Abstract
BACKGROUND: Acquired immunodeficiency syndrome (AIDS) is a pandemic disease commonly associated with respiratory infections, hypoxemia, and death. Noninvasive PEEP has been shown to improve hypoxemia. In this study, we evaluated the physiologic effects of different levels of noninvasive PEEP in hypoxemic AIDS patients. METHODS: Thirty AIDS patients with acute hypoxemic respiratory failure received a randomized sequence of noninvasive PEEP (5, 10, or 15 cm H2O) for 20 min. PEEP was provided through a facial mask with pressure-support ventilation (PSV) of 5 cm H2O and an FIO2 of 1. Patients were allowed to breathe spontaneously for a 20-min washout period in between each PEEP trial. Arterial blood gases and clinical variables were recorded after each PEEP treatment.
RESULTS: The results indicate that oxygenation improves linearly with increasing levels of PEEP. However, oxygenation levels were similar regardless of the first PEEP level administered (5, 10, or 15 cm H2O), and only the subgroup that received an initial treatment of the lowest level of PEEP (ie, 5 cm H2O) showed further improvements in oxygenation when higher PEEP levels were subsequently applied. The PaCO2 also increased in response to PEEP elevation, especially with the highest level of PEEP (ie, 15 cm H2O). PSV of 5 cm H2O use was associated with significant and consistent improvements in the subjective sensations of dyspnea and respiratory rate reported by patients treated with any level of PEEP (from 0 to 15 cm H2O).
CONCLUSIONS: AIDS patients with hypoxemic respiratory failure improve oxygenation in response to a progressive sequential elevation of PEEP (up to 15 cm H2O). However, corresponding elevations in PaCO2 limit the recommended level of PEEP to 10 cm H2O. At a level of 5 cm H2O, PSV promotes an improvement in the subjective sensation of dyspnea regardless of the PEEP level employed.
- acquired immunodeficiency syndrome
- respiratory insufficiency
- noninvasive positive-pressure ventilation
- acute lung injury
- pneumonia
- critical illness
Introduction
Acquired immunodeficiency syndrome (AIDS) is a pandemic,1 and pulmonary infections are a leading cause of morbidity and mortality.2 Respiratory failure secondary to pulmonary infection is the most common cause of intensive care unit (ICU) admission for AIDS patients.3,4 AIDS patients with hypoxemic respiratory failure frequently require invasive mechanical ventilation, which is independently associated with death.3,5
The use of noninvasive mechanical ventilation is known to improve oxygenation and dyspnea in patients with acute hypoxemic respiratory failure without AIDS,6–8 and it is possible that the use of PEEP with pressure-support ventilation (PSV) could reduce the need for invasive mechanical ventilation and its complications.7
Pulmonary histopathological examinations of AIDS patients have shown findings that are consistent with acute interstitial pneumonia and local inflammation.9 However, pulmonary physiopathological characteristics are well described only for AIDS patients with severe Pneumocystis jiroveci pneumonia,10 which is characterized by the absence of an inferior inflection point on the inspiratory limb of the pressure/volume curve. These histopathological and physiopathological findings in the lungs of AIDS patients, point to the paucity of alveolar recruitment, a key feature of the oxygenation improvement in patients using positive pressure mechanical ventilation.
Taking the above information into account, one can expect a poor response to PEEP in AIDS patients with hypoxemic respiratory failure. By contrast, continuous positive airway pressure (CPAP)11–14 and PEEP plus PSV15 have been noninvasively applied to the respiratory support in AIDS patients with respiratory failure, with promising clinical results. The cited case series11–15 have used different levels of PEEP, and have reported different degrees of hypoxemia improvement. In addition, some patients with severe respiratory failure due to pneumonia, who might be expected to exhibit poor responses to PEEP, exhibit improvements in hypoxemia using noninvasive ventilation.16
The use of high levels of PEEP without progressive increments in airway pressure could lead to distention of more compliant alveoli, which would compress the nearby less compliant respiratory units, causing alveolar derecruitment and leading to impaired blood oxygenation and lung mechanics. In contrast, stepwise elevations of PEEP during alveolar recruitment maneuvers have been associated with full recruitment in many patients with severe respiratory failure.17
Based on these uncertainties about the oxygenation responses to PEEP in AIDS patients with acute hypoxemic respiratory failure, and the reasoning that progressive airway pressurization may have potential benefits in blood oxygenation, we have hypothesized that sequentially increasing PEEP levels (up to 15 cm H2O) could improve blood oxygenation in several ways without negatively impacting hemodynamics or patient comfort. The aim of this study was to investigate the effects of different sequences of noninvasive treatments with varying PEEP levels on gas exchange, the sensation of dyspnea, and hemodynamic patterns in AIDS patients with acute respiratory failure.
QUICK LOOK
Current knowledge
Hypoxemia is a common finding in patients with AIDS and respiratory infections. Treatment with invasive ventilation results in significant morbidity and is independently associated with death.
What this paper contributes to our knowledge
Noninvasive application of PEEP results in improved oxygenation without requiring intubation. At PEEP ≤ 10 cm H2O hypercapnia and dyspnea can be alleviated by 5 cm H2O of pressure support.
Methods
Between January and December 2000, 30 AIDS patients with severe respiratory failure were prospectively enrolled. This study was conducted in the emergency department of the Emilio Ribas Institute of Infectious Diseases, a 197-bed teaching hospital in São Paulo, Brazil. This study was approved by the institutional ethics committee, and informed consent was obtained from each patient or next of kin. All enrolled patients were evaluated by an on-call investigator, who was responsible for the implementation of respiratory support. The emergency medical staff was responsible for all other medical interventions, including decisions regarding tracheal intubation, without any input from the researchers. In the emergency room, tracheal intubation has been routinely encouraged in patients with severe respiratory failure with at least one of the following findings: a Glasgow coma scale score < 13, persistent respiratory distress, PaO2 < 60 mm Hg or SpO2 < 90% despite advanced respiratory support (FIO2 > 0.6 and PEEP ≥ 5 cm H2O), or elevated PaCO2 levels.
Study Sample
Patients were included in the study if they satisfied the following inclusion criteria: dyspnea; peripheral oxygen saturation < 90% or PaO2 < 60 mm Hg in ambient air; respiratory rate > 30 breaths/min; and signs of increased respiratory work (active use of inspiratory accessory muscles or paradoxical abdominal breathing).
Patients were excluded if they had any of the following characteristics: pregnancy; age < 18 years; chronic obstructive pulmonary disease, previously diagnosed according to the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria18; hemodynamic instability (systolic arterial pressure < 90 mm Hg) at the time of admission; a Glasgow coma scale score < 14 at the time of admission; intractable vomiting; upper gastrointestinal tract bleeding; and unrelenting respiratory distress at the time of presentation that required immediate tracheal intubation.
Study Procedures
Patients fulfilling all inclusion criteria were admitted to the emergency room. Within a period of 2–5 min (the baseline time point), a blood sample for blood gas, biochemical, and hematological analysis was collected from the radial artery, with the patient breathing ambient air (FIO2 = 0.21). A second sample was also taken for blood culture. Blood gas samples were analyzed using an ABL-5 (Radiometer Medical, Copenhagen, Denmark). Clinical data, including age, sex, time of AIDS diagnosis, the use of high activity antiretroviral therapy (HAART) drugs, lymphocyte count, CD4 cell count, and risk factors for AIDS, were recorded from the patients' folders at the time of admission. Physiological data, such as heart rate, respiratory rate, body temperature, arterial blood pressure, and the subjective sensation of dyspnea, were collected at the data collection time points. After the baseline data were collected, noninvasive respiratory support was initiated by first using a PEEP of 0 cm H2O, a PSV of 5 cm H2O, and an FIO2 of 1 for 20 min. During this time, patients were randomly assigned to one of 6 groups, each of which received a different sequence of PEEP (5, 10, and 15; or 5, 15, and 10; or 10, 15, and 5; or 10, 5, and 15; or 15, 10, and 5; or 15, 5, and 10 cm H2O), using a closed box with the sequences inside of sealed envelopes. Each PEEP value was applied for a period of 20 min. Between each PEEP treatment, a washout period of 20 min using a Venturi mask with an FIO2 of 0.5 was performed. At the end of each PEEP trial, an arterial blood sample was obtained, and physiological variables were measured (PEEP = 0, 5, 10, and 15 cm H2O; for data collection time points, see Fig. 1).
Data collection time points over the course of the study. Black arrows indicate the time points of data collection. These time points corresponded to the time of admission (baseline) and 20 min after each PEEP treatment. PSV = pressure support ventilation.
After the conclusion of the study, each patient's care was conducted exclusively by the emergency department staff. All other outcome data, such as survival at 28 and 60 days, ICU admissions, ward admissions, renal replacement therapy, invasive mechanical ventilation, and the results from cultures and biopsies, were retrieved from the patient's medical record.
All patients were ventilated with a face mask and a Newport Wave-E 200 ventilator (Newport Medical Instruments, Costa Mesa, California). To ascertain each patient's subjective sensation of dyspnea, the investigators asked the patients to quantify their degree of subjective sensation of dyspnea as very intense, intense, somewhat intense, or none, in reference to their level of dyspnea at the time of admission. A chest x-ray was performed within a period of 2 hours after admission, and the characterization of lung injury was performed and recorded by 2 independent observers (CFDA and VSS). Three possible x-ray images were predefined: alveolar consolidation images, alveolo-interstitial images, and pleural effusion images.
Statistical Analysis
Based on our previous unpublished data, the minimum number of subjects required in this study to detect statistically significant differences in PaO2/FIO2 ratio ≥ 60 with a standard deviation of 50 was 24 patients. The possibility of type I error was 0.05, and of type II error 0.80. The normality of the data was tested with the Shapiro-Wilk goodness-of-fit model, and the data were presented as occurrences, percentages, the mean and standard deviation (SD) or the median with the 25th and 75th percentiles. Comparisons between the data of survivors and non-surviving patients were performed using Student t tests and Mann-Whitney U tests for parametric and non-parametric data, respectively. Categorical data were compared with the Fisher exact test or the chi-square test whenever appropriate. The time course data investigating different end-positive airway pressures were analyzed using one way analysis of variance for repeated measures (ANOVA-RM), with Bonferroni correction for 5 comparisons, which results in a significance level of 0.01. The Tukey test was used for the post hoc analysis of ANOVA-RM results. To assure the sphericity of data analyzed with the ANOVA–RM, Mauchly W tests were used, with a significance level < .05. Kaplan-Meier curves were used to present the patients' probability of survival at 60 days and to present the patients' probability of being free from invasive mechanical ventilation at 28 days. All statistical tests were performed using the commercial package SPSS 16.0 for Windows (SPSS, Chicago, Illinois).
Results
All patients completed the entire study. The randomized sequences of PEEP levels were administered to the following numbers of patients: 5, 10, and 15 cm H2O to 8 patients; 5, 15, and 10 cm H2O to 4 patients; 10, 5, and 15 cm H2O to 5 patients; 10, 15, and 5 cm H2O to 4 patients; 15, 5, and 10 cm H2O to 7 patients; and 15, 10, and 5 cm H2O to 2 patients. The demographic, etiological, and physiological data of all patients at the time of admission, the supportive measures each patient required during his or her hospital stay, and the outcome for each patient are shown in Table 1. We found that noninvasive PEEP was associated with improvements in blood oxygenation, sensations of subjective dyspnea, and heart rate (Fig. 2, Fig. 3, and Table 2). The use of 5 cm H2O of pressure support (PSV) with a PEEP of 0 cm H2O markedly improved reports of subjective dyspnea sensation, when compared to baseline (see Fig. 3). Compared to baseline, a statistical improvement in oxygenation, as measured by the PaO2/FIO2 ratio, was reached only when a PEEP of 5 cm H2O was added to a PSV of 5 cm H2O (see Table 2 and Fig. 2). The PaO2/FIO2 ratio improved linearly in response to elevations in PEEP levels, but only a PEEP of 15 cm H2O statistically increased the PaO2/FIO2 ratio when compared to a PEEP of 0 cm H2O (see Fig. 2). The PaO2/FIO2 ratio behaviors of all subgroups of patients during the study were similar, regardless of their diagnosis (pneumonia, pneumocystosis, or tuberculosis) (see Fig. 2, panel A). PEEP levels of 10 and 15 cm H2O significantly decreased alveolar-arterial oxygen difference (P(A-a)O2) when compared to a PEEP of 0 cm H2O (see Table 2). After the initial improvement of subjective dyspnea sensation observed in response to PSV application (with a PEEP of 0 cm H2O), subsequent increments in PEEP levels did not result in significant further improvements in respiratory comfort (see Fig. 3).
General and Physiological Characteristics at Admission, Diagnosis, Supportive Measures, and Outcomes of the 30 Patients
Respiratory variables of patients during the CPAP titration periods. Panel A shows the PaO2/FIO2 ratio (analysis of variance for repeated measures [ANOVA-RM] one way, P < .001). Panel B shows the PaCO2) (ANOVA-RM one way, P < .001). Panel C shows the respiratory rate (ANOVA-RM one way, P < .001). Panel D shows the tidal volume (ANOVA-RM one way, P = .044). Tidal volume was not measured at the baseline time point. Error bars represent standard deviations. * P < .05 versus baseline via Tukey post hoc analysis. † P < .05 versus expiratory positive airway pressure (EPAP) = 0 cm H2O via Tukey post hoc analysis.
Subjective quantification of the subjective sensations of dyspnea reported by patients. * P < .001 in the chi-square test for all clinical situations (contingency table 5 × 4), and in the post hoc analysis, the baseline was different from all values of PEEP (contingency table 2 × 4, P < .001).
Respiratory, Hemodynamics, and Metabolic Data of Patients During the Study*
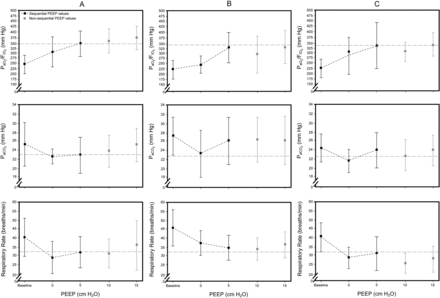
During PEEP randomization, 12 patients received an initial PEEP level of 5 cm H2O, 9 patients initially received a PEEP of 10 cm H2O, and 9 patients received an initial PEEP level of 15 cm H2O after the preliminary PEEP of 0 cm H2O was applied. Figure 4 shows that the 3 subgroups exhibit similar levels of blood oxygenation and similar respiratory rates. PaCO2 levels were somewhat higher in patients who had received an initial PEEP of 10 or 15 cm H2O than in patients who had received an initial PEEP of 5 cm H2O (see Fig. 4).
Changes in PaO2/FIO2 ratio, PaCO2, and respiratory rate from the initial baseline time point to the first randomized PEEP treatment. Panel A shows the data obtained from the 12 patients whose first level of PEEP treatment was 5 cm H2O (8 patients with the sequence 5, 10, and 15 cm H2O, and 4 patients with the sequence 5, 15, and 10 cm H2O). Panel B shows the data obtained from the 9 patients whose first level of PEEP treatment was 10 cm H2O (5 patients with the sequence 10, 5, and 15 cm H2O and 4 patients with the sequence 10, 15, and 5 cm H2O). Panel C shows the data obtained from the 9 patients whose first level of PEEP exposure was 15 cm H2O (2 patients with the sequence 15, 10, and 5 cm H2O and 7 patients with the sequence 15, 5, and 10 cm H2O). The dashed gray lines represent the PaO2/FIO2 ratio, PaCO2, and respiratory rate observed when the level of PEEP was 5 cm H2O in patients in which the first randomized PEEP was 5 cm H2O to facilitate the comparison with the means of those variables of patients with other first randomized PEEPs.
During the course of this study, 8 patients died, with the last death being recorded on day 35 of the study. In addition, several patients required intubations during the course of this study, with the last intubation occurring 7 days following ICU admission. The relevant survival data for patients in the hospital are shown in Table 3. In this study, 15 out of the 30 patients examined (50%) were using HAART. Of the 8 patients who passed away at the hospital, only 2 were using HAART, and both of these patients were using HAART only irregularly. Diagnoses of pneumocystosis, tuberculosis, and histoplasmosis were confirmed by skin (1 patient) or lung biopsy (4 patients), sputum analysis (4 patients), or bronchoalveolar lavage (3 patients). Baseline chest x-rays revealed 3 distinct patterns: unilateral pleural effusion associated with unilateral alveolar consolidation (1 case), bilateral alveolo-interstitial infiltrates (26 cases), and unilateral alveolar consolidation (3 cases). No patients exhibited barotrauma during the hospital stay, including the period of conventional or noninvasive ventilation.
Relevant Characteristics of In-Hospital Survivors Versus Non-Survivors Patients (n = 30)
Several complaints and adverse effects were associated with noninvasive mechanical ventilation use: substantial escape of air from the facial mask (4 cases), the presence of mild reddish skin on the nasal bridge (1 case), mild pain in the nasal bridge (2 cases), and psychomotor agitation (1 case). All complaints and adverse effects were resolved by protecting the nasal bridge with silicone or cotton bands or by adjusting the facial mask strips. The patient exhibiting psychomotor agitation was successfully treated with haloperidol.
Discussion
In approximately 40% of patients with AIDS, the lung is the primary site for life-threatening illnesses, and hypoxemia is common in those patients.4 The main mechanism underlying hypoxemia is the pulmonary shunt.19,20 As a result, the use of PEEP is a good option to improve blood oxygenation, possibly through alveolar recruitment.8 In our study, we observed a significant improvement of blood oxygenation in response to PEEP of 10 and 15 cm H2O, in comparison with the PEEP = 0 cm H2O. In contrast, the use of PEEP of 5 cm H2O significantly improved the oxygenation only in comparison to the baseline and not in comparison with the PEEP of 0 cm H2O. This fact could be explained by the slight improvement in blood oxygenation observed with the use of PSV of 5 cm H2O, which likely results from tidal recruitment.21
It is possible that the level of oxygenation at baseline is not comparable to that observed with a PEEP of 0 cm H2O, due to the different levels of FIO2 used at the time of measurement. The use of different levels of FIO2 results in non-linear variation of the PaO2/FIO2 ratio and the alveolar-arterial oxygen difference.22 The finding of reduced hypoxemia with a PEEP of 0 cm H2O and a higher FIO2 in comparison to the baseline level could be spurious in patients with a large amount of poorly aerated pulmonary regions, and consequently low ventilation-perfusion-ratio-associated hypoxemia. In such patients, the increase of FIO2 might induce a disproportional elevation of the PaO2/FIO2 ratio and a disproportional decrease in the alveolar-arterial oxygen difference.23 In contrast, in patients with a high pulmonary shunt level, the elevation of FIO2 may be associated with a decreased PaO2/FIO2 ratio.22 In the results shown in Figures 2 and 4, the improvements in the PaO2/FIO2 ratio observed with a PEEP of 5 cm H2O, when compared to baseline situations, occur primarily at the point at which PEEP was added, indicating that PEEP is likely an important factor for this increase in blood oxygenation.
It is interesting to note the similar degree of improvement in the PaO2/FIO2 ratio with all 3 PEEP values employed (5, 10, or 15 cm H2O, see Fig. 4). The instantaneous application of high pressure to the airway could result in regions with alveolar overdistention and areas with alveolar collapse, an effect that would result in hypoxia or a limited improvement in blood oxygenation.24 This possibility is supported by the fact that PaCO2 elevation occurs when PEEPs in excess of 5 cm H2O are applied after an initial PEEP of 0 cm H2O (see Fig. 4). The PaCO2 elevation in this scenario could be explained by the overdistention of some pulmonary regions, which would be predicted to add dead space to the airway, as has been described previously by Tusman et al in an elegant animal experiment using a swine model of acute lung injury and normal lung ventilation.25 Although this hypothesis seems plausible, we do not have any data showing the presence of alveolar dead space or alterations in pulmonary compliance. Thus, we cannot definitively exclude the possibility that an elevation of carbon dioxide production (V̇CO2) occurs with the use of higher PEEP. This elevation of VCO2 could occur due to increased anxiety or difficulty breathing, secondary to the elevation of PEEP; however, the observed reduction in respiratory rate weakens this hypothesis.
Only when the initial PEEP was 5 cm H2O did further increases in PEEP result in an elevation of the PaO2/FIO2 ratio (see Fig. 4). This subgroup of patients probably contributed to the mean progressive improvement in oxygenation of the 30 patients (see Fig. 2). The mechanism of this progressive gain in oxygenation is likely due to increased alveolar recruitment during the time of PEEP exposure, in addition to the elevation of PEEP itself. This time-recruitment mechanism is thought to collapse respiratory units in which the airway pressure is slightly higher than the opening pressure.26 This progressive improvement of oxygenation was not observed when the first PEEP used was 10 or 15 cm H2O. We suggest, as already discussed, that pulmonary overdistention might occur with these high PEEP values and could potentially explain this observation. Alveolar overdistention could cause alveolar derecruitment by altering alveolar interactions.27 The washout period of 20 min would likely partially mitigate this possibility, but it is possible that lung hysteresis could extend alveolar patency after the initial recruitment (with the initial PEEP of 5 cm H2O) throughout the entire 20 min washout period.27,28 In this case, the washout period was probably insufficient to restore pulmonary mechanics to those observed under baseline conditions. Furthermore, the initial PEEP of 5 cm H2O should be added to the physiological end-expiratory pressure and likely causes some initial alveolar recruitment that is further increased with progressively higher PEEP values. However, these arguments are only speculative.
It is also interesting to note that PaO2/FIO2 ratios improved to a similar extent during PEEP elevation in multiple subgroups of patients, including those with pneumonia, tuberculosis, and pneumocystosis (see Fig. 2, panel A). This fact indicates that PEEP may have similar beneficial effects for a wide range of different respiratory failure etiologies.
The use of PSV of 5 cm H2O and a PEEP of 0 cm H2O, in comparison with the baseline, was associated with a significant reduction in the respiratory rate and in the PaCO2. These effects are most likely due to the elevation of tidal volume and the reduction in inspiratory load, resulting in more effective alveolar ventilation, a finding similar to a previous study in patients with cardiogenic pulmonary edema.29
Regarding hemodynamic effects, we observed decreased heart rate in patients after the PSV was installed. This finding is similar to the results of previous clinical studies testing noninvasive ventilation in various settings7,30,31 and probably reflects an improvement in patients' respiratory comfort in addition to the potential cardiac benefits of positive airway pressure.32 The mean arterial blood pressure remained stable even with the highest PEEP level employed and the observed reduction in heart rate, thus showing the remarkable stability that can be achieved with high levels of PEEP. However, this finding must be extrapolated with caution.
This study is characterized by a number of limitations. First, the use of sequential PEEP can induce carry-over effects. However, we used a randomized sequence of PEEP to minimize the occurrence of carry-over phenomena. Second, we did not use a validated score of dyspnea, but, rather, had patients refer to their sensations based on their baseline level of dyspnea. Third, the sample was calculated to the primary end point of oxygenation, which could allow a type 2 error for other variables analyzed. Fourth, the mechanical ventilator used was not built specifically for noninvasive ventilation, but using this type of ventilator for this purpose was common practice at the time of data collection.33 Fifth, a substantial time delay occurred between the time of data collection and publication, and the data might be less relevant to today's patients. However, very few studies regarding noninvasive mechanical ventilation in AIDS patients have been published, a fact that stimulated this research. Sixth, our data could not differentiate between improvements in blood oxygenation resulting from increased time and those resulting from increased pressure in patients who had initially used a PEEP of 5 cm H2O. Finally, this is a short-term limited physiological study.
Conclusions
In conclusion, AIDS patients with hypoxemic respiratory failure exhibit an optimal gain of oxygenation in response to the progressive sequential elevation of PEEP from 5 to 15 cm H2O. However, the parallel elevation of PaCO2 could limit the PEEP elevation up to 10 cm H2O. The use of a PSV of 5 cm H2O promotes improvements in subjective sensations of dyspnea regardless of the use of PEEP. The use of PEEP up to 15 cm H2O was shown to be associated with a PSV of 5 cm H2O and is hemodynamically safe.
ACKNOWLEDGMENTS
We are indebted to Professor Paulo Rossi Menezes and Dr Pericles D Duarte for their inestimable help in determining the appropriate sample size and for their initial help and considerations regarding noninvasive mechanical ventilation in patients with hypoxemic respiratory failure.
Footnotes
- Correspondence: Carlos Frederico Dantas Anjos MD, Intensive Care Unit, Emergency Department, Hospital das Clinicas, University of São Paulo Medical School, Rua Cristiano Viana, 450, Apto 163, 05411-000, São Paulo, Brazil. E-mail: cfda{at}uol.com.br.
See the Related Editorial on Page 321
-
The authors have disclosed no conflicts of interest.
- © 2012 by Daedalus Enterprises Inc.