Abstract
BACKGROUND: The effectiveness of noninvasive ventilation (NIV) after extubation in preventing post-extubation respiratory failure is still controversial. METHODS: We conducted a prospective, multicenter randomized controlled study involving patients on mechanical ventilation for > 48 hours who tolerated a 2-hour spontaneous breathing trial and were subsequently extubated. The patients were randomized to NIV or standard medical therapy. Re-intubation rate within 72 hours was the primary outcome measure. Multivariable logistic regression analysis was used to determine predictors for extubation failure.
RESULTS: We randomized 406 patients to either NIV (no. = 202) or standard medical therapy (no. = 204). The 2 groups had similar baseline clinical characteristics. There were no differences in extubation failure (13.2% in control and 14.9% in NIV), intensive care unit or hospital mortality. Cardiac failure was a more common cause of extubation failure in control than in NIV. There was no difference in rapid shallow breathing index (RSBI) in extubation failure patients between control (80) and NIV (73). When using data from all patients, we found Acute Physiology and Chronic Health Evaluation (APACHE II) scores (odds ratio [OR] 1.13, 95% CI 1.07–1.20, P < .001), maximal inspiratory pressure (OR 1.04, 95% CI 1.00–1.08, P = .03), and RSBI (OR 1.03, 95% CI 1.02–1.05, P < .001) to be predictors of extubation failure. Abundant secretions were the most common reason (35.1%) for extubation failure identified by attending physicians.
CONCLUSIONS: Preventive use of NIV after extubation in patients who passed spontaneous breathing trial did not show benefits in decreasing extubation failure rate or the mortality rate.
Introduction
Noninvasive ventilation (NIV) is an effective modality to decrease the need for invasive mechanical ventilation via endotracheal intubation, especially in patients with respiratory failure due to COPD exacerbation and pulmonary edema. For patients who developed post-extubation respiratory failure, the role of NIV is not as clear. NIV has been applied as an adjunct therapy to weaning from mechanical ventilation, preventively to all extubated patients, or as a rescue therapy for patients who develop post-extubation respiratory failure.
Earlier studies in COPD patients who failed T-piece trials showed that extubation to NIV shortened the duration of endotracheal intubation.1,2 Subsequent studies used NIV as a rescue therapy in patients who developed respiratory failure after extubation. In a smaller study, Keenan et al showed that NIV did not alter re-intubation rate in patients who developed respiratory distress < 48 hours after extubation.3 In a subsequent large randomized control study, Esteban et al showed that NIV use in this patient population actually increased mortality, likely related to the delay in intubation.4 Thus it has been suggested that if NIV is to be used after extubation, it might be most effective if applied early, before respiratory distress develops. To test this hypothesis, Jiang et al showed that preventive use of NIV in all post-extubated patients did not alter re-intubation rate,5 but the study was small and included patients with unplanned extubation that is associated with high likelihood of re-intubation.6–8 Recently Nava et al and Ferrer et al found that routine use of NIV in patients who were at high risk for extubation failure decreased re-intubation rate and intensive care unit (ICU) mortality.9,10 The risk factors listed in these 2 studies, however, would have included most of the patients in medical ICU.
To determine the role of preventive use of NIV in patients after extubation, we conducted a large prospective randomized controlled multicenter trial in Taiwan. We postulated that preventive application of NIV after extubation in patients who passed the spontaneous breathing trial (SBT) would reduce the re-intubation rate. We used the re-intubation rate and ICU mortality as the primary end points.
QUICK LOOK
Current knowledge
Non-invasive ventilation to prevent extubation failure has been attempted with mixed results. The evidence base for NIV use post-extubation remains unclear.
What this paper contributes to our knowledge
Preventative use of post-extubation use of NIV to prevent re-intubation was no better than traditional treatment. In this group, the most common reason for extubation failure was excessive secretions.
Methods
Patient Population
The study was conducted in ICUs in 3 hospitals in Taiwan, from October 2002 to September 2004. Patients who required intubation and ventilator support for more than 48 hours were screened. Patients who met the weaning criteria and passed a 2-hour T-piece SBT were randomized after the informed consent was obtained from the next of kin. The ethics committee of the hospital clinic approved the study, and informed consent was obtained from all participants.
We measured respiratory mechanics parameters daily, including maximal inspiratory pressure (PImax), minute ventilation (V̇E), tidal volume (VT), respiratory rate, rapid shallow breathing index (RSBI) (respiratory rate/VT) with a portable spirometer by disconnecting the patient from the ventilator for 2 min to determine if the patient can proceed to the 2-hour SBT. If PImax ≤ −25 cm H2O, V̇E ≤ 10 L/min, VT ≥ 5 mL/kg, respiratory rate ≤ 25 breaths/min, RSBI ≤ 105 cycles/min/L, oxygen saturation (SaO2) ≥ 90% at an FIO2 of 40% or less and a PEEP of 5 cm H2O, then an SBT with T-piece, oxygen, and humidity for 2 hours was then performed.11,12 If these criteria were not met, mechanical ventilation was continued, and the patient was reevaluated the next day. A patient was considered not extubatable if respiratory rate was > 35 breaths/min or increased by 50% or more, heart rate was > 140 beats/min or increased by 20% or more, or if arrhythmias appeared, systolic blood pressure was < 80 mm Hg or > 160 mm Hg, or patients showed agitation, depressed mental status, or diaphoresis during the 2-hour SBT. These patients were reconnected with the ventilator. Patients who passed the 2-hour SBT and were able to follow commands were extubated and randomly assigned to either standard medical therapy as the control group or the NIV group. The assignments were made using a random-number table and opaque, sealed, numbered envelopes. Exclusion criteria were the presence of a tracheostomy and the absence of informed consent.
Standard Treatment and NIV Therapy
Patients assigned to standard therapy received a supplemental oxygen aerosol mask to maintain oxygen saturation at ≥ 92%. In addition, they received regular medical treatments at the discretion of the attending intensivist. These medical treatments may include diuretics, inhaled β-agonists, and mucolytic agents.
Patients randomized to NIV received ventilator support through a full facial mask (BiPAP S/T-D30, Respironics, Murrysville, Pennsylvania) in addition to standard treatment described above. The NIV support was initiated at a level of 5 cm H2O expiratory positive airway pressure (EPAP) and 10–12 cm H2O inspiratory positive airway pressure (IPAP) in a spontaneous mode. EPAP was titrated in increments of 2 cm H2O to achieve an SaO2 of ≥ 92%, while IPAP was titrated in increments of 2 cm H2O to achieve a pH of ≥ 7.35 and patient respiratory rate of ≤ 25 breaths/min.
Before NIV was begun, the head of the patient's bed was elevated to a > 45° angle. During the first hour, a respiratory therapist continuously monitored the patient's clinical condition, and provided constant reassurance to the patient. After the first 12 hours on NIV, the patient was allowed to have unassisted spontaneous breathing intermittently at increasing intervals if the patient was breathing comfortably with an SaO2 ≥ 92% and pH ≥ 7.35. The facial skin was assessed every 4 hours for any signs of pressure damage.
Criteria for Extubation Success
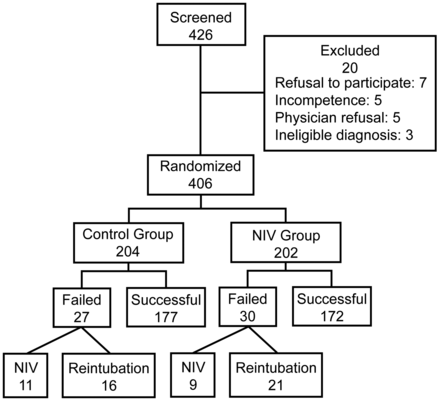
In the NIV group, if a patient was not re-intubated and able to discontinue NIV within 72 hours post-extubation, it was considered extubation success. In the control group, if a patient did not require the use of NIV or re-intubation within 72 hours post-extubation, it was considered extubation success (Fig. 1). Thus, in the NIV group, if a patient still required the use of NIV at the end of 72 hours after extubation, it was considered extubation failure. In the control group, any use of NIV within the first 72 hours after extubation was considered extubation failure. In both study groups, a patient would be re-intubated if he or she met at least one of the following criteria: cardiac arrest, respiratory arrest, apnea with loss of consciousness, or gasping for air with inability to protect the airway; lack of improvement in pH or PaCO2, or a decrease in SaO2 to 85% despite an FIO2 of ≥ 50%; a lack of improvement in signs of respiratory muscle fatigue; psychomotor agitation making nursing care impossible and requiring sedation; hypotension with a systolic blood pressure of < 90 cm H2O for > 30 min despite adequate volume challenge, the use of vasopressors, or both; and copious secretions that could not be cleared and were associated with acidosis, hypoxemia, or changes in mental status. The decision to re-intubate or try NIV was made by the treating physician, who also recorded the single most relevant reason for re-intubation from a list of 6 possible reasons.
Clinical trial profile and outcomes of patients after extubation. NIV = noninvasive ventilation.
Data Collection
We also collected baseline demographic data, the Acute Physiology and Chronic Health Evaluation II (APACHE II) score,13 clinical diagnosis, reasons for respiratory failure, and ICU and hospital stay.
Statistical Analysis
We calculated the sample size before the study. To detect a reduction in the re-intubation rate to 10% from an estimated 24%14 with a type I error of 5%, and a power of 99%, we estimated that we needed at least 129 patients in each group.
All analyses were performed using SPSS Graduate Pack 9.0 (SPSS, Chicago, Illinois). Comparison between the 2 study groups was conducted using the chi-square statistic for categorical variables and the Student t test for continuous variables. The analyses were based on 2-sided statistics, and differences between the 2 groups were considered significant at P < .05. A multivariable logistic regression model was also used to identify factors associated with extubation failure.
Results
We enrolled 426 patients from October 2002 to September 2004. As shown in Figure 1, 20 patients were excluded, so that 406 patients were eventually randomized, with 204 allocated to the standard treatment group (control) and 202 to the NIV group. The groups did not significantly differ in clinical characteristics, such as APACHE II scores, PImax, or RSBI at the time of entry into the study (Table 1). Causes for initiation of mechanical ventilation were also similar between the 2 groups.
Basic Characteristics of Patients
Compared with the control group (13.2%), the NIV group had a similar post-extubation respiratory failure rate (14.9%). There was no difference in RSBI in patients with extubation failure between the control group (80) and the NIV group (73). Fifty-six percent and 57% of these patients in the control and NIV groups, respectively, developed respiratory failure within 12 hours. In these patients, NIV prevented intubation in 30% of the patients in the NIV group and 40.8% of the patients in the control group. The remaining 37 patients required re-intubation, and the re-intubation rates in both groups were similar (10.4% in NIV vs 7.8% in control) (Table 2). There were also no differences in ICU and hospital mortality (see Table 2).
Primary Outcomes of the 2 Groups*
In both groups, we found that patients who developed post-extubation respiratory failure had lower PImax, greater V̇E, higher respiratory rate and lower VT before extubation (Table 3). The RSBI in these patients was higher (80 in control and 73 in NIV), compared to patients who did not develop respiratory failure (58 in control and 59 in NIV) (see Table 3), although the absolute RSBI values were much lower than 105. The multivariable logistic regression analysis identified high APACHE II, low PImax and high RSBI as predictors for post-extubation failure (Table 4). Major reasons for post-extubation respiratory failure identified by the attending physicians were summarized in Table 5. In both groups, excessive secretions were identified as the major cause for respiratory failure in approximately 20–26% of the patients. Cardiac failure was more common in the control group (40.7%) than in the NIV group (16.6%).
Respiration Parameters of Patients Before Spontaneous Breathing Trial*
Summary of Multivariable Logistic Regression Analysis
Main Reasons for Post-Extubation Respiratory Failure Re-intubation*
Discussion
To our knowledge this study is the largest designed to determine whether or not routine use of NIV after extubation may decrease the incidence of post-extubation respiratory failure in a non-selective patient population. We found that NIV did not decrease extubation failure rate or ICU mortality. The extubation failure rate in the control group was 13.2%, similar to that in Jiang et al's study (15.2%)5 and lower than those in Nava et al's study (24.5%)10 and Ferrer et al's study (33%).9 In the latter 2 studies, NIV reduced the extubation failure rates (8.3%, 16%, respectively) toward that in our study (14.9%). Average APACHE II score in our study was approximately 17–18, lower than that in the study of Ferrer et al (20–22).9 Average age in our study was also lower. These data indicate that our patients in general were at lower risk for extubation failure than those in the studies of Nava et al and Ferrer et al.9,10 In addition, the composition of our patient population may also explain the lack of efficacy. Our study had fewer patients with COPD (∼10%) compared to the 2 previous studies (30–35%). Respiratory failure from acute exacerbation of COPD is more response to NIV.15 NIV has been used increasingly as the first modality to support patients with acute respiratory failure, including that which occurs after extubation. In patients at high risk for extubation failure, Nava et al and Ferrer et al found that routine use of NIV decreased re-intubation rate and ICU mortality.9,10 If NIV was used as a rescue therapy in patients who developed respiratory distress after extubation, Keenan et al showed that this strategy did not alter re-intubation rate.3 This was confirmed by Esteban et al, who showed that NIV use in this patient population actually increased mortality, likely due to the delay in intubation.4 When NIV was used in all patients immediately after extubation, our results suggests that this strategy could not reduce re-intubation rate. In our study, the actual re-intubation rate in the control group was 7.8%, lower than that used for the original sample size estimation. We recalculated the sample size based on this rate. If the relative reduction in re-intubation rate remains the same (60%), given the type I error of 5% and the power of 80%, the sample size would be 124 patients per group. Thus the number of patients enrolled in our study would be large enough to detect the pre-specified difference of 60% reduction, even for the lower actual re-intubation rate. Therefore, our data do not support the preventive use of NIV following extubation.
When all data were pooled, the multivariate logistic regression analysis identified high APACHE II as one of the predictors for extubation failure. Previous studies reported that APACHE II scores did not predict hospital mortality after long-term mechanical ventilation, but a readjusted APACHE II score can be used to predict weaning outcomes in patients with < 25 days of ventilation.16 In addition, APACHE II scores were associated positively with clinical ICU outcomes such as ventilator-associated pneumonia, tracheostomy, and mortality.17 Since NIV is a supportive measure, it would not be expected to prevent respiratory failure in patients whose severity of illness was high. Our results also showed that PImax and RSBI could predict extubation failure. Both indices roughly measure the propensity to muscle fatigue. Although RSBI was greater in patients who failed extubation, the absolute values (73 and 80) were still much smaller than the recommended cutoff of 105. These data indicate that patients who have a greater APACHE II, smaller PImax, and higher RSBI (even if it is < 105) are prone to developing extubation failure. These patients should be closely monitored for signs of respiratory distress, so that NIV or re-intubation can be implemented early.
In our study, excessive secretions and cardiac failure were identified by the primary attending physicians as the major causes for extubation failure. Cardiac failure was considered the cause of extubation failure in a higher percentage of patients in the control group than in the NIV group. This is consistent with the observation that NIV benefits patients with cardiac failure,18,19 but the number of patients with cardiac failure in our study was too small to have an impact on the reduction of overall extubation failure rate. About 20–25% of the patients had abundant secretions as the cause of extubation failure. These findings underscored the importance of muscle strength in producing effective cough in preventing re-intubation.20–22 Application of NIV to these patients may add expiratory resistance and further impair the clearance of secretions.23
Generalization of the results from this study to clinical practice should be made with some cautions. First, our study enrolled a mixed patient population. The lack of effectiveness of NIV may reflect the heterogeneous effects of NIV on different patient populations. It has been shown that in certain subgroups of patients, such as COPD, early use of NIV may be beneficial.4 Second, NIV was applied continuously for the first 12 hours after extubation. The use of NIV was intermittent afterward. Since greater than 50% of the patients who failed the extubation developed respiratory failure < 12 hours after extubation, it is possible that NIV could have offered some benefits if it had been applied for a longer period of time. Third, our study included a population of patients who had a higher prior likelihood of extubation success. The differences between the 2 groups would be more difficult to demonstrate. Fourth, we did not quantify the amount of secretions or the strength of cough. Since the rate of failure due to secretions was quite high, any benefit of NIV could have been offset by its negative effects on patients with excessive secretions.
Conclusions
In this largest randomized controlled trial to date on the preventive use of NIV in non-selective ICU patients, we were unable to show reduction in extubation failure rate by NIV. NIV should not be used routinely after extubation. Patients who had higher APACHE II score, lower PImax, and higher RSBI before extubation were at higher risk for extubation failure. These patients deserve closer monitoring so that either NIV or intubation can be implemented early when respiratory failure occurs.
Acknowledgments
The authors thank the respiratory therapists, nurses, and physicians in the intensive care units of Tri-Service General Hospital, Cardinal-Tien-Ho Hospital, and Chi-Mei Medical Center, for their participation in patient care. We also thank Mr Hui-Wen Lin for his help with the statistical analyses.
Footnotes
- Correspondence: Chin-Pyng Wu MD PhD, Department of Thoracic Internal Medicine, Landseed Hospital, No. 77, Kwang-Tai Road, Ping-Jen City, Tao-Yuan County, Taiwan. E-mail: wucp{at}landseed.com.tw.
This work was supported in part by a grant (DOH 92-TD1023) from the Department of Health, Taiwan.
The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 318
- © 2012 by Daedalus Enterprises Inc.