Abstract
BACKGROUND: Nebulized hypertonic saline is a highly effective therapy for patients with cystic fibrosis (CF), yet 10% of patients are intolerant of hypertonic saline administered via jet nebulizer. Positive expiratory pressure (PEP) nebulizers splint open the airways and offers a more controlled rate of nebulization.
METHODS: In 4 consecutive adult CF patients who were intolerant of hypertonic saline via jet nebulizer, we nebulized 6% hypertonic saline via a PEP nebulizer. We measured the number of days the patients required intravenous antibiotics from enrollment to study end, compared to an equal period before PEP, and the mean time between the patients' 3 most recent infective pulmonary exacerbation episodes before PEP to their next exacerbation after PEP. Patients also completed a Likert-scale adverse-effects questionnaire on hypertonic saline via PEP versus jet nebulizer.
RESULTS: The 4 patients had severe CF pulmonary disease and all fully tolerated hypertonic saline via PEP, for 77, 92, 128, and 137 days, respectively until the study end date. There were fewer days of antibiotics in 3 of the 4 patients, from 45 to 20 days, 66 to 14 days, and 28 to 0 days (mean relative risk reduction 53%, P = .11). The other patient had 63 days of antibiotics during both the PEP and the jet nebulizer periods. There was a mean 3.6-fold longer time to next infective pulmonary exacerbation during the PEP period (P = .07). Adverse effects were less with PEP: chest tightness 68% (P = .04), bad taste 62% (P = .06), cough 47% (P = .10), and sore throat 50% (P = .20).
CONCLUSIONS: Hypertonic saline via PEP nebulizer benefits CF patients who do not tolerate hypertonic saline via jet nebulizer.
Introduction
Cystic fibrosis (CF) is the most common life-threatening autosomal recessive disease in Ireland, with an incidence of 1 in 1,461 births.1 CF is characterized by mucus retention, bacterial infection, and inflammation, leading to lung damage and ultimately respiratory failure. CF therapies aim to improve mucociliary clearance, reduce bacterial load, and lower airway inflammation.2
A mutation in the CF transmembrane receptor gene results in abnormal ion transport across the respiratory epithelium.3 The primary pathophysiological defect is thought to be depletion of the airway surface liquid. The isotonic volume hypothesis proposes that the defective CF transmembrane receptor leads to excessive absorption of fluid from the airway surface liquid and a below-normal airway surface liquid volume (but of normal tonicity),4 which impairs mucociliary clearance, and the retained mucus is a locus of infection. Nebulized hypertonic saline induces an osmotic gradient that increases the airway surface liquid, increases mucociliary clearance, and decreases CF pulmonary exacerbations.
Seven-percent hypertonic saline via jet nebulizer significantly reduces the CF pulmonary exacerbation rate and, thus, antibiotic use.5,6 As extrapolated from the original study,5 jet nebulization of hypertonic saline is associated with almost 10% treatment intolerance, predominantly secondary to cough, bad taste, and bronchospasm.
Methods
This study was approved by the clinical research ethics committee of the Cork teaching hospitals. In CF patients who have previously failed jet-nebulized hypertonic saline because of intolerable adverse effects (eg, cough, bad taste, and bronchospasm), we studied patient tolerance of hypertonic saline administered via a positive expiratory pressure (PEP) system. We compared the exacerbation rate and antibiotic use during the jet-nebulizer period and the PEP-nebulizer period.
Positive Expiratory Pressure Nebulizer System
The PEP nebulizer system we used (Pari PEP-System 1 with Pari LC Plus nebulizer chamber, Pari Respiratory Equipment, Midlothian, Virginia) is a flow-resistor device with an adjustable restricted orifice that produces exhalation resistance of 10–20 cm of H2O. PEP splints open the airways and provides a more controlled rate of nebulization.7 Nebulization of bronchodilators with PEP is well established in bronchospastic patients.8
Patients
From May 16 to July 15, 2008, we recruited all successive adult CF patients in the Cork Adult Cystic Fibrosis Centre, Cork University Hospital, Cork, Ireland, who had previously failed trials of 6% hypertonic saline via jet nebulizer (Porta-Neb compressor [Respironics, Murrysville, Pennsylvania] and Sidestream nebulizer [Respironics, Murrysville, Pennsylvania] or Pari LC Plus nebulizer [Pari Respiratory Equipment, Midlothian, Virginia]) secondary to adverse effects (ie, the patient was unwilling to continue the therapy and/or had a > 15% decline in FEV1, after 2 separate trials of hypertonic saline via jet nebulizer). Our protocol of repeated hypertonic saline trials is based on findings that airway hyper-responsiveness to hypertonic saline can be a transient phenomenon.9
We included patients who were > 18 years old, with a confirmed diagnosis of CF,10 and who had failed repeated trials of hypertonic saline via jet nebulizer. Per the American Association for Respiratory Care guidelines, we excluded patients with intracranial pressure > 20 mm Hg, hemodynamic instability, esophageal surgery, active hemoptysis, known or suspected tympanic membrane rupture or other middle-ear pathology, or untreated pneumothorax.11
Treatments
Each patient inhaled 2 puffs of albuterol (100 μg/puff) from a metered-dose inhaler, then 4 mL of 6% hypertonic saline (MucoClear, Pari Respiratory Equipment, Midlothian, Virginia) from the PEP system, which comprised a Porta-Neb compressor (dynamic pressure 11.7 psi, flow 6 L/min), a Pari PEP-System 1, and a Pari LC Plus nebulizer chamber. The respiratory therapist adjusted the resistance setting on the PEP device with a manometer attached to the PEP device's test dose port, to achieve an expiratory pressure resistance of 15–20 cm H2O. Spirometry was performed before and after the first dose. Patients who had stable spirometry (< 15% FEV1 decline) continued with hypertonic saline treatment. Patients who had stable spirometry and tolerated the treatment were instructed to do the hypertonic saline treatments twice daily, after 2 puffs of albuterol (100 μg/puff), and then to proceed with their daily routine of autogenic drainage and nebulized antibiotics. The hypertonic saline was delivered via mouthpiece, without nasal clip, and with the resistance setting determined by the respiratory therapist, throughout the study period. Patients were also advised on nebulizer maintenance, as per the manufacturer's instructions.
Exacerbations
All patients were prospectively observed from recruitment to the pre-established common study end date of October 1, 2008. We recorded the number of days on intravenous antibiotics from enrollment to study end date, and compared the data from equal period before and after hypertonic saline commencement. We also calculated the mean time between the patients' 3 previous infective pulmonary exacerbations before hypertonic saline, and the time to next infective pulmonary exacerbation that required intravenous antibiotics, after initiating hypertonic saline.
Adverse Effects
Four weeks after PEP initiation, all 4 patients completed an adverse-effects questionnaire that was based on previous studies on hypertonic saline.5,6 (Refer to the supplementary resources at http://www.rcjournal.com for the questionnaire.) On a 1–10 Likert-scale (1 = minimal, 10 = intolerable) the patients graded cough, chest tightness, sore throat, and bad taste, for both jet nebulizer and PEP nebulizer.
Statistical Analysis
With statistics software (SPSS 15, SPSS, Chicago, Illinois), we used the Wilcoxon rank test to compare intravenous antibiotic requirement before and after hypertonic saline therapy, and exacerbation-free interval before and after therapy. We used a paired-sample t test to compare the adverse effects scores.
Results
Tolerance
Of the 37 CF patients we saw in our center during the screening period who were prescribed twice-daily nebulized 6% hypertonic saline, 4 patients (12%) failed to tolerate this therapy, despite repeated testing. Two patients had failed previous hypertonic saline trials secondary to severe bronchospasm (FEV1 decrease of > 15%) and chest tightness; one patient had intolerable cough; and one patient could not tolerate the taste, despite taking the medication for several days. We recruited those 4 patients, and they all tolerated hypertonic saline via PEP nebulizer for the full study duration (Table 1). All 4 patients had severe CF-related pulmonary disease, with FEV1 < 30% of predicted and frequent infective pulmonary exacerbations (mean 9 intravenous antibiotic courses over the previous one-year period). All 4 patients were fully adherent to hypertonic saline via PEP nebulizer, for 77, 92, 128, and 137 days, to the study end date.
Patients
Exacerbations
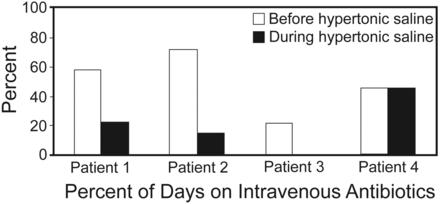
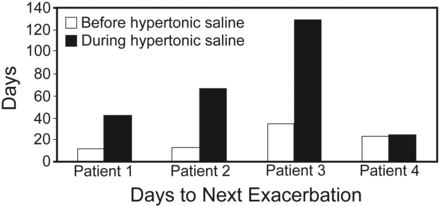
In patients 1, 2, and 3, in the hypertonic saline period, antibiotic requirement decreased (53% risk reduction in antibiotic requirement, P = .11) (Table 2 and Fig. 1), and time to next infective pulmonary exacerbation requiring intravenous antibiotics increased (mean 18 d vs 64 d, P = .07) (see Table 2 and Fig. 2).
Antibiotic Days and Time to Next Exacerbation
Percent of days on intravenous antibiotics before and during hypertonic saline therapy.
Time to next infective pulmonary exacerbation before and during hypertonic saline therapy.
Adverse Effects
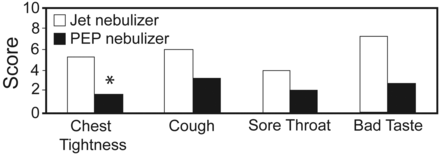
The questionnaire responses indicated a 68% reduction in chest tightness; the mean Likert-scale score changed from 5.3 with jet nebulizer to 1.7 with PEP nebulizer (P = .04). Mean cough score decreased 47%, from 6.0 to 3.2 (P = .10). Mean sore-throat score decreased 50%, from 4.0 to 2.0 (P = .20). Mean bad-taste score decreased 62%, from 7.3 to 2.8 (P = .06) (Fig. 3). No other adverse effects were reported with PEP nebulizer.
Adverse effects of hypertonic saline administered via jet nebulizer versus positive-expiratory-pressure nebulizer. (* P = .04).
Advantages patients mentioned on the questionnaire included: “PEP therapy gives a clearer feeling at the end of physiotherapy,” “It makes doing chest physiotherapy much easier and less tiring,” “You can vary the rate of flow,” “It is easier to tolerate,” “The resistance makes it easier to breath,” and “The hypertonic saline does not taste as salty.” The only subjective disadvantage noted was longer nebulization time.
Discussion
To our knowledge, this is the first report on administering hypertonic saline via PEP nebulizer in CF patients who were intolerant of hypertonic saline via jet nebulizer despite multiple trials. The benefits of hypertonic saline are well established, but almost 10% of CF patients are intolerant of hypertonic saline via jet nebulizer, predominantly secondary to cough, bad taste, and bronchospasm.5 This case series demonstrates 4 such patients who were recurrently intolerant of hypertonic saline, all of whom tolerated hypertonic saline via PEP nebulizer. In addition to tolerability and full adherence to therapy, there was a trend toward fewer infective pulmonary exacerbations and increased time to next pulmonary exacerbation. Our finding of a 53% reduction in antibiotic use during the PEP period is comparable to that of a previous study that found a 56% reduction in CF pulmonary exacerbation rate after hypertonic saline initiation.5
It is also important to highlight that all patients in this case series had very severe CF pulmonary disease (mean FEV1 26% of predicted). CF patients with severe pulmonary disease often are already receiving maximal therapy, with limited additional prophylactic strategies available. Given the established benefits of hypertonic saline,5 administration via PEP nebulizer is a useful adjunct for patients intolerant of jet nebulization, and may aid in bridging these patients to lung transplantation.
There was a 68% reduction in chest tightness with hypertonic saline via PEP nebulizer. Hypertonic saline causes transient airway narrowing in 30% of CF patients.11 PEP nebulizer probably causes bronchodilation by splinting open the airways, reducing bronchospasm, and reducing bronchoconstriction-induced cough.8 PEP also increases expiratory time, tidal volume, and minute ventilation, and decreases respiratory rate, allowing for a more optimal breathing pattern and improved pulmonary aerosol deposition.12,13 PEP can also augment collateral ventilation and improve mucociliary clearance toward central bronchioles.14,15 These benefits are supported by 2 studies that found up to 30% increased peripheral airway deposition of radio-labeled aerosol particles with pressure support and PEP nebulizer in CF patients.13,16 Given the propensity of hypertonic aerosol droplets to undergo hygroscopic growth in the respiratory tract, resulting in a more central particle deposition,17 the increased peripheral deposition of hypertonic saline with PEP nebulizer18 helps target the areas most affected by infection and inflammation in the CF lung, potentially leading to a synergistic effect between the PEP nebulizer and hypertonic saline. That said, the benefits of smaller particle size and the moderate increase in peripheral lung deposition with PEP may be offset by a significant reduction in lung particle deposition with PEP.18 This reduction in lung particle deposition could be reduced by the use of an “interrupter,” which should decrease aerosol loss through the flow-resistance orifice of the PEP system during exhalation.18 Further work on this is needed.
We found a clinically important (but nonsignificant) 47% reduction in cough with PEP. The mechanism of hypertonic-saline-induced cough is thought to be impaction and irritation of large aerosol particles in the upper airway during inhalation, resulting in a nonspecific mechanical irritation of the laryngeal rapidly adapting receptors.19 A higher aerosolization flow rate increases aerosol deposition in the oropharynx,16 which is probably a component of intolerable cough and bronchospasm in many patients with jet-nebulized hypertonic saline.12 Two of our patients mentioned the ability to vary the flow rate with PEP to be advantageous. Additionally, PEP nebulizer reduces oropharyngeal aerosol deposition, reducing irritation of the rapidly adapting receptors and cough.16
Another advantage reported by our patients was easier sputum expectoration after hypertonic saline. There are a number of mechanisms described for the therapeutic benefits of hypertonic saline in CF patients. In addition to osmotically restoring the airway surface liquid,20 hypertonic saline also increases the ionic concentration of the mucus, shielding the negative charges and resulting in a more compact mucus macromolecule, allowing for more effective airway clearance.21 Hypertonic saline also affects the ionic bonds in mucus, reducing viscosity and elasticity.22 More recent studies found significant anti-inflammatory and antimicrobial properties of hypertonic saline, including the activation of the anti-microbial peptide LL-37 through the disruption of anionic matrices,23 and an effect on Pseudomonas aeruginosa motility and quorum sensing.24 The suggestion that the benefits of hypertonic saline in CF patients are attributable to increased cough induction were refuted in clinical studies involving matched voluntary coughs.21
Limitations
Our sample size was relatively small and we did not establish whether the benefits we observed were a result of the hypertonic saline or the PEP nebulizer alone, or due to a synergistic effect. That said, the 100% tolerance of hypertonic saline via PEP nebulizer in these 4 patients with very severe CF lung disease cannot be ignored. Additionally, we used 6% hypertonic saline, as this was the only commercially available, preservative-free hypertonic saline available in Ireland at the time of the study, but the patients' previous intolerance of hypertonic saline was established on the same 6% solution.
Conclusions
This report highlights a novel method of administering hypertonic saline to CF patients who have previously failed hypertonic saline via jet nebulizer, which merits a larger, multicenter prospective study.
Footnotes
- Correspondence: Barry Plant MD, Cork Adult Cystic Fibrosis Centre, Cork University Hospital, University College Cork, Wilton, Cork N/a Ireland. E-mail: barry.plant{at}hse.ie.
-
The authors have disclosed no conflicts of interest.
-
Supplementary material related to this paper is available at http://www.rcjournal.com.
-
See the Related Editorial on Page 886
- Copyright © 2011 by Daedalus Enterprises Inc.