomized trials.73 However, the small number of premature patients studied limited statistical power, and differing primary outcome comparisons prevented meta-analysis. Some data were not available to evaluate some secondary outcomes for subgroup analysis. The primary outcome for several studies was comparison of HFNC following extubation versus NCPAP. Analysis showed mixed results. One study showed no difference, yet another revealed increased rates of reintubation with HFNC.76,77 However, the studies varied technically in terms of flow rates, gas humidification, and design. Unfortunately, none examined the effect of differing flows or cannula sizes, which affect distending pressures. No differences were found in brand of humidification system. The primary conclusion from the Cochrane group was that there was insufficient evidence to evaluate safety or efficacy of HFNC in preterm infants. They recommended further randomized studies with adequate power to better compare it to NCPAP. Outcomes should include mortality, incidence of development of chronic lung disease, and need for and duration of respiratory support. Details on technical strategies need to be better studied. Such information may allow analysis to better identify both overall effectiveness and subgroups who may benefit from HFNC. A prospective randomized controlled study published after the Cochrane reviews examined HFNC in a slightly different application. Abdel-Hady and colleagues evaluated HFNC at 2 L/min as a potential weaning or bridge therapy for preterm infants following NCPAP therapy.78 Sixty neonates were randomized to either transition from NCPAP to HFNC when their FIO2 requirement was ≤ 0.3 or remained on NCPAP until FIO2 could be decreased to 0.21. The results showed that infants with NCPAP had statistically fewer days' exposure to supplemental oxygen, and shorter duration of respiratory support than the HFNC group.78
Observational and Randomized or Quasi-Randomized Trials of HFNC for Pediatric Patients
Application of HFNC therapy to children has not received as extensive review, compared to the preterm and neonatal patient populations. One prospective observational study included 46 consecutive patients who ranged in age from newborns to 12 years of age; the median age was 2.8 years.79 Patients were assessed after being initially placed on oxygen hood, non-humidified LFNC, or oxygen face mask, and then after being switched to HFNC using Vapotherm technology. HFNC flow levels were set at 8–12 L/min for infants and 20–30 L/min for children. Tolerance was evaluated before and after change to HFNC, using a modified COMFORT scale. Analysis of both tolerance and improved arterial oxygen saturation (SaO2) within the first 60–90 min reached the significant level.79 A 5-year retrospective analysis was conducted to review 298 infants < 24 months who received HFNC in a pediatric ICU. The majority required oxygen therapy for viral bronchiolitis. In that patient group, 19% required escalation from HFNC to noninvasive ventilatory support, and 12% required intubation and mechanical ventilation. However, over the 5 year period the overall need for intubation dropped from 37% to 7% for children with viral bronchiolitis. This trend was not found in children with other pathologies, such as cardiac diseases.80
Summary
The practice of respiratory medicine has benefitted from a growing body of knowledge to support evidence-based practice of medical gas therapy and to guide clinical pathways to blend patient assessment with selection and use of equipment.14–16,23 Such themes may contrast with historical, regional/local practice variations, or imposed administrative decisions. Accurate medical information may become clouded by promulgation of often untested information by textbooks. A scientific, comprehensive, and patient-oriented approach to providing supplemental oxygen can be complex. Assessment of need requires evaluation of the patient scenario, medical-surgical history, preexisting medical conditions, treatment setting, and technical limitations of oxygen systems. There is a cognitive process of developing a differential diagnosis of hypoxemia. That process can be further complicated by comorbidities of cardiovascular or neuromuscular disorders; either or both of which may lead to concomitant hypercapnia. There are also realities of equipment availability and increasing pressures for cost constraint. Finally, human issues must be considered in terms of convenience, adherence, and patient comfort. Above all is the regard for patient safety.
Not unlike new or updated adaptations of respiratory care equipment, patient application of HFNCs preceded extensive testing and clinical trials. However, there has been a concerted effort to evaluate the efficacy and to identify clinical roles for the HFNC within the spectrum of oxygen delivery devices. The following appear to be reasonable statements based on published observational and randomized controlled studies in adults.58–63
The HFNC can effectively be used to treat patients with moderate levels of hypoxemic respiratory failure.
HFNC could be considered as an initial appliance in certain settings (eg, ED), as flow could be titrated based on response over a full range without having to change to other devices. It could also be viewed as an alternative delivery interface for situations in which hypoxemia or dyspnea was not corrected after a trial of low-flow cannula, NRB and/or air-entrainment mask with FIO2 > 0.4. Besides ABGs and SpO2, physical exam findings of sustained tachypnea and thoraco-abdominal synchrony can provide valuable clinical evidence that O2 delivery systems with lower cost and more modest technology have failed.62
HFNC systems offer independent adjustment of FIO2 and flow. That design feature can allow greater flexibility to match the needs of acutely ill patients. Higher bulk flow can match the inspiratory flow demands of tachypneic patients, which can prevent secondary air entrainment at the facial interface. It is likely that the anatomic airways serve as an oxygen reservoir with the HFNC.
Low upper airway distending pressures, similar to CPAP, can be achieved with HFNC, yet are difficult to measure and not predictable.45,46
Heated and humidified gas from HFNCs appears to provide improved comfort and allows greater tolerance, compared to oxygen delivered by face masks. This may provide an advantage to patients with marginal oxygenation, for whom removing a face mask for clear speech, eating, drinking, and/or need to frequently expectorate to clear pulmonary secretions, may precipitate hypoxemia.
Use of HFNC will likely require some additional education for nursing and medical staff. The appearance of a nasal cannula therapy may be perceived as a device that would only be associated with a low severity of illness. In reality, some HFNC patients may be receiving both high FIO2 and high flow to maintain acceptable oxygen levels.
Although adult HFNCs can provide continuous flow ≥ 40 L/min, such levels may still not meet high inspiratory flow demands of some profoundly dyspneic patients. Maximum flow capabilities of HFNCs are only about half of those that can be delivered by free-standing continuous high-flow systems, such as high-flow blenders (eg, the Downs Flow Generator or the Caradyne WhisperFlow), using either an open face mask or well-sealing CPAP masks.81 Besides better meeting higher inspiratory flow demands, designated adult CPAP systems are typically designed to provide both higher and controllable CPAP pressure. Either high-continuous-flow CPAP systems or demand-flow systems in noninvasive mechanical ventilators are standard therapy for patients with severe atelectasis or heart failure with cardiogenic pulmonary edema.64,65 HFNC therapy should also be used with caution in patients with pathophysiologic disorders that are likely to result in intrinsic PEEP, such as unstable COPD or acute asthma. Use of CPAP to counteract intrinsic PEEP is controversial and may result in unintended increase in lung volume in some patients.82,83 HFNC therapy is not appropriate for patients with acute hypercapnia for whom NIV or intubation and mechanical ventilation are indicated. The proposed mechanism and level of ability of HFNCs to clear anatomic airway dead space have not been clearly documented. There may also be a small role for HFNC as an alternative to NIV for patients for whom comfort care and do-not-intubate status have been identified.84
HFNC for use with premature and neonatal patients has been promoted as a more convenient and “gentler” method to provide NCPAP. At this time, evidence by well-powered and randomized trials is inadequate to determine if HFNC has equivalent efficacy as NCPAP for prophylaxis or treatment of RDS as well as post-extubation therapy following intubation and invasive mechanical ventilation.72 There are safety concerns for developing unintended high airway pressure; circuit pressure relief valves should be used and airway pressure monitored. Future clinical research may be better able to focus on longer-term clinically important outcomes, details on cannula size, flows, and measurement of distending pressure.
Randomized controlled trials have become a cornerstone of evidence-based practice. However, in the process of controlling variables such as patient cohorts and precise protocols, findings may lose the ability to be generalizable to clinical realities of the real worlds of the ED, ICU, or neonatal ICU.85 As physicians, nurses, and respiratory therapists incorporate the HFNC in their clinical practice, they should be encouraged to conduct research or carefully record their observations as they apply this medical gas interface within the “realities” of their clinical settings and the individual patient's needs.
Discussion
MacIntyre:
The Fisher & Paykel people are pushing hard the notion that high humidity assists secretion clearance. What's your take on that?
Jeff Ward:
I've seen that statement in a few articles, yet they never cite published data. I've not yet seen a research abstract promote that premise. Humidity in the nasopharynx and oropharynx doesn't necessarily translate to the lower airways in an open-circuit system like the HFNC. Perhaps in asthmatics there are data that airway dehydration may elicit bronchospasm.1 Roca et al found that HFNC was associated with improved patient scoring of mouth dryness and comfort.2
I have my students wander about Rochester for a day with a nasal cannula on 2 L/min. They have to leave campus, go do their laundry, and go shopping with it on, and we make sure they have the tanks with bad wheels. They have to come back the next day and write a report on the experience. I've had several students say they couldn't wear it for more than an hour before their nose stuffed up, and they had no allergic rhinitis or asthma.
The big advantage of humidification, especially at higher flow, is in patient comfort. In most HFNC devices the gas leaves the humidifier like Miami Beach on a summer day. But some systems use a heated wire circuit, which can reduce relative humidity below 100%. The humidity at the nasal prongs is probably less, because preventing condensation can result in decreasing relative humidity and dessication of secretions.3 I don't think there are data to show that heated humidified HFNC does anything for secretion retention other than prevent nasal airway drying and increased na-sal resistance. Of course, we know that if the nose is unhappy, the lungs can be unhappy too.
Branson:
I have a whole bunch of ideas about this. First, all you are doing is adding heat and humidity, and the humidity allows the cannula to change from being a low-flow device to a high-flow device: end of story. Patients have higher oxygen saturation because they get higher FIO2: no magic there. But Antonio Esquinas,4 from Spain, found that for both high-flow O2 and NIV with inadequate humidity it makes it more difficult to intubate the patient. I think the whole HFNC business—the people marketing it initially got it wrong: it improves comfort, and that is enough. They stretch for it improving secretion clearance or outcomes, or preventing intubation, but I think it clearly improves comfort and allows patients to tolerate high-flow oxygen via cannula.
Another concern is that some people say, “Oh, he's on a cannula: he must not be that sick,” but he's getting 80% O2, and I think that—just like we can with NIV—we can leave them on too long before we intervene when they really need to be intubated.
Jeff Ward:
I think Dider Dreyfuss5 and Jean-Damien Ricard6 watched their patients very carefully in the ICU and ED. When they didn't succeed on the standard default system (typically, a non-rebreathing reservoir mask), they put them on HFNC. A substantial number of their patients improved, and those who did not were put on NIV or intubated.
I think it behooves us to watch how patients respond to the devices we use. HFNC is nice because the patient can talk, eat, or drink, whereas with a mask that's not pleasant, especially if they have to be on it for a while. We found that paying attention to both FIO2 and the patient's flow demand is often valuable after extubation.
At our institution the post-extubation default device was a 40% all-purpose nebulizer with an aerosol mask. The thinking was—though with no data—that the mist would keep their airways moist, 40% would prevent hypoxemia, and the total flow of about 50 L/min would meet flow demand. However, all that would need to happen was for the patient to get tachypneic from becoming anxious and/or start to desaturate. Then their inspiratory demand pulls in room air and the FIO2 goes down. The downward spiral would worsen if the FIO2 of the nebulizer would be turned up to 0.6, 0.7, or 1.0. With high-flow systems like blenders or the HFNC we tend not to see that as much. The flow and FIO2 can be independently adjusted. Added comfort may make them less anxious and dyspneic.
Branson:
We played around with HFNC early on. If you sit a subject so they can't see the device and put them on a nasal cannula with no humidity and say, “Tell me when you can feel it in your nose,” it's at 2 or 3 L/min. Then ask, “When is it uncomfortable?” and it's 7 L/min. Then ask when they can't stand it for one more second, and it's around 15 L/min. If you heat and humidify the gas, some people can't feel it until almost 10 L/min, and it only gets so uncomfortable they can't stand it at 30 or 40 L/min.
But I agree that all these model studies are unhelpful. HFNC clearly creates CPAP in babies and neonates, and a little in adults. I have seen neonates on 15-18 L/min and you can just see the chest wall expanding, and they're on a substantial amount of CPAP. I have concerns about that. If I came into the Mayo Clinic and said, “Here's my new CPAP system,” and you asked, “How do you set the CPAP?” and I said, “Well, you don't,” and you asked, “OK, how you do you monitor it?” and I said, “Well, you don't,” and you asked, “Where's the safety?” and I said, “It's not important,” you might ask me to leave your office.
But HFNC works, and because of its simplicity people have used it, and, perhaps because they couldn't see what they were doing, may have used it inappropriately.
Jeff Ward:
I think one of the reasons you had me do this is that I've been around for a long time and seen a lot of snake oil. A lot of the companies do a disservice by making this thing sound like the best thing since sliced bread. Instead of letting the knowledge creep in first and the device/technology come second, the process is reversed. That's not what happens with a new drug, but it's part of life in some aspects of respiratory care, such as new ventilator modes.
I just think it needs to be considered. I don't think HFNCs are the end-all, but I think they provide an advantage for—not necessarily a select group, but a relatively middle group of patients, with moderate hypoxemia that can't be relieved by standard and inexpensive devices. But HFNC is a lot more expensive than a cannula and a flow meter. I don't think HFNC should be the default device for every patient in the ED with an SpO2 of > 90%.
Eiserman:*
One thing we've found as a manufacturer is that when people think of a nasal cannula, they say, “What could that hurt?” I think part of the reason for that attitude is the feeling that what's old is new again, and we're looking at the nasal cannula and applications in a different way. It might be really effective in the right patients, but it has led to less interest in researching the possible benefits and effects on outcome, and whether it's equal to or better than other therapies. I think it's because they think of it as just a simple cannula, but more research needs to be done.
Also very important is whether people are considering it O2 therapy or CPAP, because none of these devices are FDA approved or cleared CPAP-generating devices; these are O2 therapy devices. And central to the whole discussion is the critical importance of sizing the cannula. It is meant to be a non-occlusive cannula, and if it completely fills the nares, it's beyond what an HFNC is designed to do. Education is critical and randomized controlled trials are needed, because people think of it as just a cannula. It has a genuine place in the pyramid of care if it's used properly and applied to the right patients, but I look forward to more research and data to see where it fits in the arsenal.
Jeff Ward:
I think a lot of people like to paint with a roller or a spray gun: they don't like to use a small brush, which requires thinking as one integrates physical exam findings with other data. With an HFNC one can't measure the CPAP pressure or the delivered oxygen fraction. It's a lot different than using a noninvasive ventilator, with which you have a locked-in FIO2 and CPAP pressure and you can watch the patient's interaction with the machine, SpO2, and NIV waveforms. It's more of an art than slapping on a gadget and cranking it up to a number and walking away. We used to do that with IPPB [intermittent positive pressure breathing].
Branson:
How many people here use high humidity HFNC in their institutions? [About 75% of attendees indicate yes.]
Owens:
Anecdotally, in our institution the senior residents do all the triage from the floor, and I don't think they appreciate how much support patients are getting on the floor. They say, “Oh, their sats are fine,” but they roll into the ICU and need to be intubated almost immediately because their breathing frequency is 50 and they look terrible.
I would agree that more education is needed. I think it's a wonderful device and it's clearly helped people through critical illness, but it goes back to teaching people: don't look at the numbers, look at the patient. Some of the RTs also forget that, and forget how much support that is. The people who really know how much support it is are the anesthesiologists who come up to intubate these patients, and they turn white because they see the patient is on 60 L/min and an FIO2 of 1.0, because they have to take them off of it and intubate. Great technology, but it's deceptively simple in appearance.
Jeff Ward:
A couple blogs7,8 have suggested using HFNC instead of face mask to pre-oxygenate prior to and during rapid-sequence intubation.
Kevin Ward:
In emergency medicine there's new interest in using HFNC during rapid-sequence intubation, to extend the period of denitrogenation, to reduce the incidence of hypoxemia.9
Jeff Ward:
It's very difficult to quantify randomized controlled trials on patient comfort. They use visual analog scales, but if you talk to patients who've had this, they say, “This is really cool, I like it.” But it's hard to take that to the evidence-based medicine bank.
Acknowledgment
The author is grateful to James Jamieson RRT and Bryan Wattier RRT, for their assistance with digital figure images for use in this paper and at the Oxygen Journal Conference presentation.
Footnotes
- Correspondence: Jeffrey J Ward MEd RRT FAARC, Respiratory Care Program, Mayo College of Medicine, University of Minnesota, 200 1st Street SW, Siebens 10-12D, Rochester MN 55905. E-mail: ward.jeffrey{at}mayo.edu.
Mr Ward presented a version of this paper at the 50th Respiratory Care Journal Conference, “Oxygen,” held April 13–14, 2012, in San Francisco, California.
The author has disclosed no conflicts of interest.
↵* Jeri E Eiserman MBA RRT FAARC, Teleflex, Santa Fe, New Mexico.
- Copyright © 2013 by Daedalus Enterprises Inc.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵