Abstract
Ventilator-associated pneumonia (VAP) is one of the most frequent hospital-acquired infections occurring in intubated patients. Because VAP is associated with higher mortality, morbidity, and costs, there is a need to solicit further research for effective preventive measures. VAP has been proposed as an indicator of quality of care. Clinical diagnosis has been criticized to have poor accuracy and reliability. Thus, the Centers for Disease Control and Prevention has introduced a new definition based upon objective and recordable data. Institutions are nowadays reporting a VAP zero rate in surveillance programs, which is in discrepancy with clinical data. This reduction has been highlighted in epidemiological studies, but it can only be attributed to a difference in patient selection, since no additional intervention has been taken to modify pathogenic mechanisms in these studies. The principal determinant of VAP development is the presence of the endotracheal tube (ETT). Contaminated oropharyngeal secretions pool over the ETT cuff and subsequently leak down to the lungs through a hydrostatic gradient. Impairment of mucociliary motility and cough reflex cannot counterbalance with a proper clearance of secretions. Lastly, biofilm develops on the inner ETT surface and acts as a reservoir for microorganism inoculum to the lungs. New preventive strategies are focused on the improvement of secretions drainage and prevention of bacterial colonization. The influence of gravity on mucus flow and body positioning can facilitate the clearance of distal airways, with decreased colonization of the respiratory tract. A different approach proposes ETT modifications to limit the leakage of oropharyngeal secretions: subglottic secretion drainage and cuffs innovations have been addressed to reduce VAP incidence. Moreover, coated-ETTs have been shown to prevent biofilm formation, although there is evidence that ETT clearance devices (Mucus Shaver) are required to preserve the antimicrobial properties over time. Here, after reviewing the most noteworthy issues in VAP definition and pathophysiology, we will present the more interesting proposals for VAP prevention.
- ventilator-associated pneumonia
- VAP
- nosocomial infections
- mechanical ventilation
- lung bacterial colonization
- body positioning
- endotracheal tube
- modification
- medical devices
Introduction
Ventilator-associated pneumonia (VAP) is one of the most frequent hospital-acquired infections occurring in mechanically ventilated patients and is associated with increased mortality, ICU stay, and health-related costs. VAP occurrence is closely related to intubation and the presence of the endotracheal tube (ETT) itself. Thus, effective preventive strategies are of pivotal importance and a major concern in ventilated patients.1
Depending upon the VAP definition, VAP incidence ranges have been reported between near zero to 25%, with higher risk during the first days of mechanical ventilation.2 Over the years, a decreasing trend in incidence has been highlighted, and the National Healthcare Safety Network reported a reduction from 15% to 8% from 2004 to 2009.3 Recently, very low incidence rates have been reported, which estimated 1.4/1,000 ventilator days in medical ICUs and 3.5/1,000 ventilator days in surgical patients.4 This improvement can be secondary to the implementation of effective preventive strategies, diagnosis variance relying on clinical probability, and an increasing effort in surveillance programs. Reported mortality varies widely, between 20–70%.1 Nevertheless, traditional match exposed-unexposed crude mortality data may overestimate mortality, and studies have shown the actual mortality attributable to VAP could be less than usually assumed in critically ill patients.5 In a large cohort, Bekaert et al found a mortality rate of 33.0% in patients with VAP, compared to 24.3% in the group without pneumonia, showing an absolute risk reduction of almost 9%. After adjusting for the confounding factors, pneumonia accounts for only 1.5% excess in mortality at 60 days.6
However, VAP contributes to a higher morbidity, leading to longer ICU stay, duration of mechanical ventilation, and costs of hospitalization. VAP has been proposed as an indicator of quality of care in public reporting, and its prevention is a national patient safety goal. Thus, VAP has been proposed as one of the conditions considered for non-reimbursement by the Centers for Medicare and Medicaid Services. VAP derived costs are high, as it accounts for more than 50% of antibiotics prescribed in ICUs, and adds at least 10 days to mechanical ventilation and ICU stay.7,8 In 2008 a financial penalty strategy was proposed by the Centers for Medicare and Medicaid Services, in an effort to limit preventable health-related complications. This can lead to the obvious risk in changing the diagnosis rather than impacting the true disease incidence. A recent article reported a steeper trend in reduction of common healthcare infection rates from 2006 to 2011, but without any additional positive effects from the introduction of this policy.9 VAP incidence time course is very similar to that of central venous catheter related and urinary tract infections. The trend in incidence rate was not modified by this strategy, and the decrease in rates was 7.3% before and 8.2% after the policy implementation.9
Definition
The precise definition of VAP is still a matter of debate, due to the lack of criteria univocally able to distinguish it from other pulmonary conditions in critically ill patients. Each of the VAP findings is non-specific and could be consistent with other diseases. Moreover, variability is increased, as different institutions have proposed their own definitions.
In 2005, the American Thoracic Society and Infectious Diseases Society of America jointly published practical guidelines on hospital-acquired infection, and VAP was defined as a pneumonia in patients with mechanical ventilation for at least 48 hours and characterized by the presence of a new or progressive infiltrate, signs of systemic infection (temperature, blood cell count), changes in sputum characteristics, and detection of the causative agent.1 The 48-hour time frame was set to differentiate any new infection from processes already ongoing at the moment of intubation. VAP is subdivided into an early and late onset, due to the different epidemiological features and therapeutic implications of the 2 forms. Early VAP occurs within the first 96 hours of mechanical ventilation and accounts for a better prognosis, while late-onset VAP has a higher mortality and is often related to multidrug resistant bacteria.1 A European study found almost 90% of all VAP episodes to occur within the first 10 days of mechanical ventilation, with late-onset VAP accounting for more than half of the cases.10
Similarly, the Centers for Disease Control (CDC) definition shared the same structure and features with the definition described above (Table 1), but with the important difference of not requiring a window of time after intubation.11 This difference is relevant, because the CDC's definition includes pneumonia occurring in the first 2 days, which would be excluded using the American Thoracic Society/Infectious Diseases Society of America definition, leading to an increase in VAP incidence.
Centers for Disease Control Diagnosis of Pneumonia
The most common critique to current definitions is their subjectivity and low specificity. Most of the involved criteria are intrinsically linked to the observer's judgment (secretions characteristics and amount, worsening oxygenation, clinical examination). Most radiographic findings are prone to inter-observer variability and unreliability.12 Because VAP is associated with high morbidity, clinical diagnosis must prompt treatment in all suspected critically ill patients, consequently at the expense of sensitivity. Post-mortem studies assessed the accuracy of VAP diagnosis with clinical criteria plus microbiology and showed a 69% sensitivity and 72% specificity, in comparison to autopsy findings.13 Another post-mortem study compared VAP definitions based upon clinical diagnosis and the Clinical Pulmonary Infection Score14 to histological findings, and found similar results to previous studies. Moreover, inter-observer reliability was much higher for histological findings than for clinical diagnosis.15
Variability in VAP diagnosis can be also influenced by the technique used to obtain microbiological specimens of the causative agent. CDC guidelines require a lower respiratory tract protected specimen (bronchoalveolar lavage, protective brush, blind brush or lavage) and quantitative analysis to confirm VAP. Also the American Thoracic Society/Infectious Diseases Society of America advocate the use of an invasive technique for sampling, in order to distinguish causative pathogens from colonizing microorganisms. Invasive and quantitative analysis are thought to be valuable for choosing the correct antibiotic therapy and identifying true VAP cases. However, conclusive data about the effective superiority of one technique over another are lacking.16 In a post-mortem study, a sterile bronchoalveolar lavage predicted negative intra-parenchymal bacterial growth in 90% of cases.17 A surveillance study reported a sensitivity of 90% of specimens obtained via tracheal suctioning but very low positive predicting values.18 Bronchoalveolar lavage with quantitative analysis is associated with a decrease in VAP diagnosis of 76%, compared to qualitative specimens obtained via tracheal suctioning.18
The 2 major studies comparing the diagnostic sampling approach for VAP showed contrasting results. The Canadian Clinical Trials study randomized 740 VAP suspected patients (patients known to be colonized by multi-drug-resistant microorganism were excluded) to specimens obtained via bronchoalveolar lavage or tracheal suctioning. The study showed no difference in any clinical outcome.19 Conversely, a French study showed a decrease of 14-day mortality associated with invasive management in VAP suspected patients (16.2% vs 25.8%, P = .02).20 The use of a bronchoalveolar lavage and protected specimen group was associated with a decreased antibiotic use and a lower inappropriate treatment (0.5% in the invasive group vs 13% of patients in the clinical group, P < .001). However, the reduction in mortality has been shown to be more related to adequacy of the initial empirical antibiotic therapy than appropriateness of the subsequent changes.21 Cochrane meta-analysis of a total of 1,367 patients found no difference in mortality in the invasive versus noninvasive groups (26.6% and 24.7%, respectively, relative risk 0.91, 95% CI 0.75–1.11) or in quantitative versus qualitative cultures (relative risk 1.53, 95% CI 0.54–4.39).16 Similarly, no difference in antibiotic use or other clinical outcomes has been found. From a clinical prospective, Koulenti et al reported in a survey study of European ICUs that invasive diagnostic exams were performed in only 39% of patients with VAP, where bronchoalveolar lavage accounted for 13.6%, protected brush specimens for 5.8%, and blind techniques for 19.6% of the cases.10
In an effort to overcome these issues, the CDC has recently proposed a surveillance definition based upon objective and recordable data to limit definition inaccuracy and improve reproducibility of the diagnosis. The declared aim of this definition, ventilator-associated event (VAE), is for public reporting and inter-facility comparison, and it is not to be used in the clinical care of patients (Fig. 1).22 The working group looked for objective and easily recordable features to meet the definition criteria in order to reduce subjectivity and improve reproducibility. The main differences in this new algorithm are the proposal to detect all the ventilator-associated complications, not only VAP, and the omission of radiological findings, due to their unreliability and limited availability. Precise time limits are set to detect only new events directly as a consequence of mechanical ventilation. A 48-hour time lapse of stability or improvement of the respiratory function has been fixed to differentiate any new condition from the evolution of the underlying disease. Thus, this means that both PEEP and FIO2 need to be stable or decrease for at least 2 consecutive days before the report of a VAE occurrence. Moreover, the same period of time (ie, ≥ 48 h) is required for defining as sustained the rise in PEEP and/or FIO2. The likelihood of an infective process is divided into possible and probable, based upon different implications of qualitative and quantitative microbiological culture, VAP being a subcategory of a wider heterogeneous events record.
National Healthcare Safety Network surveillance definitions. WBC = white blood cells. (From data in Reference 22.)
Similar surveillance definitions have been tested in numerous studies.23–25 In 600 mechanically ventilated patients, Klompas et al assessed a similar surveillance definition in comparison to a standard clinical definition, finding VAE and VAP incidence of 23% and 9%, respectively (equal to 21.2 and 8.8/1,000 ventilator days). Only 23% (31 out of 135 subjects) of patients in the VAE group satisfied the VAP criteria. Both conditions were associated with prolonged ICU stay and days on mechanical ventilation, but only VAE showed an increased mortality risk (odds ratio [OR] 2.0, 95% CI 1.3–3.2).23 Authors addressed the quickness and reproducibility as advantages of this new definition, and pointed out a higher inter-observer reliability for the surveillance definition.23,24 One of the major strengths claimed for the new definition is its association with higher mortality. The association between respiratory worsening and mortality is no longer significant if positive microbiology results are taken into account, thus selecting only patients with confirmed lower respiratory system infections.25 In this study the overall VAE reported incidence was 12.0 events/1,000 ventilator days, including not only pneumonia but also different noninfectious causes of respiratory compromise (ie, atelectasis, pulmonary edema, thromboembolic disease, ARDS, and others).25 The risk of underestimating real VAP incidence should be taken into account, with a lower rate associated more with the surveillance definition than with clinical diagnosis. An American study reported a 20% difference between VAP cases registered by the hospital infection control service and the physician's clinical diagnosis.26 Further studies are necessary to evaluate the usefulness and cost/effectiveness of surveillance programs, even if some beneficial effects have been recently reported.27,28
Key Message
The clinical diagnosis of VAP usually relies upon 3 components: radiological findings, signs of an ongoing infection, and laboratory results. Major concern about this approach is related to the unreliability of features prone to observer interpretation. In the perspective of infection surveillance and quality improvement programs, the CDC has recently proposed a VAE definition.22 The principal differences in this definition are the choice of only objectively recordable data, rigid time thresholds, and the exclusion of radiographic imaging. Some institutions have reported a VAP zero rate in surveillance programs, which have increased the discrepancy between epidemiological results and clinical data. Consequently, VAP incidence can vary from 1.2 to 18.3/1,000 ventilator days, depending on the study population and definition.10,29 However, without any direct intervention to alleviate pathogenic mechanisms causing VAP, it is possible that the rate reduction could be attributed to a different patient population rather than to an actual improvement in clinical management.
Pathophysiology
The main risk factor for VAP is the presence of the ETT, as it impairs natural defense mechanisms such as cough reflex and mucociliary clearance, and allows for a direct communication between the oral-supraglottic space and the lower respiratory tract. The competency of anatomical barriers is disrupted, and the ETT cuff can prevent gross aspiration, but it does not assure a perfect sealing, due to the presence of folds along the cuff surface in contact with the trachea, improper inflation, and movements.30,31 Subsequently, oropharyngeal secretions pool upon the cuff and leak through. Many studies have shown how dye can quickly pass the cuff.32,33 Moreover, the presence of the ETT cuff impairs mucociliary function. Mucociliary velocity is decreased to less than half after 2 hours of intubation, and the cuff creates a mechanical obstacle to mucus clearance.34,35 Consequentially, mucus accumulates near the ETT opening unless suctioned, or it can reverse its flow and move back to the lungs as a result of gravity.36 Airway mucus accumulates into distal bronchial airways and can contribute to the subversion of respiratory system physiology leading to pneumonia. Studies have shown that secretions move into airways according to gravitational and airflow gradients, which are major factors determining original inoculum and the subsequent spread through the lungs.37,38 The influence of gravity in secretion mobilization has also been proven in animal studies, which showed better drainage of secretions from the lungs with downward position of the trachea.39
The other main pathophysiological mechanism involved in VAP is related to the bacterial colonization of the ETT. A well structured biofilm, which is an aggregate of microorganisms kept together within a complex matrix composed of polysaccharides, proteins, and DNA that forms a mechanical scaffold around bacteria, develops rapidly within hours of tracheal intubation. The biofilm constitutes a protective environment from host defenses and antimicrobial agents.40,41 Bacteria easily attach to the polyvinyl-chloride (PVC) surface of the ETT, where they multiply and differentiate their phenotype within the extracellular self-produced matrix (Fig. 2). The most common organisms associated with biofilm are Gram-negative bacteria and fungal species. The organisms can colonize the ETT at the moment of intubation, as a result of leakage of secretions outside the cuff, or following tracheal suctioning. Biofilm has been associated with increased bacterial resistance to antimicrobials, which is probably related to different cellular and extracellular mechanisms.42 First, a change occurs in cellular phenotype: bacteria can switch from the usual independent and floating form to a different phenotype that allows bacteria to “settle down” on surfaces and constitute communities, a process associated with survival advantages in a hostile environment.43 Moreover, due to the presence of the extracellular matrix and isolation from blood flow and immune defenses, the antimicrobials cannot diffuse to reach the microorganisms. Lastly, multicellular and multispecies interactions can be implicated in the increased resistance and virulence of pathogens embedded in biofilm, as it has been observed for Candida albicans and Pseudomonas aeruginosa.44 Therefore, biofilm acts as a reservoir for highly infective microorganisms that can detach and enter the lungs as a consequence of tracheal aspiration or inspiratory flow during mechanical ventilation.45
Scanning electron microscopy of an uncoated endotracheal tube after extubation. A: Luminal surface at high magnification. B: Red cells, epithelial cells, macrophages, and cocci in pairs and small chains embedded in the amorphous matrix.
Adair et al found that 70% of patients with VAP had identical pathogens in tracheal secretions and ETT biofilm. The most common isolated were Staphylococcus aureus, Enterococci, Enterobacteriaceae, Pseudomonas aeruginosa, and Candida species. Interestingly, the pathogens in biofilm showed a greater antibiotic resistance, in comparison to specimens obtained via tracheal suctioning.46 Using scanning electron microscopy, Gil-Perotin et al detected biofilm on ETT surface in 95% of patients mechanically ventilated for more than 24 hours.47 Bacterial growth was present in 83% of cases, and the most frequent microorganisms were Acinetobacter baumannii, Pseudomonas aeruginosa, and Candida albicans.47 In patients who developed VAP, the causative agent could be detected in ETT cultures after antibiotic therapy in 50% of the cases, a percentage that increased to 70% if considering only Gram-negative bacteria. In this regard it is important to take into account the interaction occurring between the 3 main factors involved in any infection process. The risk of acquiring an infection is directly correlated to the size of the pathogen's inoculum and the virulence of the microorganisms, and is inversely proportional to the immune competency of the subject. ETT biofilm is a perfect environment for bacterial colonization where high counts of resistant bacteria can be detected.46,48 Simultaneously, in critically ill patients, immunity response is usually compromised by systemic inflammation and underlying diseases. All 3 factors act together to increase the susceptibility of intubated patients to pneumonia, and must be taken into account when evaluating the patient's risk for VAP.
Key Message
Major determinants leading to pneumonia are:
The ETT, with consequent pooling and leakage of oropharyngeal secretion
Impairment of mucociliary clearance of secretions with distal bronchial obstruction and gravity dependence of mucus flow within the airways
Biofilm development, which acts as a bacterial reservoir for lung inoculum
The balance between host and pathogen characteristics
Therefore, VAP could be considered a form of aspiration (gravity) pneumonia in intubated patients. Mechanical ventilation and the ventilator are not involved in the pathogenesis of VAP, and the designation of VAP is a misnomer. Indeed, the use of noninvasive ventilation has been shown to lower the incidence of hospital-acquired pneumonia in both immunocompromised and immunocompetent patients.49 It may be better to refer to this condition as gravity-tube pneumonia, which emphasizes the real pathophysiology of the disease, instead of a non-causative association.
Preventive Strategies
Numerous measures have been addressed to prevent VAP, ranging from good general practices for infection control to pathogenic-tailored strategies. Some of these have been included into sets of procedures to be implemented together (bundles). The Institute for Healthcare Improvement bundle consists of 5 features (head of bed elevation, oral care with chlorhexidine, stress ulcer prophylaxis, deep venous thrombosis prophylaxis, and daily assessment of sedation and spontaneous breathing trial) that were shown to reduce VAP rates.50,51 Although controversial, some of these measures have been included in the guidelines and recommendations, with the aim to introduce evidence-based prevention standards into clinical practice.1,50,52
Other interventions have been proposed to modify the pathophysiological consequences of the ETT presence. From this prospective, the main strategies focus on improvement of secretion drainage by body positioning, leakage prevention through ETT modifications (subglottic secretion drainage [SSD], cuffs), and inhibition of biofilm formation (ETT coating and cleaning devices).
Body Positioning
For patients mechanically ventilated, current guidelines recommend the semi-recumbent position with head of bed elevated to 30–45°.11,50 This position is hypothesized to reduce the gastroesophageal reflux, thus limiting oropharyngeal colonization and pulmonary aspiration of gastric secretions. As opposed to healthy subjects, in critically ill patients the stomach is often colonized by pathogens secondary to alkalization of gastric contents by stress ulcers prophylaxis and enteral feeding. Bacteria may subsequently translocate from the stomach into the oropharynx and respiratory tract.53 Despite several studies demonstrating the relationship between bacterial growth and gastric pH,53 there is no persuasive evidence of this relationship in the pathogenesis of VAP.54,55
The current recommendation on body position relies upon the positive results of a single randomized study by Drakulovic et al, conducted on 86 mechanically ventilated patients assigned to supine or semi-recumbent position. Body position was assessed only once a day, and patients were dropped out of the protocol if maintained in other than study position for more than 45 min consecutively. This study demonstrated that the head of bed elevation of 45°, compared to supine position, reduces the incidence of suspected VAP by more than 75% (8% vs 34% respectively, P = .003), with similar results when VAP was microbiologically confirmed (5% vs 23%, P = .02). Supine body position and enteral nutrition were detected as independent risk factors for pneumonia, but no significant difference was found in mortality or duration of mechanical ventilation.56 The only other evidence in support of this theory derives from a previous observational study in which supine position was associated with a 3-fold increase in VAP risk.57
More recently, the study by Van Nieuwenhoven et al attempted to reproduce these results and to assess the actual feasibility of the 45° semi-recumbent position. Body position was recorded continually for 7 days, and the target 45° position was maintained only during 15% of the study time, achieving a mean of nearly 30° of backrest elevation. Moreover, the adherence to the study position changed over a 1-week period, decreasing from 28.1° to 22.6° and increasing from 9.8° to 16.1° in the 2 groups semi-recumbent and control groups, respectively.58 These results raise concern about the real feasibility of maintaining a 45° semi-recumbent position, as intended in the study by Drakulovic et al. In addition, suspected and microbiologically confirmed VAP rates did not differ between the 2 groups (confirmed VAP in 10.7% vs 6.5% for the semi-recumbent and standard of care groups, respectively). No significant differences in mortality, ICU stay, or days of mechanical ventilation were detected. A third study has been published comparing 45° and 25° backrest position, and it also did not find any significant difference.59 A meta-analysis of these studies showed a lower risk of clinically suspected VAP in the treatment group (OR 0.47, 95% CI 0.27–0.82, 337 patients), although this benefit was not confirmed with microbiological evidence for VAP (OR 0.59, 95% CI 0.15–2.35).60
From a pathophysiologic point of view, it is noteworthy that in the semi-recumbent position the trachea is oriented above the horizontal, with the risk of increased hydrostatic pressure of secretions pooled upon the cuff. This orientation may facilitate the leakage into the lower respiratory tract and increase the risk of pneumonia. Numerous animal studies performed at the National Institutes of Health support the role of gravity in the development of VAP. Animals' tracheal/ETT axis kept below horizontal allowed outward drainage of the secretions, reduced lung colonization, and decreased VAP incidence (Table 2).39,61–66 Panigada et al showed the efficacy of the “head down” position to prevent lung colonization in a sheep model, with the intervention group having the tracheal/ETT axis positioned horizontal/downward, and alternating every 6 h on each side, versus the control group positioned prone, mimicking the semi-recumbent position.39 The orientation of the trachea/ETT horizontally facilitated the sliding of secretions along the ETT and allowed a better drainage of the secretions from the airways. These results have been confirmed in all subsequent studies in sheep and pigs mechanically ventilated for up to 168 hours. Therefore, body positioning provides a strong rationale in the prevention of VAP.
Animal Studies at the National Institutes of Health Evaluating the Influence of Body Positioning on Development of Ventilator-Associated Pneumonia
In an animal study on body position and continuous suctioning of subglottic secretions (CSSS), the downward orientation of tracheal/ETT axis prevented lung colonization without any additional advantage from CSSS. In this study the CSSS “head up” group and the control group (“head up” and no CSSS) showed heavy respiratory tract colonization, while only 1 sheep out of 7 in the CSSS “head down” group showed low grade colonization.61 Similarly in pigs, animals ventilated in the group resembling the semi-recumbent position showed high lung colonization and respiratory failure, while the Trendelenburg position prevented the development of VAP for up to 168 hours.66 Finally, the effect of body position on mucociliary function was studied with radio-opaque particles, and showed that secretions are retained at the ETT lung opening secondary to cuff presence, which impaired the further movement of the secretions. Subsequently, secretions moved backward through the lungs as a result of gravity.36 This can be prevented by the Trendelenburg position, where mucus remains near the ETT opening, and then it can either enter the ETT through the effects of gravity or be removed by tracheal suctioning.
Only 2 clinical studies have evaluated the role of gravity in preventing bacterial colonization of the respiratory system.67,68 The first study is a randomized controlled trial in 60 intubated infants who were positioned supine or lateral. Cultures of specimens obtained via tracheal suctioning were significantly different on the fifth day, and had positive results in 26 infants (87%) in the supine group and in 9 patients (30%) in the lateral group (P < .01).67 The most common bacteria isolated were Gram-negative rods in both groups. Regarding safety, there were less accidental extubations in the lateral group. In adults, only a pilot study of 20 patients has been published on the feasibility of the lateral position in comparison to the semi-recumbent.68 Study position was checked every hour, and specimens obtained via tracheal suctioning were tested for pepsin as a marker of gastric aspiration. Results show that aspiration was not prevented in either of the groups, but the presence of enzyme was detected in 7 patients in the semi-recumbent and 5 in the lateral-horizontal group. A favorable trend in ventilator free days was associated with the lateral-horizontal position, despite the low number of subjects enrolled. No adverse event was associated with the lateral position, and all patients ended the study period of 64 hours, demonstrating the feasibility and safety in mechanically ventilated adult ICU patients.68
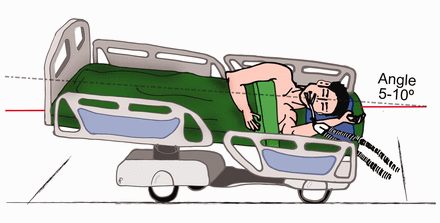
In reality, the trachea in humans has a backward orientation and the lateral position alone does not guarantee a below horizontal orientation. The trachea/ETT should be slightly below the horizontal only in the lateral position with a 5–10° Trendelenburg (Fig. 3), in order to avoid the leakage of secretions around the cuff and to better drain airway mucus out of the lungs. Based on previous findings, a large international randomized controlled trial is now ongoing to evaluate the influence of body position (lateral Trendelenburg vs semi-recumbent position) on VAP prevention in adult ICU mechanically ventilated patients (http://clinicaltrials.gov/ct2/show/NCT01138540).
The lateral Trendelenburg position adopted in the Gravity Ventilator-Associated Pneumonia trial (http://clinicaltrials.gov/ct2/show/NCT01138540) resembles the lateral safety position. The slight Trendelenburg is required to obtain the orientation of the trachea and endotracheal tube below the horizontal.
Key Message
Body positioning in intubated patients has a noteworthy importance on VAP prevention. Current guidelines recommend the semi-recumbent position (head of bed elevated 30–45°) to reduce gastric reflux. This recommendation is based upon the results of a randomized clinical trial showing the superiority of 45° head elevation compared to supine 0° positioning in VAP prevention, especially in enteral feeding patients.56 Oropharyngeal colonization and lung secretions clearance impairment play a greater role in VAP development than does the gastro-pulmonary route of bacterial translocation. Acting on gravitational forces, the lateral Trendelenburg position may enhance mucus flow out of the lungs and avoid leakage of contaminated oropharyngeal secretions. Its beneficial effects are highlighted by the results of numerous animal studies that have shown a reduction in VAP incidence and lung colonization associated with the orientation of the tracheal/ETT axis slightly below horizontal. Two clinical trials assessed its safety and feasibility on humans and now a large international randomized clinical trial is ongoing to assess the efficacy of the lateral Trendelenburg position in VAP prevention.67,68
Coated ETT
In vitro and experimental studies have tested different coatings with antimicrobial properties to reduce biofilm formation and colonization of the ETT (Table 3).69 Among the numerous studies, Berra et al showed in a sheep model that a silver-sulfadiazine and chlorhexidine coated ETT, in comparison to a standard ETT, prevents biofilm formation on the inner ETT surface and prevents distal airways colonization after 24 hours of mechanical ventilation.48 In the experimental group, only one ETT showed low bacterial growth, and all lungs were free from colonization, while in the control group all the ETTs were heavily colonized and 5 animals out of 8 showed lung bacterial growth. The ETT surfaces were visualized using scanning electron microscopy, and no structures resembling biofilm could be observed in the study group. Consistent results have been shown in another study using only silver-sulfadiazine as coating material.70 Similarly, Olson et al showed in a dog model of VAP that a hydrogel silver-coated ETT, in comparison to a standard ETT, decreases bacterial burden and histological findings of lung inflammation. The silver coating delayed colonization of the ETT surface 3.2 ± 0.8 days in the experimental group, and 1.8 ± 0.4 days in the control group (P = .02).71
Studied Endotracheal Tube Coatings
Among the numerous proposed materials, the silver-based coating has been the only material tested in clinical trials.70,72,73 Silver is an ideal coating, because it shares non-toxic, antimicrobial, and anti-adhesive properties. In a study on cardiothoracic surgical patients who were ventilated for 12–24 hours, the use of a silver-sulfadiazine ETT was associated with a lower bacterial colonization and thinner layer of biological material accumulation on the inner ETT surface, even if a structured biofilm was not present, probably as a result of the short intubation time.70 In the experimental group, neither the ETT nor the tracheal brush showed any bacterial growth, while 8 out of 23 ETTs (P < .01) and 2 tracheal brush samples were colonized in the standard ETT group. These results are consistent with a previous study in patients ventilated for more than 24 hours, in which the daily swab of the ETT and tracheal specimens obtained via suctioning were collected following bacterial colonization.71,72 As shown in animal data,71 the silver-coated ETT was associated with a delay and halt in bacterial colonization until the seventh day of intubation. Groups were similar in days of mechanical ventilation and antibiotic use. No visual biofilm structure evaluation or grading was performed in this study.72 As a result of these findings, a bigger randomized trial, with more than 1,500 patients, using a hydrogel silver-coated ETT available on the market has been conducted. This study showed a reduction in microbiologically confirmed VAP incidence (4.8% vs 7.5% in the experimental and control group, respectively, P = .03) with a relative risk reduction of 36%.73 No other difference in clinical end points was found.
A major limitation of coatings that arose from these studies is that biofilm formation can be delayed and temporarily reduced in burden, but coating efficacy would invariably decrease over time. In fact, tracheal suctioning, the standard of care to remove secretions, is inadequate to remove the layer of mucus lining upon the ETT surface that promotes bacterial proliferation and biofilm formation. In addition to the microbiological issues, the accumulated secretions reduce the effective ETT cross-sectional area available for ventilation, thus increasing airflow resistance. This problem is usually underestimated in clinical practice, where instead the increased work of breathing due to suboptimal ETT patency can be a factor in weaning the patient from the ventilator.74 In the worst case, the accumulation of thick secretions inside the ETT lumen can cause complete obstruction of the airflow and translate into a life-threatening problem. The grade of occlusion of the cross-sectional area cannot be adequately predicted only upon the duration of mechanical ventilation.75
The Mucus Shaver is a device constituted of an expandable silicon rubber balloon with 2 or more shaving rings that adhere to the surface of the ETT.62 The Mucus Shaver showed the ability to effectively remove secretions and bacterial biofilm, thus allowing a silver-coated ETT to retain its anti-microbial efficacy. Twelve sheep intubated with silver- sulfadiazine coated ETTs were divided into 2 groups: the control group was suctioned with standard catheters, while the study group was suctioned and cleaned with the Mucus Shaver every 6 hours. All the ETTs in the control group showed heavy colonization, while the study group had no bacterial growth detected. ETTs treated with the device were clean at visual inspection, and the absence of bacterial biofilm was confirmed at scanning electron microscopy.63 In a recent study, the Mucus Shaver was shown to be safe and efficient in a clinical setting in which 24 patients expected to require prolong mechanical ventilation were randomized into a control group or to tracheal suctioning plus ETT clearance every 6 hours until extubation.76 Only one ETT showed colonization in the study group, compared to 10 out of 12 in the control group (8% vs 83%, P < .01). Scanning electron microscopy showed little secretions on the surfaces cleaned with the Mucus Shaver. No adverse event was reported during the nearly 400 maneuvers performed in the study by the nursing staff. No difference was detected in days of mechanical ventilation or VAP incidence, but the study was not powered for these outcomes, due to the small number of patients enrolled.76
Key Message
Accumulation of secretions within the ETT lumen is a common finding at the moment of extubation in critically ill patients, and it is the major limiting factor of the utility of coated ETTs. Good results can be found in the literature on coated ETTs, but ETT colonization may be only delayed because biofilm will invariably develop over time as secretions accumulate upon the active surface. Thus, it is of pivotal importance to maintain ETT patency in order to reduce biofilm burden. The ETT clearance device (Mucus Shaver) has been shown to be effective in preventing bacterial colonization and partial or total occlusion of the ETT, with a safe and easy profile of use.76
Leakage Prevention: Subglottic Secretions Drainage and ETT Cuff Modifications
ETT modifications have been proposed to target the prevention of micro-aspiration. One strategy is based upon the preemptive evacuation of the fluid collected upon the cuff through suctioning before the fluid passes the cuff. Others innovations apply to the cuff itself, in attempting to improve its performance in sealing the tracheal lumen.
Subglottic Secretions Drainage.
SSD is performed through a specially modified ETT equipped with a suctioning channel opening just above the inflated cuff. Suctioning can be delivered continuously (CSSS) or intermittently (SSD) to remove the secretions. The benefit of one technique over the other has not been established. In a meta-analysis, the relative risk reduction was similar for the continuous (relative risk 0.50, 95% CI 0.37–0.66) and intermittent (relative risk 0.59, 95% CI 0.47–0.74) suctioning. CSSS, despite using lower negative pressure than the intermittent strategy, has been associated with tracheal mucosal lesions (ranging from erythema to erosions and necrosis with cartilage exposure) in all animals treated with CSSS for 72 hours,61 and no study has shown its safety in humans. Most of the literature confirms some beneficial effect on pneumonia development associated with SSD, but low impact has been found on clinical outcomes.77,78 Despite the usefulness of SSD as a prevention tool in VAP management, controversial evidence about its utility and concerns about safety may limit its use in clinical settings.1,11
A recent meta-analysis of 13 randomized clinical trials, including a total of 2,442 patients, showed an overall risk reduction for VAP associated with SSD (relative risk 0.50, 95% CI 0.46–0.66). Moreover, the use of SSD was associated with improvement in other outcomes such as ICU stay (relative risk −1.52, 95% CI −2.94 to −0.11), duration of mechanical ventilation (relative risk −1.08, 95% CI −2.04 to −0.12), and delay in VAP onset (2.7 d on average). No effect on mortality was reported, or in ICU and hospital stay.77 Similar benefits have been shown by Dezfulian et al, who included only 4 studies. In patients treated with SSD, ICU stay was reduced by 3 days, mechanical ventilation was reduced by 2 days, and VAP occurred 7 days later.79 In a study with more than 700 cardiac surgical patients, CSSS was associated with a reduction in VAP rate only in patients mechanically ventilated for more than 48 hours (26.7% vs 47.5% in the CSSS and control groups, respectively, P = .04). Although no difference in mortality was found, CSSS was associated with reduced ICU stay, days of mechanical ventilation, and antibiotic usage. In the multivariate analysis, the only protective factor was CSSS, while only reintubation was associated with an increased risk of VAP.80 In patients ventilated for more than 48 hours, Lacherade et al found a risk reduction of 42.2% in microbiologically confirmed VAP. VAP occurred in 25 out of 169 patients in the SSD group, while it occurred in 42 of the 164 patients in the control group (P = .02). Moreover, the beneficial effect of SSD was present both in early- and late-onset VAP, defined by a 5 days threshold.78
A different approach has been proposed by Li Bassi et al with the “Mucus Slurper,” a custom-made ETT equipped with multiple holes at the very tip that allows direct suctioning of secretions from the tube lung opening and very near to the cuff.64 In sheep mechanically ventilated for 72 hours with the tracheal/ETT axis oriented below the horizontal, the Mucus Slurper was shown to prevent accumulation of secretions within the ETT.65 The study group was intubated with the Mucus Slurper, while a standard ETT with tracheal suctioning every 6 hours was used in the control group. Mucus Slurper was automatically timed to perform a negative suctioning pressure every 2 minutes, in synchrony with the early expiratory phase. At the end of the study, the tube and the trachea were free of secretions in the Mucus Slurper group while the control group showed mucus accumulation within the ETT lumen. The use of the Mucus Slurper was safe, as no lesion of the tracheal mucosa was detected, and the suctioning did not entail a decrease in PEEP during the suctioning.64,65
ETT Cuffs.
ETT cuffs made of PVC were introduced in the 1970s, and replaced latex cuffs, which required high and poorly controllable pressure to guarantee tracheal sealing, often resulting in tracheal damage.81 PVC cuffs are called high-volume, low-pressure cuffs because they are bigger than the tracheal diameter and can close the trachea lumen without stretching. PVC is an inelastic material, so the pressure exerted inside the cuff is equal to that upon the tracheal wall. If the cuff internal pressure is maintained lower than 30 cm H2O, it can be assumed to have a good sealing to prevent macro-aspiration and preserve tracheal capillary perfusion.82 Due to the larger surface, an inflated high-volume, low-pressure cuff creates longitudinal folds that lie upon the tracheal surface, creating channels for leakage of oral-pharyngeal secretions into the lungs.83 Micro-aspiration cannot be completely avoided, even with appropriate cuff pressure, as it may be enhanced by ETT movements, manual checking of cuff pressure, and in any situation in which pressure difference is favorable for flow to the trachea. Guidelines recommend maintaining a cuff pressure above 20 cm H2O, based on evidence that lower values have been reported to be a risk factor for VAP development.1,84 However, the use of an automatic controlled system for maintaining the cuff pressure above 20 cm H2O was not associated with any beneficial effect.85 In patients in the semi-recumbent position, the micro-aspiration could not be avoided even if the cuff pressure was constantly maintained above 20 cm H2O. Cuff pressure was found lower than the target in only 0.7% of the total study determinations in the automated group, but it was found lower than the target in 45.3% of the total study determinations in the control group.85 Most recently, in patients with continuous control of cuff pressure, Nseir et al found a lower incidence of micro-aspiration of gastric contents, as detected by the presence of pepsin in the specimens obtained via tracheal suctioning. Continuous control of cuff pressure was efficient, and the 2 groups did not differ in body positioning (average 40° backrest position), enteral nutrition, or use of stress ulcer prophylaxis. VAP rate was lower in the interventional group, compared to the control group (9.8% vs 26.2%, respectively, P = .03). However, no differences were found in clinical outcomes such as ICU stay, days of ventilation, and antimicrobial use in the 2 groups.86
Different materials such as polyurethane30 and Lycra,87,88 have been tried to overcome PVC limitations. Microcuffs are made of a very thin layer of polyurethane that has been designed to prevent leakage by creating smaller folds when inflated inside the trachea. In an in vitro study, a polyurethane cuff prevented the leakage of subglottic secretions at clinically safe pressures (20–30 cm H2O). In the artificial tracheal model, the Microcuff showed not to fold in channels along the tracheal wall in computed tomography analysis, whereas the standard PVC cuff had folds clearly visible when inflated to 20 cm H2O.30 In a clinical study, Lorente et al tested a new ETT equipped with both the ultrathin polyurethane cuff and subglottic secretion drainage. VAP rate was reduced from 19.9/1,000 ventilator days in the control group to 7.5/1,000 ventilator days in the study group. Risk of both early (OR 3.3, 95% CI 1.19–9.09) and late-onset VAP (OR 3.5, 95% CI 1.34–9.01) was reduced in the experimental group.89 Although not commercially available, the polyurethane Lycra cuff was demonstrated to seal the trachea without leakage at appropriate inflation pressure. In vitro studies showed no folds upon its surface when inflated, and no dye shedding.88 Moreover, Young et al developed a pressure-limited cuff that does not create folds upon contact with the trachea. The low elasticity of the material allows an effective pressure exerted upon the tracheal wall.90 This low-volume, low-pressure cuff was shown to prevent leakage in both in vitro and in vivo studies, but further clinical studies are required to better evaluate its utility and safety.33
A different approach entails changes in cuff shape in order to achieve a better sealing. Tapered-shaped cuffs are designed to assure that a part of the cuff surface matches the tracheal diameter and to prevent folding and channels formation. Tapered-shaped cuff proved to be more efficient in sealing in an in vitro study and to prevent leakage, even after a prolonged period of time.91 However, no clinical trial has evaluated the benefit of this device in mechanically ventilated patients.
Key Message
Different ETT innovations have been proposed to improve ETT performance. Polyurethane and tapered-shaped cuffs are promising in improving a better sealing cuff profile, although trials are required to evaluate their impact in clinical practice. In the meanwhile, trying to maintain PVC cuff pressure in the appropriate range is fundamental, even if it does not avoid the occurrence of micro-aspiration. Lastly, SSD showed mixed results in regard to its utility in clinical outcomes, and doubts about its safety in humans also limits its use for preventing secretions leakage around the cuff.
Summary
Although the exact incidence and impact of VAP are open to debate, it still presents an important challenge to caregivers. Pneumonia development is a multifactorial process that follows the disruption of respiratory system physiology caused by intubation. Preventive strategies focus on better secretion management and on reduction in bacterial colonization. The influence of gravity on outward movement of secretions proved to be efficacious in animal models and safe in humans. However, clinical studies are required to evaluate the effects of lateral Trendelenburg body positioning on VAP reduction. Additionally, numerous ETT innovations have been proposed, although no modification implemented alone proved to have an effective impact on relevant clinical outcomes. Further research on targeted interventions is needed to effectively reduce VAP incidence. In this perspective, diagnostic accuracy and reproducibility are of great importance in selecting the correct population and comparing various inter-facility measures and results.
Footnotes
- Correspondence: Lorenzo Berra MD, Department of Anesthesia, Critical Care, and Pain Medicine. Massachusetts General Hospital, 55 Fruit Street, Boston MA 02114. E-mail: lberra{at}partners.org.
Dr Berra presented a version of this paper at the 51st Respiratory Care Journal Conference, “Adult Mechanical Ventilation in Acute Care: Issues and Controversies,” held September 7 and 8, 2012, in St Petersburg, Florida.
The authors have disclosed no conflicts of interest.
- Copyright © 2013 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
Discussion
Gajic:
I have a question about the definition. If I understand correctly, arterial blood gas values are not required to diagnose new hypoxemia. Correct?
Berra:
Yes, that is correct.
Gajic:
Is it a change in FIO2, regardless of the reason for the change?
Berra:
That is also correct.
Gajic:
How about a temporary increase in FIO2 for suctioning, let's say.
Berra:
No, it needs to be a sustained increase in FIO2. The patient has to be stable for 2 days.
Hess:
Turning up the FIO2 to do bronchoscopy or suctioning wouldn't result in a VAC [ventilator-associated condition]. There needs to be an increase in the minimum FIO2 by at least 0.2, or an increase in the minimum PEEP by 3 cm H2O, and this must be sustained for 2 consecutive calendar days.
MacIntyre:
So we're going to have to report to somebody every time we raise FIO2 or PEEP for more than 2 days? We'll have to have some kind of reporting mechanism, and somebody's going to have to keep tabs on this, and somebody else will put it in the newspaper, and everybody's going to misunderstand it. Is this really what's happening? Will we be required to report every FIO2 or PEEP increase?
Hess:
It depends on where you live. This will be reportable in some states, but not others. I suspect that eventually it will be all of us. I will also point out that a VAC is not VAP.
MacIntyre:
Exactly: that's my point. We're going to be reporting all of these ventilator changes, and I don't know who's going to look at these data and how they're going to interpret them.
Hess:
I certainly hope that there will not be many of our patients who meet these criteria. Many of our patients, I would hope, by day 3 on the ventilator are getting better, not worse.
MacIntyre:
But if they are getting worse, we have to report it, and it's going to be measured by somebody as being bad practice at my hospital.
Kacmarek:
Neil, look closely at the definition: after 2 days of stability, then you see the increase. You have a patient who's going along appropriately for 48 hours and then all of a sudden you have to bump the FIO2 or PEEP for a prolonged period.
MacIntyre:
Let me remind you that ARDS has a mortality rate of somewhere around 30%. That means 30% of my patients almost certainly are going to have increasing PEEP or FIO2 requirements over the course of their hospital stay. I find this an incredibly onerous burden, looking for something that nobody's going to know what the heck to do with!
Kallet:
Before you pop a blood vessel, I was on one of the conference calls for this, and I think they're just trying to get a denominator. Obviously, if somebody gets intra-abdominal sepsis, it won't count.
MacIntyre:
Why won't it count?
Kallet:
Because then they're going to drill down to what they are culturing from the lungs.
MacIntyre:
Who's going to drill down?
Kallet:
You are.
MacIntyre:
And how am I supposed to use that to protect myself from having to say this is a VAC?
Hess:
I see this as an opportunity for all of us to do the things we're talking about around this table today. To avoid VAC we follow what Ognjen was saying this morning about prevention of ARDS by how we choose VT and PEEP, if we use noninvasive ventilation appropriately, and if we listen to you tomorrow and we get our patients promptly extubated—that's how you avoid VAC.
MacIntyre:
Dean, let me be clear: I'm not complaining about measuring and monitoring these things in your own hospital. What I'm having difficulty with is this public reporting, because I have no idea what the public reporters will do with the data. I can record this in my hospital, and hopefully I will use it to change practices, evaluate, and figure out what's going on. What I'm worried about is that some bureaucrat in Washington is going to take these numbers and use them in twisted ways.
Turner:
Ultimately, data such as these will probably play a role in reimbursement decisions for all of us.
MacIntyre:
Exactly! So if I take more patients in my unit who are sicker than the hospital down the street, and therefore my ARDS mortality rate is going to be higher because they're sicker, that means I'll have more patients whose FIO2 and PEEP go up because they're sicker and they're going to die, and I'm going to get dinged for that. Pay-for-performance is going to go against places like Duke, where we're taking the sickest of the sick.
Kacmarek:
Neil, as you're well aware, eventually, we're going to have to report everything we do, regardless of the circumstances. People want data to compare one institution to another and to national standards. Why? Because some of us do not perform as well as we could or should. This is just an evolution of being transparent about what we do. In your situation the number of ventilator days will be so high that the number of events as a percentage is going to be very low, and I doubt that in the long run it will be impacting you any differently than any other institution, unless you are doing a crummy job.
MacIntyre:
My number of VACs at Duke is going to be worse than at community hospitals in the area, mainly because we take their patients who are not doing well. If you take it at face value, the newspaper would say, “Duke Hospital has more VACs than the community hospital and therefore is a worse hospital.”
Kacmarek:
Let me ask you about those patients you get from the community hospitals. They're very sick and unstable and on high FIO2 and high PEEP. Do they have 48 hours of stability before you have to change anything? That's what has to happen for it to move along the path to eventually be called a VAE [ventilator-associated event] or VAC. Those patients you're talking about will not fit this definition, because they're too sick, they already have very high FIO2 and PEEP, and they won't meet the criteria, unless after they get better they develop a secondary process, and then they should be reported.
MacIntyre:
I think this group here ought to argue strongly for quality improvement assessments and monitoring. But I don't think this public reporting of potentially misleading information to bureaucrats is the way to go. I've probably belabored the point enough.
Marini:
I agree with you, Neil. Practicing in a busy referral hospital, we have a lot of people who get volume overloaded, who have heart attacks, who are older, et cetera. The initial admitting diagnosis is usually layered 4 or 5 problems deep, and they get intubated for who knows what reason. They may be stable for 48 hours, but a lot of things can destabilize them. Like most of our problems in intensive care, we have lousy definitions, and it will get us into trouble. Maybe not at this stage, but I agree with Neil that this could be onerous.
MacIntyre:
These numbers will make it look like our hospitals are the worst in the country.
Marini:
Unless there's some modification to what is reported.
Kallet:
I also agree. I'm not in favor of it. I'm certainly not in favor of calling it a VAC, and I made that clear on the CDC [Centers for Disease Control and Prevention] call I was on. I do think it's meant to try to get a better epidemiologic hold. Public reporting is going to be a huge thing. However, I think if we truly decrease VAP so there are no positive tracheal cultures associated with this, or whatever they require us to do, it turns out that most of these are sepsis or cardiogenic pulmonary edema or something that will wash out. Like anything else, it could blow up in our faces, and I think, politically, the critical care organizations need to circle the wagons on this and make it very clear that this could backfire.
Hess:
This discussion is interesting, because Bob and I are part of the group who started looking at this and thinking about it at Massachusetts General Hospital. I think it's fair to say that we did not think this would be a big problem for us at all, because the majority of our patients are extubated before the third day, so there will be very few patients who will ever make it this far.
MacIntyre:
What about the 30% of your ARDS patients who are going to die?
Kacmarek:
They represent a minute percentage of the patients we mechanically ventilate. When I look at the denominator, the number of days of mechanical ventilation, the number of reportable events is going to be small. I sit with the infection control nurses and try and sort through the VAP rates for the entire institution, and it is incredibly laborious, it's almost impossible to come up with anything objective in the current definition to really say with any certainty that it's a VAP. We're guessing every time we do it—every time we try to fit subjective findings into the current horrible definition.
Hess:
Correct me if I'm wrong, Bob, but I don't think we're concerned that we're going to get dinged. We're concerned about how difficult it is to track all this information.
MacIntyre:
It's going to be very difficult and costly, and it's going to have no benefit.
Branson:
Lorenzo, you're an expert in this.
Berra:
I love to watch the excitement of American politics. It's actually a beautiful discussion.
Branson:
Should I use a silver-coated ETT, and, if so, when? Should I use a subglottic tube, and if so, when? And what's your impression of the antibiotic-coated ETTs? Don't they just kill one group of bacteria while making it possible for others to proliferate?
Berra:
First of all, I would emphasize that, before any exotic preventive measures, a good starting point would be to implement the measures approved by the Institute for Healthcare Improvement: the famous bundle for VAP prevention. If we just followed that, we might be in a different position right now. Having said that, VAP is a multifactorial disease. There's not just one specific mechanism you can point out. For example: a coated ETT might be useful for patients ventilated for a short period, with whatever coating: silver, hydrogel, ultraviolet light. Over time, secretions deposit and colonization occurs. An ETT is not like a central venous catheter that's constantly flushed by the immune system or by antibiotics. The ETT does not have those characteristics. So cleanliness, basic nursing oral care, and good care of the patient, more than anything else, will avoid pneumonia. Overall, I think we cannot say that technology will prevent pneumonia; rather, it is the care of the patient.
Hess:
So should we abandon elevating the head of the bed for VAP prevention?
Berra:
No, I didn't say that. The semi-recumbent position may decrease the incidence of gastric reflux contaminating the lungs, compared to the supine position. However, I hypothesized that the lateral head down position may be even more beneficial, preventing secretions from entering the lungs and preventing pneumonia. A large randomized controlled trial is going to test this hypothesis. Ideally, with a patient who fainted and lost consciousness, we always put him or her in the recovery position (lateral position) for this reason, to prevent aspiration. Since the ETT cuff does not prevent aspiration and micro-leakage, one could keep these patients in the lateral position to prevent aspiration past the cuff.
Hess:
In my view, evidence for elevation of the head of the bed is not very strong; it's very weak, considering all the emphasis that's placed on it.
Berra:
Yes, the most important study is a Lancet paper in 1999.1
Hess:
This is anecdotal, which admittedly is the lowest level of evidence. A few years ago at Massachusetts General Hospital we went around every so often and checked if the head of the bed was elevated at least 30 degrees. An observation I made at the time was that the head of the bed was almost always elevated at least 30 degrees, but the head of the patient often was not.
Marini:
I'm surprised that the x-ray has nothing to do with the proposed definition. In our hospital the reported VAP rate has plummeted to nearly zero. And for many hospitals, as Neil suggested earlier, maybe it's an administrative redefinition, or whatever. But, clinically, I don't see pneumonia. I see plenty of fevers that don't pan out to be pneumonia. I'm wondering if maybe this VAP issue is something we get emotionally attached to rather than a really critical thing. We jump in with antibiotics very quickly, very early. How much morbidity is associated with that I don't know. In the post-extubation phase, that's when I get worried, because they're no longer being suctioned and they have impaired reflexes. You take the ETT out, and they probably have a slow recovery of the mucociliary escalator. Lorenzo, have any studies looked at the incidence of pneumonia in the post-extubation phase?
Berra:
A study from a large database should be published soon, of anesthesia patients who were otherwise healthy requiring surgery, so they had only a few hours of intubation. The idea was to track the number of rehospitalizations for lung infections after intubation in the operating room. A recent French study instead looked at attributable mortality for VAP in ICU patients.2 They studied many ICUs in France, and found ICU mortality attributable to VAP of about 1-1.5%.
Gajic:
What is the role of oral chlorhexidine in patients with tracheostomy? I'm trying to use chlorhexidine on mechanically ventilated patients with tracheostomy and I get pushback because there's no evidence for it. How do we prevent oral contamination in patients with tracheostomies? A tracheostomy tube is not going to completely abolish it, as you know.
Berra:
One caveat is that most of the studies on VAP prevention used 3% chlorhexidine, whereas at the bedside the most popular is 0.5%. Another interesting point is the relationship between dental plaque and VAP. Patients without teeth have a much lower incidence of VAP. So I think oral care is number one when it comes to bacterial challenge to the lungs, while the ETT is just the continuation of the dental plaque (the oral pathogens) inside the carina.
Kacmarek:
I'm assuming in the ICU that you interpret the chest x-ray yourself; you don't depend on radiology. If after the fact you review the chest x-ray reports over a period of time and try to make sense of what's going on with the patient, it's impossible. One chest x-ray is interpreted as pneumonia, the next is interpreted as lungs clear, the next as atelectasis. The interpretations make no clinical sense if they are made out of the context of the clinical situation. I spend about 2 hours a week going through this VAP interpretation stuff, and the chest x-rays frequently confuse the interpretation of the clinical data.
Marini:
Bob, don't all pneumonias have some identifiable infiltrate?
Kacmarek:
You would think chest x-rays taken at noon, at 2 o'clock, and at 4 o'clock would have very similar interpretations, but you'll have noon pneumonia, 2 o'clock clear lungs, and 4 o'clock the pneumonia's back. Not what I see when I look at a chest x-ray; I'm talking about the written interpretation you see when you follow the interpretations sequentially to try to determine by chest x-ray whether there has been a new pneumonia.
Marini:
But if you look at it?
Kacmarek:
That's a different story. But you can't look at every x-ray for every person who's mechanically ventilated in your institution.
Marini:
In a retrospective study, maybe.
Gajic:
Every chest x-ray called VAP by the surveillance nurse comes back to me for review, and I say, this is not working: it's every x-ray. So I'm taking them and saying, well, wait a second: there was infiltrate before the patient even came to the hospital. The admission x-ray had the same infiltrate. At least somebody who knows the clinical situation looks at it. Obviously, this is subjective, because I have a very different agenda than some other people on this.
Kacmarek:
And it's a different story, looking at the 5 cases per month in your unit that are under discussion, versus the 100-150 patients each month who are ventilated in your unit. It's impossible to do that.
Berra:
I would like to share an interesting observation from when I was working in the laboratory on a VAP animal model.3 From sheep and dogs with pneumonia we took biopsies from the atelectatic part of the lungs, from abscesses, and from lung areas that appeared nice and pink and inflated, and there was no difference in bacterial count from the different areas. Very interesting. We did about 20 biopsies in the atelectatic parts versus the non-atelectatic parts, and they had the same counts. So the chest x-ray can give you a false positive, because you see beautiful images, but the lungs are heavily colonized with pathogens.
Marini:
So where the x-ray clouds up in a focal area, it may be edema that was not present elsewhere?
Berra:
I don't know the mechanism, but what struck me was that the x-ray did not correlate with bacterial colonization. You might have “spared” areas of the lungs that indeed are extremely colonized anyway.