Abstract
BACKGROUND: Double-triggering (DT) is a frequent type of patient-ventilator asynchrony and has potentially severe consequences, such as alveolar overdistention or the generation of intrinsic PEEP. However, the first breath of DT could be patient-triggered (DT-P), auto-triggered (DT-A), or ventilator-triggered (DT-V).
OBJECTIVE: To differentiate DT-P, DT-A, and DT-V using airway pressure or flow changes during the trigger-delay phase in ventilated patients.
METHODS: Fourteen mechanically ventilated patients with DT were included. All patients were on flow-triggered ventilation modes and received either continuous mandatory ventilation or pressure support ventilation. Breaths in which the first breath was associated with an esophageal pressure drop of > 1 cm H2O were categorized as DT-P. Breaths in which the first breath occurred at the ventilator set cycle were categorized as DT-V. Breaths in which the first breath occurred earlier than the ventilator set cycle without esophageal pressure drop were categorized as DT-A. The pressure drop and flow change at 0.13 s (PD0.13 and F0.13, respectively) in the trigger-delay phase were calculated from the nadir.
RESULTS: There were 507 double-triggered breaths: 271 DT-V (53%), 50 DT-A (10%), and 186 DT-P (37%). The PD0.13 for DT-V, DT-A, and DT-P were 0.16 ± 0.12 cm H2O, 0.25 ± 0.17 cm H2O, and 1.34 ± 0.67 cm H2O, respectively. The F0.13 for DT-V, DT-A, and DT-P were 2.11 ± 2.31 L/min, 2.64 ± 2.07 L/min, and 16.51 ± 8.02 L/min, respectively. The best discriminatory criteria for differentiating DT-P from DT-V and DT-A, based on the Youden index (sensitivity + specificity – 1) was PD0.13 ≥ 0.49 cm H2O, which had a Youden index of 95%. CONCLUSION: DT-P can be distinguished from DT-V and DT-A by using airway pressure deflections in the trigger-delay phase.
Introduction
Patient-ventilator asynchrony is a frequently observed phenomenon in ventilated patients. According to a recent study,1 at least 50% of patients who receive mechanical ventilation for more than 24 hours display some conflict with the ventilator. Aside from an ineffective triggering, double-triggering (DT) is another major type of patient-ventilator asynchrony.1 DT is usually defined as 2 ventilator insufflations delivered within one patient inspiratory effort, and the first trigger should be patient-triggered (DT-P),1–4 but can be ventilator-triggered (DT-V) or auto-triggered (DT-A).5 DT-P is likely to occur in patients with short inspiratory times, lower PaO2/FIO2, high maximum inspiratory pressure, high PEEP, and greater expiratory trigger settings.1,4 In our opinion, a direct prolongation of inspiration time in a continuous mandatory ventilation mode or a change of expiratory trigger setting or pressurization rate in pressure-support ventilation is a reasonable strategy for abolishing DT-P.4 DT-V is usually due to disharmony between the patient's breath rate and the set ventilator rate. Prolonging the inspiratory time may not be helpful. An adjustment of trigger sensitivity may be needed for DT-A. DT is likely to be associated with high tidal volume, which may create intrinsic PEEP in patients with expiratory flow limitation, and increase the work of breathing. In patients with acute lung injury, high tidal volume may cause alveolar overdistention and exacerbate lung injury. DT may also disrupt sleep.6
Distinguishing DT-V, DT-P, and DT-A can be done only by examining the inspiratory effort on the tracing of the esophageal pressure, transdiaphragmatic pressure, or diaphragmatic electromyogram. Unfortunately, these invasive procedures are usually not available in clinical practice. Differentiating DT-P from DT-V or DT-A based on airway pressure and flow tracings may be problematic with a flow-triggered ventilation mode.4 A subjective criterion of abrupt airway pressure drop of > 0.5 cm H2O before a ventilator-assisted breath is prone to be inaccurate because the onset of inspiratory effort is hard to determine.1 Although a triggered breath can easily be identified with a modern ventilator, auto-triggering may also be hard to identify. In view of the different pathophysiological bases between these types of DT, we sought to establish objective criteria for differentiating DT-V, DT-P, and DT-A, based on 2 variables universally available on current ventilators: airway pressure and flow. As DT-P implies obvious inspiratory drive,1 we hypothesized that airway pressure and flow changes in the trigger-delay phase should be significantly different between the first breath of DT-P and that of DT-V or DT-A. In addition to establishing an objective criteria, we also studied the effect of changes of ventilator settings on DT-P and DT-V, to test our hypothesis. We used esophageal pressure deflection as an objective measure of inspiratory effort.
Methods
This study was performed at National Cheng Kung University Hospital, Taiwan, and approved by the hospital's ethics committee. All subjects or their next of kin gave written informed consent.
Subjects
Between March 2006 and September 2008, all mechanically ventilated patients in our 10-bed respiratory intensive care unit were screened daily for the appearance of patient-ventilator asynchrony. The inclusion criteria were: hemodynamically stable; no inotropic agents; ventilated with an FIO2 of < 0.5 and PEEP ≤ 10 cm H2O, and agreement on the placement of an esophageal balloon. The exclusion criteria were pregnancy, unstable angina, myocardial infarction or aortic dissection as a cause of admission, and nasal or oropharyngeal lesions that prohibited the placement of an esophageal balloon. A total of 762 patients were ventilated during the study period, and we included 68 patients. Ventilators used included Puritan-Bennett 840 (Tyco International, Princeton, New Jersey), Galileo (Hamilton Medical, Bonaduz, Switzerland), and Servo-i (Maquet, Solna, Sweden). To validate our hypothesis for management of DT-P and DT-V we studied an additional 12 patients with DT-P or DT-V, before and after changes of ventilator settings. In those 12 patients we did not place an esophageal balloon.
In 68 patients we placed an esophageal balloon in the lower third of the esophagus, which we inflated with 0.5 mL of air. Proper balloon placement was verified with the occlusion test.7 Air flow was measured using a pneumotachograph (PN 155362, Hamilton Medical, Bonaduz, Switzerland), connected to a differential pressure transducer (MP 45, Validyne, Northridge, California). The flow sensor was placed between the endotracheal tube and the Y-piece. Tidal volume was calculated by integrating the flow signal. Airway and esophageal pressure were measured individually, with 2 differential pressure transducers (P300D, Validyne, Northridge, California). All signals were sampled and digitalized at 100 Hz, and data were stored in data-acquisition software (AcqKnowledge, Biopac, Goleta, California). All patients were studied in a semi-erect position if possible. If clinically required, endotracheal suctioning was performed before the measurement. The initial ventilator settings were selected by the attending physician and respiratory therapist. The ventilator triggering type could be changed to flow-triggering during the recording period if the initial setting was pressure-triggered. We recorded the pressure and flow data for an average of 10–20 min with each patient. If deemed necessary, adjustment of the ventilator settings was allowed during the recording period. Breaths with a questionable esophageal pressure signal were not included for further analysis.
Definitions of DT-P, DT-V, and DT-A
DT was defined as 2 consecutive triggers separated by an expiratory time < 50% of the mean inspiratory time. If the first trigger had an esophageal pressure drop of > 1 cm H2O, the breath was categorized as DT-P.8 Breaths in which the first breath occurred at the ventilator set time trigger, without concomitant esophageal pressure drop, were categorized as DT-V. Breaths in which the first trigger occurred earlier than the ventilator set time trigger, without concomitant esophageal pressure drop, were categorized as DT-A (Fig. 1). The first trigger of the DT breath was used for further analysis.
Typical flow, airway pressure, and esophageal pressure tracings of double-triggering, by the patient (DT-P), the ventilator (DT-V), and auto-triggering (DT-A).
Estimation of the Inspiratory Swing in the Inspiratory Trigger-Delay Phase From Airway Pressure and Flow Tracings
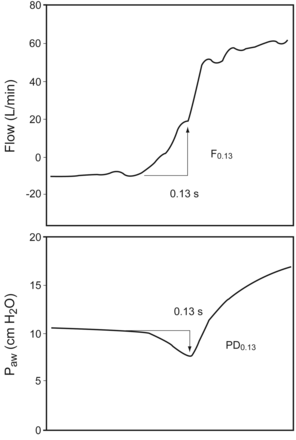
Bench studies of modern ventilators showed that trigger delay may vary with inspiratory flow rate, the type of ventilator, and respiratory mechanics.9–11 It may be difficult to determine the onset of inspiratory effort from airway pressure and flow tracings, so any arbitrary definition of an inspiratory effort from airway flow and pressure tracing is subject to criticism. However, in bedside ventilator simulation of inspiratory efforts,9 we found an inspiratory-trigger-delay range of 0.07–0.13 s, when both inspiratory effort and ventilator type (PB840, Servo, or Galileo) were taken into consideration. The upper limit was 0.1 s with lower effort, and 0.13 s with higher effort.9 We thus selected 0.13 s from airway pressure and flow tracings for estimating the inspiratory trigger delay in our patients, because we believe that DT-P is associated with stronger inspiratory effort. We calculated the pressure drop at 0.13 s (PD0.13) and the flow change at 0.13 s (F0.13) during the trigger-delay phase (retrograde from the nadir of the airway pressure tracing) for DT-V, DT-P, and DT-A (Fig. 2).
Pressure drop (PD0.13) and flow change (F0.13) at 0.13 s during the trigger-delay phase.
Statistical Analysis
Results are given as mean ± SD, unless otherwise specified. We used the paired t test to compare continuous variables between 2 matched groups. We created scatter plots of pressure and flow changes to characterize their distributions. P values < .05 were considered significant. We calculated airway pressure and flow changes for detecting DT-P, DT-V, and DT-A with receiver operating characteristic curves, to reveal the detection power, and the areas under the curve. We used the Youden index12 (sensitivity + specificity – 1) to determine the optimal criteria for detecting DT-P. We used statistics software for the analysis (Prism 4, GraphPad Software, San Diego, California).
Results
We recorded data from 68 patients. Fifteen patients were excluded: 7 because they had no asynchrony; 5 because of poor esophageal pressure tracing; and 3 because of frequent hiccup. In the 53 included patients, the mean recording period was 18.6 ± 6.8 min. We analyzed 21,810 breaths, and 4,134 breaths showed patient-ventilator asynchrony: 3,222 (78%) showed ineffective triggering in the expiratory phase; 233 (6%) showed ineffective triggering in the inspiratory phase; 507 (12%) showed various forms of DT; 135 (3%) were auto-triggered; and a few breaths were short-cycled.
Table 1 describes the 14 subjects who demonstrated DT. All the patients were on continuous mandatory ventilation or pressure support modes with flow-triggering set at 2 L/min (or flow-trigger scale 2 with Servo-i), which is a common setting in our respiratory intensive care unit. We excluded recordings on pressure-triggered modes. There were a total of 507 breaths with DT-V, DT-A, or DT-P, among 14 patients: 271 DT-V, 50 DT-A, and 186 DT-P. The mean accumulated tidal volume for all the DT breaths was 773 ± 211 mL (13.2 ± 3.5 mL/kg ideal body weight), the mean tidal volume for the normal breaths was 493 ± 94 mL (8.5 ± 1.5 mL/kg ideal body weight).
Subjects, Ventilators, Modes, Tidal Volume, Breaths, and Double-Triggering Events*
Airway Pressure and Flow Changes in the Trigger-Delay Phase for Discriminating the Double-Triggering Types
Table 2 shows the PD0.13 and F0.13 data. The PD0.13 and F0.13 of the DT-P breaths were higher than those of the DT-A and DT-V breaths. For distinguishing DT-P from DT-A and DT-V, the area under the receiver operating characteristics curve was 0.99 for PD0.13 and 0.97 for F0.13. Table 3 shows the best PD0.13 and F0.13 values for discriminating DT-P from DT-V and DT-A. PD0.13 ≥ 0.49 cm H2O is the best discriminator as a whole, with minimal difference among the 3 ventilator types. F0.13 was a poor discriminator with the Servo-i ventilator. The best PD0.13 and F0.13 values for differentiating DT-A from DT-V were PD0.13 ≥ 0.28 cm H2O (sensitivity 38%, specificity 91%) and F0.13 ≥ 2.43 L/min (sensitivity 46%, specificity 76%). It is difficult to separate DT-V from DT-A based on airway pressure and flow changes during the inspiratory trigger-delay phase.
Airway Pressure and Flow Changes in the Trigger-Delay Phase of Double-Triggered Breaths
Optimal PD0.13 and F0.13 for Distinguishing DT-P, DT-V, and DT-A
Esophageal Pressure Drop and Duration of Neural Inspiration Between the First DT-P Breath and Its Previous Normally Triggered Breath
Data on the esophageal pressure drop between the first DT-P breath and its previous, normally triggered breath were available from 6 patients (54 breath pairs). Esophageal pressure drop was significantly greater in the first DT-P breath in 5 patients (9.5 ± 4.4 cm H2O in the normally triggered breath vs 11.4 ± 4.8 cm H2O in the first DT-P breath, n = 54). Neural inspiratory time, calculated from the onset of rapid decline of esophageal pressure to the nadir,13 was significantly longer in the first DT-P breath than in the previous normally triggered breath in all 6 patients displaying DT-P (0.79 ± 0.28 s in the normally triggered breath vs 0.93 ± 0.22 s in the first DT-P breath).
Management of DT-P and DT-V
We studied 12 patients with DT-P and DT-V but without placement of esophageal balloon before and after readjustment of ventilator settings. We examined the airway pressure and flow tracings for at least 5 min before and after ventilator adjustment in 6 patients with DT-P and 6 with DT-V. In the patients with DT-P, 3 patients were on pressure support ventilation, 2 were on a pressure control mode, and 1 was on a volume control mode. Their number of DT breaths averaged 7 per minute, which decreased to 0.2 per minute after prolonging the inspiratory time, by decreasing the expiratory flow cycle threshold in 3 patients on pressure support, and by increasing the inspiration time in 2 patients on pressure control ventilation. In the patient with DT-P on a volume control mode, DT was abolished by a change to a pressure support mode, with an increase in inspiratory time by appropriately adjusting the expiratory flow cycle threshold. Although not used in the present study, the inspiratory time can also be prolonged by reducing the pressurization ramp.4
All 6 patients with DT-V were on pressure-control ventilation, and the number of DT breaths averaged 6 per minute. Their initial mandatory respiratory rate averaged 17 breaths/min, and DT-V breaths were completely abolished by decreasing the mandatory respiratory rate to an average of 8 breaths/min.
Discussion
We demonstrated in this study that DT-P can be reliably separated from DT-V and DT-A based on airway pressure or flow changes in the trigger-delay phase in mechanically ventilated patients under flow-triggering, in which the airway-pressure drop may be extremely low. Pressure-drop change was more powerful than flow change for distinguishing DT-P from DT-V and DT-A. This information could be of value for objective classification of various forms of DT.
Some limitations to our study need to be addressed before further discussion. The first is the criterion we used for inspiratory effort: an esophageal pressure drop of > 1 cm H2O was taken as the objective criterion of inspiratory effort.8 As esophageal pressure is an indirect estimate of neural drive, the onset of neural inspiratory time may be different if transdiaphragmatic pressure or diaphragm electromyogram signal is used. However, the use of esophageal pressure as the indicated onset of neural inspiration should be acceptable, as the bias errors are the lowest, according to a recent study.13 Therefore, we are confident that our classification of various forms of DT should be accurate.
The second limitation is the small number of patients monitored and the variations in distributions of DT-P, DT-A, and DT-V in individual cases. In addition, only patients with esophageal pressure monitoring were included, and more than one type of ventilator was used. Although the trigger delays are not the same for different ventilators10 and the trigger delay was apparently affected by patients' effort and respiratory mechanics, airway pressure drop and flow change in the trigger-delay phase were apparently higher in the DT-P breaths with all 3 ventilator types. The optimal discriminating criteria for separating DT-P from DT-V and DT-A were similar when P0.13 was used. Therefore, our selected criteria for distinguishing the various forms of DT should be applicable to most patients, with modern ventilators.
In our study, P0.13 was much higher in DT-P than in DT-V or DT-A, and DT-P was usually associated with greater and longer inspiratory efforts, compared to non-DT breaths in the same patient. This finding implicated greater inspiratory drive when DT-P occurred. Although flow-triggering may be associated with minimal pressure deflection in the trigger-delay phase, a recent bench study that used the most sensitive trigger thresholds found that inspiratory trigger pressure (an analog to our pressure drop) averaged 2.2 cm H2O in modern ventilators.10 Therefore, it is not surprising that the first DT-P breath was associated with a significantly higher pressure drop in the inspiratory trigger-delay phase, in comparison to those with DT-V or DT-A.
In addition to pressure drop, flow change in the pre-inspiratory phase was also much higher in DT-P breaths, and could also be used to separate DT-P from DT-V and DT-A. As DT-P is associated with a greater inspiratory effort that usually starts well before the end of expiration, it unsurprisingly leads to an increased flow change in a flow-triggered mode. In contrast, DT-V and DT-A are not associated with inspiratory effort, which are destined for smaller flow changes.
There are some clinical implications from our findings. DT-P is the second most frequent cause of patient-ventilator asynchrony, and identification of DT-P based on ambiguous airway pressure and flow change preceding the first assisted breath is subject to conflict.5 However, DT-P can be distinguished from DT-A and DT-V in the majority of cases, according to our pre-selected criteria. In our opinion, a fixed timing for calculating the pre-inspiratory pressure drop is needed for correct classification of DT. Once DTs are correctly classified, their management plans differ, as their underlying pathophysiologies are different. DT-P is associated with short inspiratory duration, which is a more frequent event when the pressurization ramp is rapid in pressure support ventilation. Directly prolonging the inspiratory time or manipulating the expiratory flow cycle threshold or pressurization time may abolish DT-P. In contrast, DT-V is associated with a faster mandatory respiratory rate, and reducing the mandatory rate or changing to a spontaneous breathing mode will synchronize the ventilator with the patient's breathing. Successful management of our 12 patients with DT-P or DT-V confirmed this principle of ventilator adjustment.
Conclusions
DT-P can be easily differentiated from DT-V and DT-A with the pre-inspiratory pressure or flow change at 0.13 s. Based on our recommended criteria, DT-P can be distinguished from DT-V and DT-A in the majority of cases. The high discriminatory power of our defined criteria may allow objective identification of DT when only airway pressure or flow is used.
Footnotes
- Correspondence: Chang-Wen Chen MD MSc, Medical Intensive Care Unit, Department of Internal Medicine, National Cheng Kung University Hospital, 138 Sheng-Li Road, Tainan 704, Taiwan. E-mail: cwchen{at}mail.ncku.edu.tw.
-
Chang-Wen Chen MD MSc presented a version of this paper at the 22nd Annual Congress of the European Society of Intensive Care Medicine, held October 11–14, 2009, in Vienna, Austria.
-
This study was partly supported by grants from the National Science Council of Taiwan (98-2320-B-006-002-MY2, NCKUH-9704017 and NCKUH-9804001.
-
The authors have disclosed no conflicts of interest.
- Copyright © 2011 by Daedalus Enterprises Inc.