Abstract
BACKGROUND: Percutaneous endoscopic gastrostomy (PEG) tube placement in amyotrophic lateral sclerosis (ALS) patients with impaired respiratory function is associated with an increased risk of peri-procedural and post-interventional complications. It was the aim of the study to analyze peri- and post-interventional complications and survival after PEG tube placement under noninvasive ventilation (NIV) in ALS patients with various degrees of respiratory impairment.
METHODS: Twenty-six subjects were included in this retrospective case study. Prior to PEG tube placement, training with ventilatory support via an oronasal mask was performed with ALS subjects on the pneumology ward. PEG placement was then performed under continuous NIV. FVC, sniff nasal inspiratory pressure, and demographic data were assessed. Complication rates and 1-month and overall survival rates were analyzed.
RESULTS: There were no deaths within 24 hours after PEG placement. One subject died within the first month. The mean survival rate after PEG was 12 ± 10 months (range 0.6–42 months). There was no difference in post-PEG survival between subjects with moderately (> 50%) and severely (< 50%) impaired FVC.
CONCLUSIONS: In this case series, PEG tube insertion was associated with minimal peri- and post-procedural complications. The low complication rate might be due to the systematic use of procedural NIV in ALS subjects.
- percutaneous endoscopic gastrostomy
- PEG
- amyotrophic lateral sclerosis
- ALS
- respiratory
- noninvasive ventilation
- NIV
- complications
Introduction
Several studies have shown that malnutrition and weight loss are negative prognostic factors in patients with amyotrophic lateral sclerosis (ALS).1 However, evidence that percutaneous endoscopy gastrostomy (PEG) prolongs survival is limited,2 and the optimal time of PEG placement is still an unresolved issue. The American Academy of Neurology practice and European Federation of Neurological Societies guidelines state that PEG should be offered in patients with dysphagia and accelerated weight loss percentage.3,4 This recommendation is based on the assumption that complication rates increase with impaired respiratory function, but no controlled studies have been performed.5–7 According to the recently published Guidelines of the European Federation of Neurological Societies, and according to the guidelines of the National Institute for Health and Clinical Excellence, noninvasive ventilation (NIV) should be offered as soon as symptoms of impaired respiratory function are present4,8 and at least one laboratory test (eg, FVC < 80% of predicted value, sniff nasal pressure < 40 cm H2O, maximum inspiratory pressure < 60 cm H2O, substantial nocturnal desaturation on overnight oximetry, morning blood gas PCO2 > 45 mm Hg) is abnormal.4 The indication for NIV is independent from the indication for PEG placement.3 Although rates of PEG tube insertion in ALS patients steadily increased within the last decade, only up to 20% of the patients who met the criteria received a feeding tube.2,9 Several reasons may account for this discrepancy, including the increased risk of complications associated with PEG placement, especially in patients with impaired pulmonary function and FVC measures below 50%.10
More recently, NIV was proposed to optimize respiratory function before and during PEG placement.5 However, only limited data exist for comparisons of outcomes from PEG placement under NIV in moderately (FVC > 50%) versus severely (FVC < 50%) respiratory impaired ALS patients.5,11,12
With the current retrospective chart review of our ALS patients supported by NIV during PEG placement, we hypothesized that peri-procedural NIV permits safe PEG placement in all patients, even if severe respiratory muscle impairment is present. We therefore analyzed peri-procedural complications and post-interventional survival in patients with various degrees of respiratory impairment.
QUICK LOOK
Current knowledge
Malnutrition and weight loss are negative prognostic factors in amyotrophic lateral sclerosis (ALS). Percutaneous endoscopic gastrostomy (PEG) can prolong survival, but the optimal time of PEG tube placement is unresolved. Noninvasive ventilation (NIV) has been proposed to optimize respiratory function before and during PEG tube placement, but the impact of perioperative PEG NIV on outcomes is unknown.
What this paper contributes to our knowledge
In ALS patients with moderately to severely impaired ventilation, NIV before, during, and after PEG tube placement reduced complications, compared to PEG tube placement without NIV.
Methods
The procedures were conducted in accordance with the ethical standards of the World Medical Association Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects. The scientific relevance and analysis of results were approved by our local ethics board.
Subjects
Data from ALS patients who were treated at the Neuromuscular Diseases Unit/ALS Clinic and received a PEG tube at our institution were analyzed (chart review). Patients fulfilling the following criteria were included in this analysis: clinically definite, probable, probable laboratory-supported, or possible ALS by El Escorial criteria13; age 18 or older; and impaired oral food or fluid intake leading to a > 10% decrease of body weight and/or impaired quality of life due to swallowing problems.3 The degree of bulbar impairment was evaluated with the revised ALS Functional Rating Scale, which is a validated questionnaire that evaluates the subject's degree of functional impairment.11 The questionnaire covers 12 different items of daily function, including bulbar function, rated on a 5-point scale, from 0 = “can't do” to 4 = “normal ability.” The individual item scores were summarized. The lowest score was 0 = worst, and the highest was 48 = best.14 In addition, body mass index was calculated as mass (kg)/(height (m)2.
Respiratory Measurements
Spirometers (EasyOne Diagnostic 2.15, ndd Medical Technologies, Chelmsford, Massachusetts, and MicroRPM, Micro Medical, Kent, United Kingdom) were used for the FVC and sniff nasal pressure measurements. FVC was performed as described elsewhere,14,15 and the values were normalized and are expressed as percent of predicted. Sniff nasal pressure was also performed as described elsewhere,16 and the values were age- and sex-corrected17 and are presented as percent of the lowest predicted. All pulmonary function tests were performed before the procedure by an experienced respiratory technician who was familiar with the applied methods. Three consecutive measurements of FVC were recorded, and the best value was documented. Repeated sniffs in an upright position were performed at least 5 times, until a consistent value of sniff pressure was reached, and the highest value was recorded.
Procedures
All subjects were hospitalized on the pulmonary ward before PEG tube insertion. After initial clinical assessment, a specially trained nurse adapted a nasal or oronasal vented mask, depending on the subject's ability to keep the mouth closed, attached a pulse oximeter finger probe (Pulsox-300i, Minolta, Osaka, Japan), and initiated NIV (BiPAP Synchrony or BiPAP Synchrony ST, Philips Respironics, Murrysville, Pennsylvania). The usual initial setting was a spontaneous mode with an inspiratory pressure of 15 cm H2O and an expiratory pressure of 5 cm H2O. The following nights, the subjects were monitored with nighttime oximetry recordings with a target nighttime SO2 of > 90%. They were instructed to use NIV as long as possible during sleep time. Pressure settings were adapted according to the subject's tolerance and SpO2 readings, taking into account the readout of the stored information in the NIV device for mask leaks, breathing frequency, and estimated minute ventilation. Respiratory secretions were managed with a respiratory therapist assessing the subject in the ward. If necessary, manual physiotherapy was complemented with mucolytic agents such as acetylcysteine and with the use of mechanical devices (eg, CoughAssist, Philips Respironics, Murrysville, Pennsylvania). In subjects with marked hypoventilation, arterial blood gas analysis was performed and NIV settings were increased until PaCO2 levels and HCO3– levels were within normal limits during NIV.
As soon as the subject felt comfortable with NIV, PEG insertion was performed. Prophylactic antibiotics (ceftriaxone 2 g intravenous) were administered to all subjects at least 1 hour before the procedure. Sedation during the procedure was performed with midazolam or propofol. The intervention was performed by 2 experienced gastroenterologists and an experienced nurse.
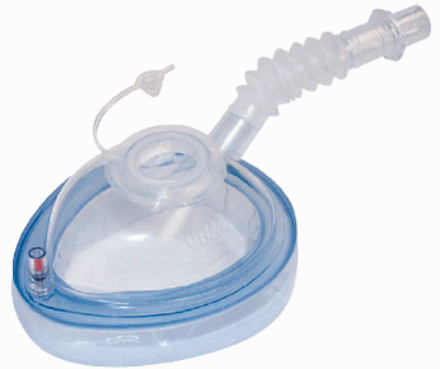
The safety standards included the presence of drugs and equipment necessary for the maintenance of airway patency and hemodynamic stability, as well as monitoring systems, including pulse oximetry, electrocardiogram, and blood pressure. A qualified nurse and an anesthesiologist participated in the procedure. Endoscopy was performed using a special mask (Fig. 1) (Endoscopy Mask, VBM Medizintechnik, Sulz, Germany) with an opening that allows insertion of the endoscope during NIV. All subjects were treated with a bi-level pressure controlled mode of ventilation. The settings varied according to the subject's needs, with inspiratory pressure between 15 and 25 cm H2O, expiratory pressure of 4 cm H2O, breathing frequency of 14 breaths/min, inspiration time of 1.5 s, and flow trigger setting of 2–5 cm H2O/mL, adapted to maintain oxygen saturation at > 92%. The PEG tube (Freka PEG gastric set CH 15, Fresenius Kabi, Bad Homburg, Germany) was inserted by the pull-through-method, under sterile conditions.
Endoscopy Mask, which allows CPAP during percutaneous endoscopic gastrostomy (PEG) tube insertion.
After the procedure, NIV was continued until the subject was fully awake and breathing spontaneously. It was ascertained that saturation was above 90% at all times without supplementing additional oxygen to avoid exacerbation of hypoventilation.
As soon as feeding via the PEG tube was possible without pain or discomfort, the subject was discharged. All subjects were encouraged to continue the use of NIV and maneuvers for the control of secretions at home.
Statistics
Pre- and post-procedural outcome measures were analyzed retrospectively. Stratification of subjects based on pre-interventional FVC as high FVC (≥ 50%) and low FVC (< 50%) was carried out based on the published nutrition management algorithm in the recent practice parameter update for ALS patient therapy.3 Comparison of parameters between the 2 groups was performed with a 2-tailed, unpaired t test.
Results
Data from 26 subjects (13 males and 13 females) with a mean age of 65 ± 14 y (range 32–83 y) were entered into the analysis. The pre-PEG mean disease duration was 26 ± 14 months (range 3–68 months). Sixteen subjects (61.5%) had bulbar onset disease, 9 had limb onset disease, and one subject had thoracic onset disease. The mean BMI before PEG was 22 ± 3 kg/m2 (14–27 kg/m2), and the mean ALS Functional Rating Scale score was 30 ± 9 (18–41). Pre-PEG FVC values were available from 21 subjects (mean 59 ± 27% of predicted), and sniff nasal pressure measures were available from 12 subjects (mean 32 ± 14% of predicted). After PEG tube insertion the subjects continued to use nighttime NIV at home.
In the group of subjects with FVC > 50%, 4 subjects experienced complications. One subject developed signs of peritonitis, one subject developed upper-respiratory-tract infection, and procedural complications occurred in another subject (failure to place the PEG tube). One subject experienced laryngospasm 3 hours post PEG tube insertion and required intubation. This particular subject recovered and survived for 18 months. There were no deaths within the first 24 hours after PEG, and only one (67-year-old) subject died, 18 days after the procedure, because of respiratory failure. He had the PEG placement 34 months after onset of the disease. He was the only subject in this study with familial ALS and a fast progression of the disease. The mean survival after PEG was 12 ± 10 months (range 0.6–42 months).
In 5 subjects, no respiratory function tests were available, because they were seen by a pneumologist in private practice, but they were declared normal and referred to the high FVC (≥ 50%) group. Twenty-six subjects were stratified in either the high FVC (≥ 50%, n = 16) or low FVC (< 50%, n = 10) groups. Pre-PEG subject characteristics in the 2 groups did not differ regarding age, symptoms at onset, and BMI (Tables 1, 2, and 3). All subjects experiencing post-procedural complications were in the high FVC group, and there was no difference in post-PEG survival between the groups (high FVC 12 ± 9 months, low FVC 14 ± 12 months, P = .62) (see Tables 1, 2, and 3).
Subject Characteristics Prior to Percutaneous Endoscopic Gastrostomy Tube Placement
Outcomes After Percutaneous Endoscopic Gastrostomy Tube Placement
Subjects With Respiratory Insufficiency and FVC > 50%
Subjects With Respiratory Insufficiency and FVC < 50%
Discussion
PEG tube placement in patients with respiratory impairment, especially with ALS, is challenging and requires a specialized team of physicians and nurses familiar with this particular disease. In addition, procedures have to be adapted to the particular needs of the patient. For the patient it is very challenging to adapt to various devices during the progression of ALS disease. It has been suggested that in ALS patients with an FVC < 50% the risk of complications increases.3 However, no formal study comparing patients with FVC below and above 50% exists. Furthermore, it is known that FVC is not a good predictor of diaphragmatic weakness, which is the main cause of respiratory symptoms in ALS patients.18,19 We therefore introduced procedural NIV for PEG tube placement in ALS patients who suffered from respiratory symptoms, irrespective of their FVC values.8
The most important finding of this study is that the outcomes in subjects with FVC < 50% and FVC > 50% did not differ with respect to long-term survival. The mean time from PEG tube insertion to death was 12 months. Post-PEG survival in our cohort was longer, compared to other studies, with an average of 6 months survival after PEG-tube insertion.18–20 The nutritional status in our cohort (BMI 22 ± 3 kg/m2) was comparable to other studies.5,9,10 It seems possible that in the present study the mean FVC before PEG insertion was higher than the FVC in previously published studies,9,10 implying that the nutritional status cannot explain this imbalance. However, even when considering only those subjects with an FVC < 50%, the complication rate was less and the survival time longer, compared to other studies.5,9,10 This suggests that peri-procedural NIV may help to minimize the risk of PEG placement in subjects with an FVC < 50%. It is unclear whether the complication rate is also lowered in subjects with an FVC > 50%. The adverse outcomes in the “stronger” (FVC > 50%) cases is difficult to explain, but may be a result of the small sample size. It challenges the view that FVC is the major determinant of the risk of complications.
The overall complication rates of PEG placement with NIV were low (4/26, 15%). No subjects died within 24 hours post tube insertion. All complications, including one death within the first month, occurred in patients in the high FVC group. In other studies utilizing procedural NIV in respiratory impaired ALS patients (FVC range 33–36%, lower than ours), the post-procedural 30-day mortality was up to 6%.9,11,12 Importantly, the 24 hour and 1 month mortality rate in patients without NIV and variably impaired respiration (mean FVC% 31–69%) was markedly higher (up to 11.5%).8,9,21–23
Independently of the use of NIV during PEG placement, one must consider that all existing guidelines referring to NIV are not evidence-based, but based on expert opinion, since there is a lack of prospective controlled studies. The fact that some patients with symptoms of nocturnal hypoventilation but with normal respiratory function tests and blood gases24 may also benefit from NIV has led to a modification of the most recent guidelines, which now recommend that NIV should be initiated whenever respiratory symptoms are present.4 The patients in our ALS clinic are referred for NIV evaluation even when they complain about symptoms of nocturnal hypoventilation, which may be the reason why, in our study, the mean FVC before PEG placement was higher than in other studies, and could also have led to the good outcomes in this study. Recently it was shown that early implementation of NIV in ALS prolongs survival and improves respiratory symptoms.25,26
We conclude that the use of NIV for PEG placement in ALS patients with moderate to severe impaired ventilation may be an advantage, compared to PEG placement without NIV. Our results suggest that NIV before, during, and after PEG placement may improve outcomes, even in ALS patients with severe respiratory impairment. However, there are several limitations to our study. First, this was an observational study with a small number of treated subjects, because the health insurance system in Switzerland only covers the costs of in-patients who live within a particular canton. This precluded many patients who were taken care of in the ALS out-patient clinic and received PEG placement at our institution. In addition, the retrospective design of the study has well known limitations, such as retrieval bias and incomplete data. However, our data are in line with data of a previously published important study in this field.5
Conclusions
Our results support the use of NIV during PEG placement in ALS patients with moderate and severe respiratory impairment. To determine if early implementation of NIV and early secretion management therapies improve outcomes in ALS patients, a prospective controlled study is warranted.
Footnotes
- Correspondence: David Czell MD, Neuromuscular Diseases Unit/ALS Clinic, Cantonal Hospital, Greithstrasse 20, St Gallen 9007, Switzerland. E-mail: david.czell{at}gmx.ch.
The authors have disclosed no conflicts of interest.
- Copyright © 2013 by Daedalus Enterprises