Abstract
BACKGROUND: Endotracheal tubes (ETTs) impose a substantial respiratory work load on mechanically ventilated patients. Automatic tube compensation (ATC) should overcome this flow-dependent resistive work load; however, ETT resistance can be increased by tracheal secretions or ETT deformities. Our bench study investigated whether ATC provides effective respiratory work load relief in used ETTs.
METHODS: We enrolled 20 critically ill patients requiring mechanical ventilation for longer than 48 hours. After extubation, we collected the used ETTs and measured the pressure-time products (PTPs) by using a bellows-in-a-box lung model that simulated spontaneous breathing, at a respiratory rate of 10 breaths/min, inspiratory time of 1.0 s, and tidal volumes (VT) of 300 mL, 500 mL, and 700 mL. The ventilator was set at ATC 100% with PEEP of 5 cm H2O and FIO2 of 0.21. The flow and airway pressure at the proximal (Paw) and distal (Ptr) ends of the ETT were recorded, and the PTP integrated from Ptr analyzed.
RESULTS: PTP values increased with VT during ATC. Even at 100% ATC the ventilator did not completely compensate for the PTP imposed by the ETT. In used ETTs, peak flow and peak Paw were lower and PTP values were greater than in new ETTs. As VT increased, the percentage difference in the PTP values between the new and used ETTs increased.
CONCLUSIONS: ATC does not necessarily compensate for an ETT-imposed respiratory work load. ETT configuration changes and tracheal secretions can increase ETT resistance and decrease the ability of ATC to compensate for the increased respiratory work load.
- automatic tube compensation
- respiratory work load
- mechanical ventilation
- endotracheal tube resistance
- pressure-time product
Introduction
In tracheally intubated patients, work of breathing (WOB) consists of the work necessary to overcome the impaired mechanical properties of the patient's respiratory system as well as the work due to the resistance of an endotracheal tube (ETT).1,2 An ETT is the narrowest component of the whole pneumatic connection between a patient and a ventilator.3 The resistive properties of the ETT substantially influence inspiratory and expiratory WOB.1–3 In clinical practice, pressure support ventilation (PSV) is commonly used to compensate for the WOB of the ETT during the weaning process. However, WOB during spontaneous breathing is variable because the pressure drop across the ETT is flow-dependent in a non-linear way.2 The pressure support level required to eliminate the WOB related to an ETT has been shown to vary from 5 to 22 cm H2O.4
Automatic tube compensation (ATC) was developed to overcome the ETT-imposed respiratory work load.5–9 ATC is designed to increase airway pressure by continuously calculating the pressure drop across the ETT (ΔPETT) during inspiration.5–9 Theoretically, it simulates spontaneous breathing without the ETT, and appears to be suitable for use during the weaning period. Studies have shown that success rate for extubation with ATC is as good as that with PSV, a T-tube, and CPAP alone.10–13 However, the lumen of the ETT can be gradually narrowed by deposits of bronchial secretions on its inner wall, especially in patients with pneumonia, or by deformities of the ETT itself.14–17 Partial obstruction of the ETT can also increase the resistance of the ETT.17 In ATC mode, a ventilator measures flow and calculates the pressure required to overcome the flow-dependent resistive work load of the ETT.5–9 The ATC calculations/algorithms are assuming a new, clean, and not deformed tube; because the calculation is based on the inner diameter and length of the ETT as well as on the measured flow, changes in the inner diameter of the ETT can hamper the accuracy of this calculation. Decrease in inner diameter induces increase in resistance, thus pressure drop is underestimated, and compensation is insufficient.
The purpose of this study is to estimate the impact of ATC in a real clinical situation (ie, with a dirty ETT being partially obstructed or kinked) on alleviating the inspiratory work load imposed by a used ETT, compared with a new ETT of an identical size, and, finally, how much ATC is compensating for the ETT-related additional WOB in a real clinical situation.
QUICK LOOK
Current knowledge
Automatic tube compensation is a technique that uses the known resistance of the endotracheal tube, the flow, and the airway pressure to eliminate a percentage of the work of breathing caused by the tube. The in vivo resistance of endotracheal tubes is greater than the in vitro resistance.
What this paper contributes to our knowledge
In subjects ventilated for > 48 hours, both a reduction in the internal diameter of the endotracheal tube from secretions, and alterations in the shape of the tube in the airway reduce the ability of automatic tube compensation to overcome the additional work of breathing.
Methods
Subjects
Adult patients whose lungs were mechanically ventilated for at least 48 hours at the ICU were enrolled in this study. We excluded patients who were intubated transnasally or who had gross hemoptysis. Medical gases were heated and humidified using a hygroscopic heat and moisture exchanger (HME) (Hygrobac 352 filter, Dar, Mirandola, Italy) or a heated humidifier with a heated wire circuit (MR 730, Fisher & Paykel, Auckland, New Zealand). The water temperature was set at 37°C and the temperature of the heated wire at 39°C. All ETTs were made of polyvinyl chloride and were equipped with a low-pressure cuff (Evac, Mallinckrodt, Athlone, Ireland). The study protocol was approved by the ethics committee of Tokushima University Hospital (institutional review board number 980), and informed consent was obtained from the participants.
Lung Model and Ventilator
A custom-made 2-bellows-in-a-box lung model was used to simulate spontaneous breathing (Fig. 1). Details of the lung model have been described previously.8,9 Briefly, the lung model consisted of 2 bellows placed in a plastic airtight box. The upper bellows, lower bellows, and the space between the bellows and the box represented the lung, diaphragm, and pleural cavity, respectively. The diaphragm bellows was connected to a T-tube, into which a gas jet flow was injected to create a negative pressure in the diaphragm bellows. Duration and flow of the gas injection were created by a wall-based gas source, pressure regulator, and proportional solenoid valve, which were regulated by a computer. During the expiratory phase the diaphragm bellows opened to the atmosphere and returned to its original position. The compliance of the lung model was set at 30 mL/cm H2O, and spontaneous breathing was simulated at a respiratory rate of 10 breaths/min, with an inspiratory time of 1.0 s, and tidal volumes (VT) of 300 mL, 500 mL, or 700 mL. The ventilator (840, Nellcor Puritan-Bennett, Carlsbad, California) was connected to the lung model via a standard ventilator circuit (SpA, Dar, Mirandola, Italy). In this commercial ventilator, the flow-adaptive tube compensation was applied as the ATC algorithm, and gas flow rate and airway pressure were continuously measured within the ventilator. The ventilator was set at ATC 100%, with no PSV, with a PEEP of 5 cm H2O and an FIO2 of 0.21. The trigger sensitivity was set at 1.0 L/min, with a rising time of 0.2 s. The expiratory sensitivity was set at 5%.
Experimental setup. Spontaneous breathing was simulated with a bellows-in-a-box lung model. Pressure was measured both at the inlet and outlet of each endotracheal tube (ETT). Flow was measured at the outlet of the ETT. All data were recorded on a personal computer via an analog-digital converter. HME = heat and moisture exchanger. Ptr = tracheal pressure. Paw = airway pressure.
We inserted the used ETT or the size-matched new ETT between the ventilator and the lung model via the simulated trachea (standard 20-cm length smooth bore tube with an inner diameter of 18 mm). ETTs were collected from subjects at the time of extubation. Extubation was performed in a standardized way that included suctioning to remove mobile secretions in the airway. We set the used ETT in the lung model as soon as possible after it was removed from the subject, and took care to ensure that the ETT maintained the same shape as when it was removed, because curvatures or deformities in the ETT or deposits of bronchial secretions on the inner walls of the ETT could affect our measurements. Two HMEs (Hygrobac 352 filter) were inserted into the proximal and distal ends of the ETT to avoid dehumidification during measurement, and to protect the ventilator and lung model from bacterial contamination. Pressure sensors were also connected to the proximal and distal ends of the ETT. A flow sensor was connected to the distal end of the ETT.
Measurements
The airway pressure at the proximal (Paw) and distal (Ptr) ends of each ETT was measured with differential pressure transducers (TP-603T, ± 50 cm H2O, Nihon-Koden, Tokyo, Japan). The flow at the trachea (Ftra) was measured with a pneumotachometer (model 4700, 0–160 L/min, Hans-Rudolph, Shawnee, Kansas) connected to a differential pressure transducer (TP-602T, ± 5 cm H2O, Nihon-Koden, Tokyo, Japan). All flow and pressure signals were amplified, digitized, and recorded at 100 Hz per signal, using data acquisition software (WINDAQ, Dataq Instruments, Akron, Ohio). The same measurements were made on used and new size-matched ETTs.
Data Analysis and Statistics
The negative deflection of Ptr was defined as the maximum pressure drop from baseline during inspiratory triggering, and it was reported as a positive value (Fig. 2). The pressure-time product (PTP) was calculated as the integral of the Ptr timed from the point where Ptr began to decrease from baseline to the point where Ptr returned to baseline, which was used as an index of inspiratory work load (see Fig. 2). The percentage difference in the PTP values between the new and used ETTs was calculated as follows: the difference in the PTP between the new and used ETTs divided by the PTP of the new ETT. Delay time was defined as the time between the beginning of the Ptr decline and negative deflection (see Fig. 2). To evaluate the resistance of the used ETTs, we calculated the pressure drop across the used ETT (Paw − Ptr) at a flow rate of 30 L/min, and compared this with a size-matched new ETT. The percentage difference in the PTP values between the new and used ETTs was determined for patient populations stratified by presence or absence of pneumonia. Data were expressed as median and interquartile range. The Wilcoxon rank sum test was used to compare each variable between new and used ETTs at each VT setting. The Spearman rank test was used to correlate the percentage difference in the PTP values between the new and used ETTs with the duration of mechanical ventilation. All statistical analysis was performed with statistical software (SPSS 11.01, SPSS, Chicago, Illinois), and P values of < .05 were considered statistically significant.
Definition of measured parameters. The negative deflection (PI) of the tracheal pressure (Ptr) is the maximum pressure drop from baseline during inspiratory triggering, and is reported as a positive value. The pressure-time product (PTP) is calculated from the tracheal pressure as the integral of the negative deflection from the point where the pressure begins to decrease to the point where pressure returns to the baseline. Delay time (DT) is the time interval between the beginning of pressure decline and the lowest value.
Results
Subjects
A total of 20 subjects were enrolled in the study. The clinical features of the subjects are summarized in Table 1. The median duration of mechanical ventilation was 5 days (range 2–42 d). The primary reasons for mechanical ventilation included pneumonia (no. = 9), postoperative respiratory failure (no. = 7), and others (no. = 4). The inner diameters of the ETTs ranged from 7.0 mm to 8.5 mm. An HME was used for 18 subjects.
Clinical Features of the Subjects
Comparison of the Waveforms of the New and Used ETTs
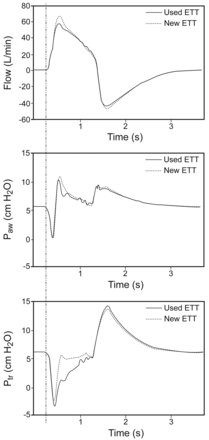
Figure 3 shows representative tracings of Ftra, Paw, and Ptr for the new and used ETTs during ATC mode at a VT setting of 700 mL. The vertical line shows the timing of the inspiration start, indicating that Paw and Ptr changed in parallel with Ftra. With a used ETT, after the triggering of the inspiratory effort, Ptr gradually increased and then returned to baseline at the end of inspiration. With a new ETT, Ptr rapidly returned to and remained close to baseline during the latter phase of inspiration. Peak Ftra and peak Paw were higher in a new ETT, compared with a used ETT.
Representative tracings of tracheal flow, airway pressure (Paw), and tracheal pressure (Ptr) in used and new endotracheal tubes (ETTs) during automatic tube compensation at a tidal volume setting of 700 mL. In the used ETTs, Ptr was gradually increased and was close to the baseline at the end of inspiration. In a new ETT, Ptr increased close to baseline during the latter phase of inspiration. Peak tracheal flow and peak Paw were slightly higher in the new ETTs than in the used ETTs. The vertical line shows the timing of the inspiration start, indicating that Paw and Ptr changed in parallel with tracheal flow.
PTP in the New and Used ETTs
Table 2 shows the PTP values with the new and used ETTs. As VT increased, PTP also increased in both ETT types (P < .001). PTP values were greater in the used ETTs than in the new ETTs. As VT increased, the percentage difference in the PTP values between the new and used ETTs also increased (see Table 2). In subjects with pneumonia, the median percentage difference in the PTP values between the new and used ETTs tended to be higher than those in subjects without pneumonia (Table 3). The percentage difference in the PTP values between the new and used ETTs was not associated with the duration of mechanical ventilation. Table 4 presents the delay time, negative deflection, peak Paw, and peak Ftra values during ATC. The peak Paw was greater in the new ETTs than in the used ETTs at the 700 mL and 500 mL VT settings. The peak Ftra was greater in the new ETTs than in the used ETTs at all VT settings. The delay time did not differ significantly between the new and used ETTs at all VT settings, but the negative deflection values were greater in the used ETTs at the VT 700 mL setting. The peak Ftra was greater in the new ETTs than in the used ETTs at all VT settings. The delay time values differed only at the VT 700 mL setting; the negative deflection values were greater at the VT 700 mL and 500 mL settings (see Table 4). The median pressure drop across the ETTs at a flow rate of 30 L/min was increased by 19.3% in the used ETTs, compared with the size-matched new ETTs: 2.5 cm H2O in the used ETTs and 1.9 cm H2O in the size-matched new ETTs (Table 5).
PTP for New and Used ETTs and Their Percent Differences
Percent PTP Difference Between New and Used ETTs in Patients With and Without Pneumonia
Delay Time, Deflection of Tracheal Pressure, Peak Airway Pressure, and Peak Tracheal Flow
Pressure Drop Across ETT at a Flow of 30 L/min
Discussion
The major finding of the present study was that ATC compensated less for the work load with used ETTs than with new ETTs. To our knowledge, this study is the first to evaluate the effects of ATC on respiratory work load compensation in ETTs used for longer than 48 hours.
The findings revealed that ATC provided lower pressurization, and PTP values were greater in used ETTs, compared with new ETTs. As VT increased, the percentage difference in the PTP values between the new and used ETTs increased. During spontaneous breathing, ATC compensates for the flow-dependent ΔPETT on the basis of the calculated pressure, not on the actual pressure measured at the distal end of the ETT.7 The ΔPETT can be approximated with the following equation8:
in which K1 and K2 are the tube-specific coefficients, and V̇ is the flow. The calculation of K1 and K2 is based on the diameter, length, and type of ETT used. Tracheal secretions, condensate, and kinks in the ETT decrease the inner diameter of the ETT, sometimes to a value that is less than the input value, causing a ventilator to underestimate the ΔPETT. Ideally, a ventilator should provide a large degree of pressure support in cases where the ETT is partially obstructed. However, as resistance in the ETT increased in such cases, the flow of inspired gas decreased and ATC provided less pressurization. PSV or CPAP, on the other hand, would provide constant inspiratory support regardless of the inner diameter of the ETT. In the PB840 ventilator, PSV is not active during ATC, and the study was done without PSV. We calculated PTP, delay time, and negative deflection, which are known to estimate the trigger performance of the ventilator. By using PSV zero, these indexes also estimate the performance of the ventilator, even if we actually do not use PSV zero in clinical practice.
In a clinical setting, tracheal secretions and ETT deformities often result in a gradual reduction in the inner diameter of the ETT in patients with acute respiratory failure.14–17 Shah and Kollef reported an average percentage difference in minimal ETT segment diameters between new and used ETTs of 10.2% (range 0–84.2%) after mean 4.7 days of use.16 Wilson et al reported that 73–79% of extubated ETTs had a pressure drop of > 3 standard deviations, compared with size-matched controls, and a pressure drop of 48–56% was equivalent to the next smaller size ETT.17 In the present study, although we did not directly evaluate the inner diameter of each ETT, the pressure drop across the ETTs increased 19.3% in used ETTs, compared with size-matched new ETTs.
Increased flow resistance within an ETT occurs especially when airway humidification is inadequate.14 The reduction in the ETT diameter is greater with hydrophobic HME than with hydroscopic HME or heated humidifier with a heated wire circuit.14 Although we used hygroscopic HME (no. = 18) and heated humidifier with a heated wire circuit (no. = 2) in the present study, the pressure drop across the ETT was larger in used, compared with new, ETTs. In the present study, 9 of 20 subjects suffered from pneumonia, and bronchial secretions were increased in these subjects; the percentage difference in the PTP values between the new and used ETTs tended to be higher in subjects with pneumonia, compared with subjects without pneumonia. Shah and Kollef reported that the minimum inner diameter of ETTs from subjects with ventilator-associated pneumonia was smaller than that of ETTs from subjects without ventilator-associated pneumonia (24% vs 8%), and our findings are consistent with their results.16 In this situation, ATC would inadequately compensate for the ETT's resistance and would provide inadequate support. Previous studies have shown a trend toward more patients who received ATC tolerating a spontaneous breathing trial, and have suggested that successful extubation could be achieved equally with PSV, a T-tube, or CPAP alone.10–13 However, in cases where an ETT is partially obstructed, ATC may compensate less for the ETT resistance and increase the risk for respiratory muscle fatigue or hypoventilation during the weaning period. Thus, minute ventilation should be closely monitored.
This study has several limitations. First, it was performed mainly in an in vitro setting with a lung model, to simulate the clinical situation. Second, although we evaluated the resistance of the ETT and PTP in used ETTs, we did not evaluate the effects on respiratory status during the weaning process or on clinical outcome. The majority of the patients enrolled in the study were weaned from mechanical ventilation successfully, and we could not evaluate the effects of ETT resistance on weaning outcome.
Conclusions
ATC did not entirely compensate for the ETT-imposed respiratory work load. ATC was less effective in compensating for the respiratory work load imposed by used ETTs, compared with new ETTs. In cases where the ETT was partially obstructed, ATC undercompensated for the ETT resistance. Therefore, care should be taken when using ATC during the weaning period in patients who have ample tracheal secretions and undergo long-term mechanical ventilation.
Footnotes
- Correspondence: Jun Oto MD PhD, Department of Emergency and Critical Care Medicine, University of Tokushima Graduate School, 3-18-15 Kuramoto-cho, Tokushima City, Tokushima, 770-8503, Japan. E-mail: joto{at}clin.med.tokushima-u.ac.jp.
The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 813
- Copyright © 2012 by Daedalus Enterprises Inc.