Abstract
Airway pressure release ventilation (APRV) and bi-level positive airway pressure (BIPAP) are proposed to reduce patient work of breathing (WOB) sufficiently and to obviate issues related to patient-ventilator synchrony, so that spontaneous breathing can be maintained throughout the course of acute lung injury (ALI). Thus, APRV/BIPAP should reduce requirements for sedation and muscle paralysis, and thereby reduce the duration of mechanical ventilation. Only 17 human, animal, or lung-model studies have examined these claims, either directly or indirectly. Most did not target patients with ALI. Studies on sedation use have serious methodological limitations. Other studies found that APRV/BIPAP either increased WOB and asynchrony, or had no effect on energy expenditure. To supplement the discussion of patient WOB during APRV/BIPAP in ALI, 4 clinical examples showed marked elevation and wide variation in patient WOB. One plausible explanation is that spontaneous breathing is superimposed upon the mechanical ventilation pattern. Thus a variety of “breathing environments” exist during APRV/BIPAP that affect patient WOB and respiratory drive differently and perhaps unpredictably. This characteristic of APRV/BIPAP makes WOB comparisons with traditional modes problematic. Furthermore, the theoretical benefits of APRV, in terms of controlling patient WOB, appear particularly limited when lung-protective ventilation is used for ALI patients with high minute ventilation demand. Future research should focus on issues of WOB and synchrony, so that reasonable ventilation protocols can be devised to test clinical outcomes against traditional modes. To date, low-level evidence suggests that promoting spontaneous breathing with APRV/BIPAP may not be appropriate in patients with relatively severe ALI/ARDS.
- acute lung injury
- acute respiratory distress syndrome
- airway pressure
- release ventilation
- patient-ventilator synchrony
- work of breathing
Introduction
Airway pressure release ventilation (APRV) is a time-triggered, time-cycled, bi-level, pressure-regulated ventilation mode that allows a patient's spontaneous breathing pattern to be superimposed upon the mechanical ventilation pattern.1,2 This is accomplished by an expiratory value design that is responsive to a patient's active expiratory efforts, so that the bi-level airway pressure provides 2 levels of continuous positive airway pressure (CPAP). Therefore, APRV does not require synchronization between patient and ventilator that is essential for the safe delivery of mechanical ventilatory support with traditional modes. APRV is the term given to the mode when the time period at the upper CPAP level exceeds that at the lower level and produces an inverse-ratio ventilation pattern. When the timing pattern is adjusted so that the timing ratio is equal, or when time at the lower CPAP exceeds the upper level, the mode has been referred to as “BIPAP” or bi-phasic positive airway pressure.3 These are the terms and related definitions that will be used in this paper. In passing, it should be noted that in the absence of spontaneous breathing efforts, APRV and BIPAP are indistinguishable from pressure-controlled ventilation (PCV).
In theory, during APRV the ability of patients to breathe spontaneously, unencumbered by the ventilator's valving system, should result in lower levels of sedation and less need for neuromuscular blocking agents (NMBAs).4 This may be an important feature of the mode, as there is now convincing evidence that high levels of sedation and NMBA prolong the duration of mechanical ventilation.5,6 In addition, advocates of APRV implicitly accept the long-held belief of intermittent mandatory ventilation (IMV) proponents, that facilitation of spontaneous breathing throughout the course of critical illness prevents respiratory muscle de-conditioning and weakness that contributes to ventilator-dependence.7 In this paper I will review the purported advantages and disadvantages of APRV and BIPAP for management of acute lung injury and acute respiratory distress syndrome (ALI/ARDS), both in regard to patient work of breathing (WOB) and synchrony with the ventilator.
APRV in the Management of Patients With ARDS/ALI
There are several proposed mechanisms by which APRV may be an effective alternative mode of mechanical ventilation for patients with ALI/ARDS. The application of a sufficient amount of CPAP promotes alveolar recruitment and improves functional residual capacity, thereby increasing respiratory-system compliance (CRS) and reducing the elastic WOB.2,8,9 Gas exchange may be improved by the effects of APRV on alveolar recruitment, as well as by maintaining active diaphragmatic contractions that promote dorsal-caudal distribution of ventilation, thereby improving ventilation-perfusion matching.10,11 Periodic airway pressure release, either to ambient or to a lower CPAP level, promotes carbon dioxide excretion,2 thus limiting the minute ventilation (V̇E) demand. In addition, the promotion of spontaneous breathing should enhance venous return by increasing the mean systemic right-atrial pressure gradient and thus increase cardiac output.1,4
In essence, APRV is inverted IMV: rather than supplementing V̇E by adding positive-pressure breaths above PEEP, tidal ventilation is achieved by brief reductions in functional residual capacity. As originally intended, release times of approximately 1.5 seconds9,12 are believed to prevent the collapse of unstable alveoli. Augmenting carbon dioxide excretion may reduce the V̇E demand placed upon the patient, thereby limiting that portion of respiratory muscle power output related to flow-resistive work. More recently, it has been proposed that the addition of low-level pressure support ventilation (PSV) or automatic tube compensation could further reduce resistive WOB.13 Proponents of APRV also have speculated that sedation and NMBA requirements should be less than those needed during conventional modes of mechanical ventilation because synchrony with the ventilator during APRV is not necessary.4
Literature Review
Relatively few studies have examined the effects of APRV on breathing-pattern and work-related variables, sedation and NMBA requirements. Since 1987, 17 papers have reported data on these issues in some fashion.13–29 Of these studies, 6 reported on aspects of breathing effort and synchrony13–16,26 or spontaneous ventilatory pattern,22 whereas 3 investigated energy expenditure,18,24,25 and 6 reported on sedation requirements and/or duration of mechanical ventilation.17,19,20,23,28,29
Breathing Effort and Synchrony
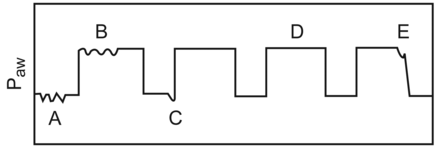
In 1994 the first 3 studies examining synchrony and WOB during APRV and BIPAP were published.14–16 Conceptually, the most informative study compared BIPAP to PSV in patients recovering from cardiac bypass surgery.14 Its main contribution was describing the different breath types that occur during APRV or BIPAP. These have been defined as (Fig. 1):
Spontaneous breaths that occur during low CPAP (Type A)
Spontaneous breaths that occur during high CPAP (Type B)
Quasi-assisted breaths that are synchronized with the ventilator cycling to the high CPAP level (Type C)
Completely passive breaths (Type D)
To these breaths, must be added: Spontaneous breaths that occur as the ventilator cycles to the lower CPAP level (Type E).
Breath-type schematic during airway pressure release ventilation. A: Spontaneous breath during low continuous positive airway pressure (CPAP). B: Spontaneous breath during high CPAP. C: Quasi-assisted breath that is synchronized with the ventilator cycling to the high CPAP level. D: Completely passive breath. E: Spontaneous breath that occurs as the ventilator cycles to the lower CPAP level.
In the first of those studies14 the pressure-time product (PTP), the mechanical correlate of respiratory muscle oxygen consumption,30 was higher during BIPAP than PSV. However, that finding reflected the preponderance of unsupported breaths at lower CPAP (Type A) in the BIPAP group. Interestingly, Type-B breaths at the higher CPAP were so rare that they were excluded from the analysis. Thus, one of the most important aspects of the effect of APRV on WOB could not be determined from that study.
Putensen et al16 used a mechanical lung model to examine the mechanism by which V̇E was noted to decline clinically during the transition to APRV. They found that simulated patient power of breathing increased when the pressure-release to the lower CPAP level occurred during a spontaneous breath, and this was associated with a decline in V̇E. In a study of 16 patients without obstructive lung disease, who were being weaned from mechanical ventilation, Chiang et al15 reported that 5 patients (31%) felt more uncomfortable during APRV, compared to synchronized IMV or PSV. In 2 of those patients gross asynchrony was noted by the investigators. The remaining 11 patients stated no preference for any mode in terms of comfort. Breathing discomfort was not associated with either the time at lower CPAP or the number of cycles per minute.
Hering et al21 assessed the effects of spontaneous breathing on renal perfusion during APRV. In ALI/ARDS patients with relatively mild disease the mean inspiratory esophageal pressure change (ΔPes) was 9 ± 3 cm H2O. This suggests a reasonable degree of inspiratory effort that is partly explained by a substantial low CPAP level of 16 cm H2O, which probably caused a high degree of lung recruitment. Likewise, Wrigge et al13 reported a mean ΔPes of 10.2 ± 3.2 cm H2O at the same mean lower CPAP of 16 cm H2O in a similar group of patients with mild lung injury. In an animal model of lung injury, Hering et al26 reported that APRV reduced PTP by 35% and diaphragmatic blood flow by 40%, compared to spontaneous breathing.
In the most comprehensive study to date, Henzler et al27 compared respiratory drive and breathing effort in an animal model of ALI between assist-PCV, PSV, and BIPAP. Pigs ventilated at the same level of inspiratory pressure-assist had a significantly lower respiratory rate, respiratory drive, WOB, PTP, ΔPes, intrinsic PEEP, and power output with assist-PCV, compared to BIPAP. PSV also produced lower WOB, compared to BIPAP, but at a substantially higher respiratory rate. Synchrony scores also were higher (ie, better synchrony) during assist-PCV and PSV. Interestingly, there was a significant indirect relationship between respiratory drive and synchrony scores, so that higher respiratory drive was associated with more asynchrony. That respiratory drive and WOB were higher during BIPAP also suggests a direct link between asynchrony and increased WOB.
Breathing Pattern
Neumann et al22 examined how APRV influenced patient breathing pattern when time at lower CPAP was systematically reduced as time at higher CPAP was increased in equal increments. This study was done primarily on postoperative patients, only 25% of whom had ALI/ARDS, whereas some had COPD. As time at lower CPAP was reduced below the standard setting of 1.5 seconds, driving pressure for mechanical ventilation decreased, as noted by an inadvertent increase in lower CPAP caused by incomplete lung emptying. As lower CPAP time decreased, so did tidal-volume variation, whereas both the spontaneous respiratory rate and the arterial carbon dioxide partial pressure increased both in patients with ALI/ARDS and those with obstructive lung disease. Although mean arterial carbon dioxide partial pressure increased modestly (3 mm Hg), the increase at the high end of the range was substantial (15 mm Hg) and probably reflected the effects of air-trapping.
Energy Expenditure
Three studies compared differences in oxygen consumption, carbon dioxide production, and energy expenditure between either APRV and PSV25 or BIPAP and PSV.18,24 Uyar et al25 studied patients without significant lung disease in a crossover design and found no difference in energy expenditure between the modes. Staudinger et al18 studied patients without risk factors for lung injury, and likewise reported no differences in energy expenditure between BIPAP and PSV. Similarly, Elrazek24 found no difference in oxygen consumption, carbon dioxide production, or energy expenditure between BIPAP and PSV during short-term mechanical ventilation in surgical patients. None of these studies provides indirect calorimetric evidence, suggesting that APRV provides an advantage in WOB over conventional modes of assisted ventilation.
Sedation, Duration of Mechanical Ventilation, and Other Outcomes
Putensen et al20 published the most widely referenced study regarding the effects of APRV versus PCV on sedation and weaning outcomes. This prospective randomized trial reported that in severely injured trauma patients at risk for ALI/ARDS, those managed with APRV required significantly less time on mechanical ventilation (6 days), as well as reduced analgesic and sedative requirements. These findings have been criticized31 because of serious flaws in the study design. After enrollment, patients randomized to APRV were allowed to breathe spontaneously and had their sedation adjusted to facilitate this goal. In contrast, patients randomized to receive PCV were paralyzed and deeply sedated for the first 72 hours of the study, irrespective of whether such treatment was necessary for clinical management. Thus, patients in the PCV arm were at a significant disadvantage by design. This raises the question of whether any difference in outcomes would have been found if patients in the PCV group had been managed in a similar manner to those in the APRV arm.
Varpula et al23 compared APRV to synchronized IMV with pressure support in a prospective randomized trial that examined both sedation requirements and duration of mechanical ventilation in patients with ALI/ARDS. No difference was found in either ventilator-free days or sedation and analgesic requirements. These results may suggest that APRV confers no special advantage, compared to assisted modes of ventilation, as long as spontaneous breathing efforts are promoted.
In contrast, a prospective non-randomized study with approximately 600 postoperative cardiac surgery patients, by Rathgeber et al,17 reported that BIPAP was associated with reduced analgesic and sedative consumption. In addition, duration of mechanical ventilation was reduced by a mean of 3–5 hours, compared to synchronized IMV and assisted volume control ventilation (VCV), respectively. Unfortunately, there was a large imbalance in the distribution of patients, with only 42 patients (7%) managed with BIPAP, so that selection bias may have influenced the results.
A prospective observational study28 comparing APRV and assisted VCV on day-1 of ALI/ARDS found that the median total doses of sedatives and analgesics were lower in patients managed with APRV. In addition, fewer patients in the APRV group required deep sedation, compared to VCV (38% vs 80%, respectively). However, there was an imbalance in illness severity, with patients in the VCV group having a significantly higher Acute Physiology and Chronic Health Evaluation (APACHE II) score (25 vs 17) and a trend toward worse oxygenation than the APRV group. Moreover, the groups were starkly imbalanced in other respects: The VCV group had 148 patients, compared to only 17 patients in the APRV group. In addition, the VCV group consisted of 98% medical patients, whereas the APRV group was 94% surgical or trauma patients. Interestingly, patients in the APRV group had a substantially higher incidence of restlessness or agitation (18% vs 1%). As the lung-injury scores32 were relatively low in each group (mean of 2.3), the study provides little insight into sedation requirements and tolerance of APRV in patients with more severe ARDS.
In a poorly documented observational study19 involving an unknown number of patients with severe ARDS, sedative and NMBA use were compared between PCV with inverse-ratio ventilation and APRV. As expected, APRV allowed inverse-ratio ventilation without use of NMBA, and a consequential reduction in sedation requirements. However, PCV-inverse-ratio ventilation, a relatively popular mode of mechanical ventilation for severe ARDS in the late 1980s,33 is seldom used in contemporary practice,34 and the results of that study cannot be generalized to contemporary management of ALI/ARDS.
Finally, there was a recent post hoc data analysis from a prospective multicenter international cohort study of mechanical ventilation practices. Case-matched, controlled comparisons were made between 234 patients managed with assist control ventilation and 234 patients managed with APRV/BIPAP.29 No difference was found in duration of mechanical ventilation, weaning time, weaning failure, or intensive care unit stay. Likewise, there was no difference in intensive care unit or hospital mortality.
Can APRV Be Compared to Conventional Modes of Assisted Mechanical Ventilation?
As described earlier, APRV advocates proposed several theoretical mechanisms by which patient WOB may be decreased with that mode. However, there are also several mechanisms by which patient WOB theoretically may be increased (Table 1). These potential problems are discussed below, both in terms of clinical relevance, and how they would make the design and interpretation of any comparative clinical study challenging. Both WOB and power output of the respiratory muscles (ie, work-per-min) during APRV are dependent upon several factors related to the variety of “breathing environments” a patient encounters during spontaneous breathing.
Methodological Issues Potentially Influencing the Results of a Study Comparing the Effects of Airway Pressure Release Ventilation to Assisted Volume Control or Pressure Control Ventilation on Patient Work of Breathing During Lung-Protective Ventilation
Minute Ventilation Support
A major concern during APRV relates to V̇E support, as the number of CPAP cycles per minute creates the baseline V̇E. If a standard APRV frequency of 8–15 cycles/min is used,3,12,35–38 then a substantial amount of V̇E could be shifted abruptly to the patient. This could result in an acute rise in arterial carbon dioxide level and a marked increase in respiratory drive, WOB, and respiratory muscle power output. This may have negative consequences, particularly when trying to maintain a release-volume of 6–8 mL/kg in patients with relatively high V̇E demand. For example, a V̇E of approximately 13 L/min is common in patients with early ALI/ARDS.39 In an average-size 70-kg predicted-body-weight patient with a target release volume of 7 mL/kg (490 mL), a cycle frequency of 8–15 cycles/min would yield a V̇E between 3.9 and 7.4 L/min, or 30–56% of baseline demand. Immediately, this patient would be required to assume up to 70% of their V̇E demand. In a critically ill patient with marginal gas exchange and respiratory muscle impairment this may result in acute clinical deterioration.
In any study comparing WOB, the sudden shift in V̇E demand to unsupported breaths would probably increase WOB more during APRV than during assisted VCV or PCV. Maintaining lung-protective ventilation (LPV) goals seemingly would necessitate decreasing the time ratios, at least transiently, thus transforming APRV to BIPAP. In fact, some studies have allowed for substantially higher frequencies of 20–24 cycles/min during APRV,12,37,38,40 such that the cycling ratio could reach 1:1.37 Interestingly, the original study describing APRV used a cycling frequency of 20 cycles/min with a cycling ratio of only 1.3:1.1
Release-Volume and Ventilator-Induced Lung Injury Risk
The alternative approach would be to use a substantially larger release volume or to increase the cycle frequency. Larger release volumes would probably produce a more satisfying breath and may relieve dyspnea while lessening the power demands on the respiratory muscles. Unfortunately, it also would violate LPV goals and increase mortality risk. Of the APRV studies that have measured release volumes, mean values have been reported between 550 to 840 mL12,13,21,22,35,40 and 9 mL/kg by measured body weight,23,37 which probably translates into 11 mL/kg predicted body weight.41 In many studies these values exceeded current LPV targets.
Furthermore, patients can augment the tidal volume that occurs during the change-over from lower to higher CPAP, which can increase the risk of ventilator-induced lung injury,31 as well as complicate the assessment of the actual release-volume generated from the CPAP gradient. Ventilator-induced lung injury results from excessive transpulmonary pressure in concert with high end-inspiratory lung volume that causes severe mechanical stress.42,43 Thus, the superimposition of spontaneous tidal volumes at the higher CPAP level in theory could increase the likelihood of stretch-related injury. However, experimental models have shown that the addition of spontaneous breaths during APRV improves aeration in the dorsocaudal lung regions,10,11,44 so that the risk of ventilator-induced lung injury could be questioned. Yet in the experimental studies the timing ratios were fixed at 1:1,10,44 so that while overall intrapulmonary gas distribution may have favored dorsocaudal lung regions, it does not exclude the possibility of less favorable spatial ventilation patterns if inverse timing ratios and/or higher pressure levels are used. Interestingly, the percentage of minute ventilation distributed to areas of high-ventilation-perfusion (ie, a marker for lung tissue most at risk for over-distention and stretch-related lung injury) was unchanged between passive ventilation and spontaneous breathing during APRV.11 Furthermore, in clinical practice, improved dorsocaudal ventilation distribution may not be fully realized if those lung regions have extensive consolidations (eg, pneumonia, aspiration, and blunt chest trauma).
Spontaneous Breaths That Occur During Low CPAP (Type A)
Type-A breaths occur at lower CPAP and should cause relatively higher WOB, as CRS probably would be lowest. In addition, patients must assume the entire resistive WOB, unless pressure support or automatic tube compensation is added. If time at lower CPAP is prolonged, a larger proportion of spontaneous breaths should occur in this environment, thus increasing respiratory muscle power output. Furthermore, when release times at the lower CPAP level are reduced below 1.5 seconds, there may be a higher incidence of intrinsic PEEP, from incomplete lung emptying, that would increase breathing effort.27 However, the number of such breaths might be limited, due to the smaller portion of the APRV cycle time.
Spontaneous Breaths That Occur During High CPAP (Type B)
At the higher CPAP level it is presumed that CRS will improve so that the elastic WOB is diminished. This assumption, however, was based largely on the concept of “optimal CPAP,”45 in which a constant level of CPAP improved CRS and reduced the power output, compared to breathing through a T-piece. Although these findings were generalized enthusiastically to the management of patients with ARDS,46 the external validity of such declarations was never verified and remains suspect. For example, early investigations on the impact of CPAP on chest mechanics noted that improvement in functional residual capacity and CRS seemingly were related to time-dependent changes in the viscoelastic properties of both the lung and chest wall (“creep”)47 effected by the application of a constant distending pressure.48 Therefore, the use of repetitive pressure-releases may limit the effectiveness of the higher-level CPAP on lung recruitment.
Furthermore, since the advent of APRV, research on lung recruitment in ARDS suggests that inflation pressure of at least 40 cm H2O sustained for 30–40 seconds, followed by a transient period of high-level PEEP, is required to sustain improvement in lung function.49 Although these conclusions are based largely on the assessment of oxygenation improvements, there is a consistent, direct link between improvement in functional residual capacity, CRS, and oxygenation in ARDS.8,50,51 Therefore, the notion that CRS can be improved enough to reduce the elastic WOB when lower inflation pressures are held briefly, with the lungs then allowed to partially deflate 8–15 times per minute, should be viewed with a certain degree of skepticism.
In contrast, an excessive level of CPAP may cause lung over-distention that paradoxically increases elastic WOB. Also, at increased lung volume, resting respiratory muscle length is altered abnormally, thereby requiring greater activation and tension development to achieve the same tidal volume.25 Moreover, a high level of end-expiratory pressure may induce expiratory efforts, resulting in expiratory WOB that may counter the effects of lung recruitment.48,52,53
Quasi-assisted Breaths That Are Synchronized With the Ventilator Cycling to the High CPAP Level (Type C)
Modern versions of APRV allow synchronization of patient effort to cycling between CPAP levels. This introduces assisted breathing to APRV that may function similarly to patient-triggered PCV or PSV breaths.22 As the number of CPAP cycles increases, there is a greater likelihood that a patient's spontaneous effort would be synchronized and supported. Therefore, WOB during APRV may be similar to assisted VCV, or PCV, if a large number of spontaneous breaths are converted to Type-C breaths. However, such a strategy would necessarily decrease the time ratios and probably transform APRV to BIPAP.
Spontaneous Breaths That Occur as the Ventilator Cycles to the Lower CPAP Level (Type E)
Lastly, spontaneous breaths occurring during the pressure-release transition to the lower CPAP increases WOB16 and may create the most discomfort, as airway pressure would be decreasing, and gas flow would be escaping the circuit just as the patient was trying to inspire.31 These Type-E breaths would tend to occur more frequently as the APRV cycle frequency is increased.
Clinical Examples of Patient-Ventilator Interactions During APRV and BIPAP in ALI/ARDS
Previously, only Calzia et al14 and Henzler et al27 have measured patient effort during BIPAP. In patients who had participated in a previous study on WOB in ARDS/ALI,54 we also had examined the effects of APRV or BIPAP on the breathing pattern and WOB during brief (ie, 20 min) trials to gather preliminary data on how patients might respond when these modes are used for LPV. Scalar waveforms and data from 4 of those patients are presented here to illustrate the different breath types that can occur during APRV and BIPAP, and how such patient-ventilator interactions affect measurements of patient WOB. The waveforms and accompanying data presented here underscore the difficulty and ambiguity inherent when trying to compare WOB between traditional modes of assisted ventilation with APRV and BIPAP. This appears to be particularly problematic in patients with significant impairment in chest mechanics, increased V̇E demand, and elevated central respiratory drive (Table 2, Figs. 2–5).
Characteristics and Ventilator Settings of Patients on Airway Pressure Release Ventilation or Biphasic Positive Airway Pressure
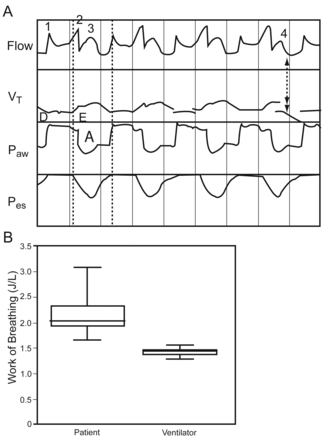
A: Scalar flow, tidal volume, airway, and esophageal pressure tracings from Subject 9, a 39-year-old male with severe pneumonia-induced acute respiratory distress syndrome. All spontaneous efforts are captured as Type-B breaths, wherein the patient's inspiratory efforts consistently occur immediately after the ventilator time-triggers to the upper continuous positive airway pressure level, noted by the small, secondary increase in inspiratory flow (labels 1 and 2). This pattern also is a relatively common form of asynchrony found during lung-protective ventilation when traditional modes are used, wherein a patient's inspiratory effort always follows the time-triggered mechanical breath. There also appears to be a 2-phased expiration whereby exhalation of the Type-B breath (label 3) is immediately followed by the pressure-release (label 4). B: The translation of this type of patient-ventilator interaction into distribution of patient and ventilator work of breathing measurements shows no overlap between patient and ventilator. The ventilator performs an extraordinarily high level of work that does not appear to off-load the inspiratory muscles. VT = tidal volume. Paw = airway pressure. Pes = esophageal pressure.
A: Scalar flow, tidal volume, airway, and esophageal pressure tracings from Subject 10, a 41-year-old female with sepsis-induced acute lung injury. All spontaneous breathing efforts appear as hybrid breaths that commence at the lower continuous positive airway pressure level (Type A), but are sustained into the cycling to the upper continuous positive airway pressure level (Type C or B). With the Type-C breath there is an enhanced peak inspiratory flow (label 1), similar to what occurs during patient-triggered pressure-controlled ventilation. In contrast, the diminishing patient effort that coincides with the transition from lower to higher pressure level results in a secondary flow spike occurring during the decay of the sine wave generated during the Type-A breath (label 2). Finally, the Type-E breath creates a characteristic sine wave typically observed during spontaneous, unassisted breathing (label 3). B: The translation of these patient-ventilator interactions into distributions of patient and ventilator work of breathing measurements shows wide disparities, with the patient assuming an extraordinarily high level of work. The very low level of ventilator work of breathing probably reflects a high proportion of hybrid Type-A breaths commencing from lower CPAP.
A: Scalar flow, tidal volume, airway, and esophageal pressure tracings from Subject 13, a 72 year-old female with trauma-induced acute lung injury. All spontaneous breathing efforts occur as hybrid breaths that commence at the cycling from the higher to the lower continuous positive airway pressure level (Type E), but are sustained into the lower CPAP (Type A). In this figure, 3 distinct inspiratory flow spikes can be observed. Label 1 denotes the time-triggered ventilator breaths to the higher CPAP that is characterized by the sharp initial flow spike at the beginning of each mechanical breath. Labels 2 and 3 denote what appear to be patient-initiated discreet breaths, but apparently this is a single inspiratory effort interrupted by the time cycling of the ventilator to the lower CPAP. This Type-E breath produced the second, higher and slightly wider, flow peak profile that is truncated prematurely by the pressure-release, and then immediately reappears as a small, quasi-sine wave that transforms into expiratory flow. It also appears to cause an abrupt drop in lung volume at end-inspiration (label 4). This interpretation is supported by the observation that the esophageal pressure deflection is one consistent signal, with no evidence of a secondary effort. Therefore, the coupled inspiratory flow spikes represent the interactions of the ventilator's valving function at the transition from higher to lower CPAP with a sustained inspiratory effort. B: The translation of these patient-ventilator interactions into distributions of patient and ventilator work of breathing measurements for Subject 13 again show a very narrow distribution of ventilator work of breathing, whereas the corresponding patient work of breathing is substantially higher, with a wider range of work values.
A. Scalar flow, tidal volume, airway, and esophageal pressure tracings from Subject 4, a 24-year-old male with pancreatitis-induced acute lung injury. This patient had near-normal muscle strength (92 cm H2O) and was able to generate high peak flow rates and tidal volumes (labels 1, 2, and 3) during both Type-B and Type-C breaths, making them difficult to distinguish. Asynchrony from active expiratory muscle contraction is apparent from the very brief inspiratory spike and volume change (label 4) during the Type-D breath. B: Despite the majority of breaths ostensibly occurring during a high level of ventilatory support, patient work of breathing was extraordinarily high, with a wide distribution of values, compared to the corresponding ventilator work of breathing. This example underscores the problem of promoting spontaneous breathing while simultaneously trying to off-load the respiratory muscles in patients with markedly reduced respiratory-system compliance, elevated minute ventilation demand, and near-normal respiratory muscle strength.
Specific details for each example are presented in the accompanying figure legends. Overall, what is striking about the scalar waveforms (A panels) and corresponding WOB distribution between patient and ventilator (B panels) is the consistency with which the ventilator's pressurization pattern seemingly has little impact on reducing patient WOB. For example, in Subject 9 all spontaneous efforts appeared to be Type-B breaths (see Fig. 2A), so that there was a complete dissociation between patient and ventilator WOB. The ventilator performed an extraordinarily high level of work that did not appear to off-load the inspiratory muscles (see Fig. 2B). This peculiar pattern is at odds with the push-pull theory of assisted mechanical ventilation.55 Under these circumstances the ventilator seemingly provides V̇E support only.
This type of asynchrony also was detected in the same patient during VCV,54 and has been reported in other patients during LPV with traditional modes.56,57 Interestingly, we have observed that this phenomenon disappears when the set tidal volume is increased (usually above the patient's spontaneously generated tidal volume). It suggests that patient-ventilator tidal-volume mismatching (ie, displacement-related feedback) may be responsible for this particular type of asynchrony.
Another impression from reviewing the scalar waveforms is the appearance of “hybrid breaths” that do not conform neatly with the breath classification scheme of Calzia et al14 for APRV and BIPAP. When spontaneous breathing efforts are sustained, the resulting breaths may transpire over more than one “breathing environment” so that the theoretical effect on patient WOB becomes uncertain. These hybrid breaths were captured in scalar tracings from Subject 10, in whom spontaneous efforts began at lower CPAP (Type A), but appeared to continue well into the transition to higher CPAP (see Fig. 3A). In this circumstance the effect on patient WOB is difficult to predict. Although this Type-A breath should cause relatively higher patient WOB, it may have been partially off-loaded because a portion of her inspiratory effort occurred at the transition to the higher CPAP (quasi-Type-C). However, as more breaths are not initiated strictly as patient-triggered Type-C breaths, they do not function as ventilator-assisted breaths. Therefore, the anticipated benefits in reducing patient WOB during APRV or BIPAP from intentionally creating frequent Type-C breaths would not be fully realized either. This interpretation appears to be supported by the corresponding patient-ventilator work distribution, wherein ventilator WOB is considerably lower and more narrowly distributed, compared to the patient's WOB (see Fig. 3B).
Some additional observations are in order. Most salient were the marked differences between patient and ventilator WOB, coupled with the striking intensity and variability in patient WOB. This coincided with highly abnormal levels of respiratory drive in 3 subjects (see Table 2). As noted above, a previous lung-model study16 demonstrated, and discussions from a Journal conference31 speculated on the impact of what I have termed Type-E breaths. Scalar waveforms captured such breaths in 2 subjects (10 and 13). However, it was impossible to isolate the associated WOB for these breaths. In addition, these scalar waveforms also might be interpreted as hybrid breaths, as inspiratory effort continued into low CPAP. Scalar waveforms from Subject 4 reveal how frequently breath types vary over a brief period of time during APRV, and this probably explains some of the wide variation in patient WOB found in these cases.
Implications for Clinical Study Designs
In reviewing all of the possible breath-types during APRV and BIPAP, it is obvious that these permutations in “breathing environments” may have an unpredictable impact upon patient WOB. These considerations are of vital concern to clinical researchers attempting to design studies examining WOB during APRV. Cataloging the frequency of each breath type and its corresponding level of patient WOB is important to improve our understanding of APRV/BIPAP. The clinical examples presented above demonstrate the methodological difficulties involved in such an observational study. Specifically, our measurements were made with an instrument (Bicore CP-100, Viasys, Yorba Linda, California) that did not allow us to simultaneously identify different breath types from scalar waveforms and isolate them to measure WOB. Nonetheless, from a pragmatic standpoint, the primary focus should be on the power output and energy expenditure of patients with different severities of lung injury. Ultimately, clinicians are concerned primarily with determining when, and under what conditions, these modes can be used to provide LPV with an acceptable degree of respiratory muscle exertion.
In our small sample of patients with ALI/ARDS, only 2 patients appeared more comfortable on APRV, and their WOB was either lower or comparable to LPV using traditional modes. Consistent with other studies13,15,21 discussed in this review, these 2 patients had both relatively minor lung injury and pathologic alterations in chest mechanics at the time of study. Therefore, APRV/BIPAP may be a suitable ventilation mode in this and similar patient populations. However, for the entire sample, WOB, PTP, and ΔPes were comparable to the 4 subjects described above (1.98 ± 0.96 J/L, 298 ± 150 cm H2O·s/min, and 19 ± 7 cm H2O, respectively), as was V̇E and CRS (11.8 ± 3.8 L/min and 29 ± 16 cm H2O, respectively). Thus, the clinical data presented here suggest that APRV and BIPAP should be avoided when attempting to implement LPV in patients with ALI/ARDS who have substantial impairment in chest mechanics and elevated V̇E demand.
Negative Impact of Spontaneous Breathing in ALI/ARDS
The principal counter-argument to proponents of APRV is that spontaneous breathing efforts may exacerbate clinical instability and may impede recovery from life-threatening conditions. For example, loaded breathing during severe respiratory failure causes respiratory muscle oxygen consumption to exceed 25% of total body oxygen consumption even when V̇E demand is normal.58 Consequently, systemic perfusion and oxygen delivery may be diverted away from vital organs. In animal models, severe respiratory muscle loading causes a 2,500% and 900% increase in perfusion of the diaphragm and external intercostals, respectively, whereas expiratory muscle perfusion increases approximately 300–400%.59 High degrees of respiratory muscle perfusion may have particularly negative consequences for patients with hemodynamic instability, as hypoperfusion of the gastrointestinal tract facilitates translocation of bacteria into the systemic circulation.60 The same concern extends also to patients suffering from extensive injuries, as sustained hypoperfusion impedes healing and may increase the likelihood of infection.61
In addition, spontaneous breathing during ALI/ARDS is associated with large negative deflections in intrathoracic pressure.62 In the setting of altered permeability of the alveolar-capillary membrane, strenuous breathing efforts increase the transcapillary pressure gradient that enhances pulmonary edema formation.57 This effect is magnified particularly in circumstances when aggressive fluid therapy is used.52,63 What impact spontaneous breathing might have on pulmonary edema formation during APRV or BIPAP probably would be dependent upon the CPAP level and timing ratio employed.
Over the past 2 decades there has been mounting evidence that mortality from ALI/ARDS is associated with the inability of the lungs to resolve pulmonary edema.64 Strategies to limit fluid intake/promote diuresis are associated with improved clinical outcomes.65–69 Thus, in the acute phase of ALI/ARDS the promotion of spontaneous breathing may be at odds with other strategies to keep the lungs dry and improve clinical outcomes. However, vigorous inspiratory efforts also occur during assisted VCV or PCV, particularly during LPV,54,70 that also may accentuate pulmonary edema formation.57
Ultimately, ventilator management of patients with severe ARDS requires the clinician to balance complex and competing needs for ensuring LPV. These include providing adequate gas exchange, promoting alveolar edema clearance, unloading the respiratory muscles, and preventing ventilator-induced diaphragmatic dysfunction and critical illness polyneuropathies, while ensuring adequate systemic oxygen delivery.
Concluding Observations
The purported advantages of APRV are that it provides adequate mechanical support to off-load the respiratory muscles and obviates issues related to patient-ventilator synchrony, so that spontaneous breathing can be maintained throughout the course of ALI/ARDS. Theoretically, APRV and BIPAP should maintain respiratory muscle strength and endurance while simultaneously reducing both the need for NMBA and a high level of sedation administered to critically ill patients. In theory, the consequence should be reduced duration of mechanical ventilation.
Unfortunately, despite more than 2 decades of research on APRV and BIPAP, relatively few studies have subjected these claims to rigorous examination and even fewer have focused on the role of these modes in patients with significant lung injury. Those studies that have suggest that APRV/BIPAP may be an acceptable approach to managing patients with mild, and perhaps moderate, non-obstructive lung disease.
Nonetheless, there is no convincing evidence that APRV or BIPAP reduces sedation requirements or duration of mechanical ventilation in critically ill patients with relatively severe ALI/ARDS. Moreover, the theoretical claims that APRV adequately supports patient WOB can be challenged by equally plausible counter-arguments that unrestricted spontaneous breathing during mechanical ventilation actually may increase patient WOB and asynchrony, particularly during LPV. In fact, these counter-arguments are supported by several of the studies reviewed in this paper, along with the clinical examples wherein APRV or BIPAP was associated with markedly elevated WOB in patients with relatively severe ALI/ARDS.
Furthermore, the debate over the role of APRV and BIPAP in treating patient-ventilator asynchrony and reducing patient WOB is plagued by inconsistent methodological approaches, particularly in regard to setting the timing ratios and release frequencies. A recent review found substantial overlap among studies in timing ratios used between APRV and BIPAP.3 Approximately 50% of studies on APRV used a timing ratio between 1:1–1.9:1, whereas only 9% of BIPAP studies used a timing ratio greater than 1:1. This should not be surprising, as the original study on APRV1 also used only a mildly inverted timing ratio. Both published recommendations4 and clinical studies12,37,38,40 have allowed cycling frequencies of 20 or higher, that overlap with conventional mechanical-ventilation frequencies. This ambiguity regarding what constitutes APRV or BIPAP, and its overlap with conventional pressure-regulated mechanical ventilation, is complicated further by proprietary naming rights imposed by manufacturers. Thus, APRV also is known as Bi-Level Ventilation (Covidien, Mansfield, Massachusetts), Bi-Vent (Siemens, New York, New York), and Duo-PAP (Hamilton, Reno, Nevada).
Finally, to this confusion of terminology must be added the daunting task of direct comparison of WOB between APRV and traditional mechanical ventilation modes. This is related primarily to the variety of “breathing environments” encountered during APRV, and the unpredictable effects these may have in individual patients. Until a well designed and executed prospective clinical trial is done, animated debates regarding the role of spontaneous breathing and APRV in managing asynchrony and WOB in patients with significant lung injury will continue.
Ultimately, the most pertinent clinical measures are the power of breathing (WOB per minute) and PTP, which relate to respiratory muscle perfusion/oxygen consumption, and probably signify the potential for oxidative stress, inflammation, and structural damage71–74 to the muscles from excessive exertion. Paradoxically, unrestricted spontaneous breathing during severe respiratory failure may promote ventilator-dependence. To this must be added the role of ΔPes in promoting pulmonary edema formation during the acute phase of lung injury.
Issues regarding sedation and NMBA use between APRV/BIPAP and traditional mechanical ventilation modes should be delegated to a lower priority until more basic issues related to asynchrony and WOB are more fully illuminated. In this manner, reasonable mechanical ventilation study protocols can be established that should facilitate better studies comparing APRV to traditional modes of assisted mechanical ventilation on sedation and NMBA requirements, as well as clinical outcomes such as duration of mechanical ventilation.
Discussion
Kacmarek:
You didn't mention the extent of esophageal pressure swings in APRV. Newman et al1 illustrated the perfect setup for APRV, and when the patient breathes spontaneously on the high CPAP [continuous positive airway pressure] level, esophageal pressures decreased in some cases by up to 15 cm H2O. The patient is putting forth a lot of effort to breathe and is potentially developing a very high transpulmonary pressure. If the high CPAP level is 30 cm H2O, the transpulmonary pressure could be 45 cm H2O. That is clearly not a lung-protective ventilatory strategy.
Kallet:
Bob, I agree with that. That's the study that really needs to be done. Someone needs to look at pressure-regulated modes and cytokine production with variable degrees of lung-protective ventilation. What happens if you set the pressure control for 20 cm H2O and then you let the VT [tidal volume] go where it will, or you try to restrict the pressure control and make them pull harder to get the volume they want? In either case, what happens to cytokine expression? That hasn't been answered yet.
I think the bottom line is that ventilator-induced lung injury is stress on the lung tissue itself, which is transpulmonary pressure. Obviously, the airway pressure we set is not necessarily the alveolar pressure, so it's really difficult to gauge the transpulmonary pressure when the patient is simultaneously pulling down to let's say –15 cm H2O. My thinking is that it's probably not good.
Kacmarek:
I agree.
MacIntyre:
Rich, APRV is allowed in the current ARDS Network studies [http://www.ardsnet.org]. The rule is that the VT needs to be considered the combination of the ventilator-delivered VT plus whatever additional VT the patient adds during the amplified inflation or “high” pressure. That concept goes back to what Bob [Kacmarek] was talking about. I think you can get misled by the fact that the ventilator's VT is at 6 mL/kg, but we forget that during the high-pressure phase the patient may be adding another 2, 3, or 4 mL/kg.
Kallet:
I think it depends whether it's synchronized or not; it's so variable with what happens.
MacIntyre:
Of course it's variable. My point is that it can often be additive.
Kallet:
So I'm assuming you have [ARDS Network investigator] Roy Brower bound and gagged in a closet somewhere?
MacIntyre:
No, Roy actually bought into that idea. Terminology is an issue that has permeated all the Respiratory Care Journal Conferences I've been to. They've taken a mode and given it 2 different names—both of which are trade names—depending on the inspiratory-expiratory ratio. I find that very conflationary.
Kallet:
I'll own that! The issue I had in designing this study protocol was patient safety. I did not think I could take patients with severe ARDS who required ventilation of 10–12 L/min and compare them to APRV, where they suddenly had to pick up 60% of their minute ventilation. I thought it would bias the study against APRV! The load changes would be different. What's good about this data is that it has some good graphics to show how asynchrony looks during APRV. My study design was well intentioned, but it needs to be done as a different study, with better equipment that could capture and isolate breath types A, B, C, and E and figure out what's going on. If you compare APRV to conventional modes, the real salient point is the power of breathing. The power of breathing in most of these patients was astronomical—sometimes 20-25 J/min, and they looked very, very uncomfortable! I think you're caught in a difficult situation trying to use APRV for lung-protective ventilation and to actually enforce it.
MacIntyre:
I was just concerned about the terminology.
Kallet:
OK, I'll clear up the terminology. I'm not rigid about that. It's confusing to me and it annoys me that when we talk about APRV we have to jump through all these hoops of classification, if it's one thing or the other.
MacIntyre:
When you think about it, every form of positive-pressure ventilation is bi-level, for crying out loud. You have an inspiratory pressure and an expiratory pressure. This notion that bi-level is this magical new approach to supporting patients with mechanical ventilation seems crazy to me.
Kallet:
I propose that Dave Pierson take a vote, and whatever you guys want me to call it, I will call it.
Hess:
Let me just say that, as the editor, it really becomes problematic when every author comes up with their own terms of what they call things. I think we confuse our readers sometimes. We try to make a concerted effort to have it be as consistent as possible.
Kallet:
What's standard terminology?
Hess:
Well…
Kacmarek:
We have a standard terminology that's different than your standard terminology!
Hess:
And therein is the problem, because every author wants to do it his way, and I think it confuses our readers.
Kacmarek:
But what is the accepted terminology across the board? There is none.
Hess:
There is standard recommended terminology that some people don't want to conform to.
Branson:
Obviously, I agree. We've spent a long time trying to do that. I think it's funny, though, that Neil takes offense at “bi-level” when we had a whole Journal Conference and some of the titles of the papers were about bi-level ventilators. Or that somehow the ventilator used for noninvasive ventilation is different than a regular ventilator because it's bi-level. I agree: we have to have some kind of consensus. But even to Bob's [Kacmarek] point, APRV is a trade name. If you ask “What does the ventilator do?” it's very easy if you're willing to accept that every breath has a trigger, a limit, and a cycle. And even if you want to call it bi-level or CPAP with release, when you show me the picture and tell me how it works, we ought to understand what's going on.
Kacmarek:
But if you have a term that the literature repeatedly uses, that should be the default term for that mode. APRV, whether I like it or not, is what's out there: 90% of the papers use it, it's understood by the respiratory care community to mean X, and if you change it to “time-cycled whatever,” people aren't going to know what the hell that is.
Branson:
I don't think you have to change the name, I just think you just have to have a description.
Kacmarek:
I have no problem with calling it APRV and then saying “this is A, B, C, and D,” but losing the term APRV causes much more confusion than trying to standardize to some other term that nobody uses in general discussions about mechanical ventilation.
Hess:
I don't have a problem with APRV.
MacIntyre:
It's a trade name.
Hess:
I'm not picking on anybody in this room, but people outside this room call things all kinds of different names.
Kacmarek:
It was a term used by John Downs in the original paper describing this. Before it was a trade name it was his term that he used to describe this approach.
Hess:
I don't think we're arguing about APRV. It's a bigger discussion than that.
Pierson:
If we confused the readers only with the terminology, this conference would be a wonderful success.
Branson:
I'm struck by the same discussion as yesterday. The first time I put somebody on APRV was in 1985, and we didn't even use a ventilator to do it, and our results were exactly your results: if the patient's PaO2/FIO2 ratio is 200/250 and the high pressure has to be 20 cm H2O, they do perfectly fine. If they get really sick, it won't support them. If APRV was the answer, we'd know it by now. Having said that, you could travel all around the country and go to places that use nothing but APRV.
The Shock Trauma Center in Baltimore puts everyone on APRV and prones every trauma patient with respiratory failure, and they swear their mortality rate in ARDS is 15%. I believe them, because young healthy trauma patients will survive as long as you fix the surgical problem and then don't do something untoward in the intensive care unit; you just have to get them over the problem.
It's hard, and I'm not trying to dismiss APRV. If people like it and use it and know what they're doing—and that's probably the most important thing about this whole conference—I have no problem. The problem is when a therapist in the medical intensive care unit has a hypoxemic COPD patient and thinks, “This guy needs to be recruited: I'm gonna put him on APRV”—that's where we get into a problem.
MacIntyre:
Mike, you were maybe about to say the same thing?
Gentile:
Yes: don't steal my comment.
MacIntyre:
I think you've illustrated the terminology problem yet again. You said that over half of your studies use APRV at less than 1:1, and when that occurs, spontaneous breathing during the inflation phase is unusual, if it occurs at all. Indeed, spontaneous breathing will occur if you give them enough expiratory time during the expiratory phase, in which case it is identical to pressure-targeted IMV [intermittent mandatory ventilation], and if they don't breathe at all, it turns into pressure assist control ventilation. So the term APRV morphs into other things, depending on how you've set it up.
Branson:
What Neil's describing, and what you showed me, is that in those patients APRV was failing. If you have to go to where the inspiratory-expiratory ratio is 1:1, you're no longer doing APRV, and it's because APRV failed to support that patient.
Hess:
But a lot of people still call that APRV, because that's what the machine says on the screen.
Branson:
I agree.
Kacmarek:
Your comments concern me about APRV. I think it's a dangerous mode because it's a mode, as you just implied, where less sick patients—trauma victims, who will usually do well regardless of the ventilatory approach, as long as we don't do something foolish—will do quite well. But if the patient is very sick, they can't be managed on APRV. So how can you promote a ventilation mode where if you use it on a patient who's a little sicker, you get into all kinds of problems—where you can only use it on patients who are marginally ill?
MacIntyre:
But Bob, the point is that if you take the APRV and you create a VT of 6, 7, or 8 mL/kg and you give it 20 breaths a minute and a short inspiratory-expiratory ratio, it's pressure assist control ventilation.
Kacmarek:
I agree, but that's different.
MacIntyre:
Well, the mode selector still says APRV.
Kacmarek:
The primary proponents of APRV recommend an inverse ratio, forcing the patient to breathe spontaneously at the high CPAP level, which is not well tolerated by sick patients.
Gentile:
This conference reminds me of my family reunion, where you've really got to jump to get a word in edgewise.
Kacmarek:
Damn right.
Gentile:
I'm interested in designing a survey about the actual use practices of APRV. We could have a whole Journal Conference to argue what's best and how to use it.
Sassoon:
Listening to this debate is amusing. All of you have previously stated that we should abandon IMV Well, APRV is actually the reverse of IMV; doesn't that mean that we should abandon APRV?
Branson:
I'm not trying to promote APRV or NAVA or IMV, but I'm not going to call the people who use it routinely in a patient population of no chronic obstructive airways disease and relatively stiff lungs and who seem to report good results and tell them that they can't do it any more because Bob said so.
Kallet:
I have a biased response about this issue in particular, because I feel like I'm being hustled. The most vociferous proponents of APRV have made very misleading claims that their outcomes in patients with ARDS are much better than the ARDS Network study results.1 It's disingenuous to compare the mortality of a population of trauma-induced ARDS to the general ARDS population, where pneumonia and sepsis are the predominant etiologies, and carry a higher mortality!2 San Francisco General Hospital, which is a level-one trauma center, has a large population of trauma patients with ARDS, and our mortality in 1996 through 2007, using volume-control or dual-mode pressure control (with or without lung-protective ventilation) was 23%.3 It's not really different from the 21% mortality reported by the Shock Trauma Center in Baltimore.1 Then on top of that we get into these terminology debates where APRV proponents will claim, “It's not really inverse-ratio ventilation because you're going from functional residual capacity downwards, so there can't be any ventilator-induced lung injury.” There are a lot of distorted arguments made by APRV proponents, who, in my opinion, are overly enthusiastic, and it causes problems.
Hess:
I agree with that. I've heard it argued that the mortality is 10% using APRV in trauma patients, compared to the ARDS Network mortality of 30% in the low-VT group.1 However, Eisner et al did a subgroup analysis of the ARDS Network data and reported a mortality of 11% in patients with trauma-related ARDS (regardless of VT).2
Hurford:
It's reasonable to assume, since we're talking about definitions, that maybe trauma patients don't get ARDS. They get lung contusions and atelectasis.
Gentile:
Also, is the cause of death hypoxic respiratory failure or is it another injury? It's also interesting that people who use this mode advocate air-trapping, and we all work really hard to eliminate air-trapping in all other modes. But it's sort of OK to do it this way, but it's very uncomfortable to breathe on.
Branson:
Mike, that's a bad argument, because you have normal lungs. You'd be uncomfortable on 10 of CPAP too, but a patient with cardiogenic pulmonary edema will pull the facemask back on because it gives them relief. We don't use APRV, we've probably used it twice in the past year in patients who weren't responding. My biggest concern is where people decide to set the low pressure, and how much air-trapping they get and that they don't understand how much it is. Then you end up with a group of different PEEPs based on the local regional compliances: not a single PEEP. That's my biggest concern about APRV and the people who use it, especially people who use it on every single patient. I don't think there's a mode that works on every patient.
Younes:*
I think we shouldn't be blaming Rich [Kallet] for the nomenclature problem; we should be blaming the industry because I think they are the ones who are not playing fair. If you want to call it APRV, it has to stick: you should not be able to change the settings outside the range that was originally proposed for APRV. So either they should limit the inspiratory-expiratory-ratio setting to what it was supposed to be, or if you go down to a lower ratio, it should change the mode automatically from APRV to pressure assist control or whatever. It is really their problem: they have made it possible for people to use a different mode under the name APRV.
Footnotes
- Correspondence: Richard H Kallet MSc RRT FAARC, Respiratory Care Services, San Francisco General Hospital, NH:GA-2, 1001 Potrero Avenue, San Francisco CA 94110. E-mail: rkallet{at}sfghsom.ucsf.edu.
-
Mr Kallet has disclosed relationships with Viasys, Covidian, and Puritan Bennett.
-
Mr Kallet presented a version of this paper at the 46th Respiratory Care Journal Conference, “Patient-Ventilator Interaction,” held March 19-21, 2010, in Cancún, Quintana Roo, Mexico.
↵* Magdy Younes MD FRCP(C) PhD, Department of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
- Copyright © 2011 by Daedalus Enterprises Inc.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.
- 67.
- 68.
- 69.↵
- 70.↵
- 71.↵
- 72.
- 73.
- 74.↵