Abstract
BACKGROUND: Re-intubation is associated with high morbidity and mortality. There is limited information regarding the risk factors that predispose patients admitted to the surgical ICU to re-intubation. We hypothesized that preoperative comorbidities, acquired muscular weakness, and renal dysfunction would be predictors of re-intubation in the surgical ICU population.
METHODS: This was a prospective observational study in 2 surgical ICUs of a large tertiary hospital. All patients who were extubated during their surgical ICU stay were included. Demographic and clinical data were collected before and after extubation. The primary outcome was re-intubation within 72 h. Using multivariate logistic regression analysis, independent risk factors of re-intubation were determined, and a prediction score was developed.
RESULTS: Between December 1, 2012, and January 31, 2014, we included 764 consecutive subjects. Of these, 65 subjects (8.5%) required re-intubation. Independent risk factors of re-intubation were blood urea nitrogen level of >8.2 mmol/L (odds ratio [OR] 3.66, 95% CI 1.97–6.80), hemoglobin level of <75 g/L (OR 2.10, 95% CI 1.23–3.61), and muscle strength of ≤3 (OR 2.03, 95% CI 1.16–3.55). The presence of all 3 risk factors was associated with an estimated probability for re-intubation of 26.8%.
CONCLUSIONS: In noncardiac surgery, surgical ICU subjects, elevated blood urea nitrogen level, low hemoglobin level, and muscle weakness were identified as independent risk factors for re-intubation. The presence of these risk factors can potentially aid clinicians in making informed decisions regarding optimal airway management in patients considered for an extubation attempt. (ClinicalTrials.gov registration NCT01967056.)
Introduction
Over the last decade, a systematic approach for liberation from mechanical ventilation and tracheal extubation, including spontaneous awakening and breathing trials, has been recommended and has resulted in decreased duration of mechanical ventilation.1–4 The incidence of extubation failure reported in the literature varies from 6 to 40%.5–19 Re-intubation in nonsurgical critically ill patients is associated with increased mortality5,9,13,14,16–18,20 and morbidity, including prolonged mechanical ventilation support,9,14 ventilator-associated pneumonia,13,14,21 organ failure,13,14 and prolonged length of stay (LOS).5,9,14–17 The most common reason for re-intubation reported is respiratory distress due to either primary respiratory failure or secondary causes, such as congestive heart failure, hemodynamic instability, airway obstruction from postextubation stridor or airway edema, inability to clear airway secretions, or altered mental status.6,7,11,13–17,19,20,22
In critically ill patients, some predisposing factors for re-intubation have been proposed in the literature. Acquired muscle weakness is associated with aspiration,23 prolonged weaning from mechanical ventilation, and weaning failure.22,24–26 The high mortality seen in critically ill patients with renal failure has been attributed to the need for mechanical ventilation,27 and renal failure per se has been identified as an independent risk factor for prolonged mechanical ventilation and weaning failure.27–30 In surgical patients, the etiologies leading to tracheal intubation and mechanical ventilation might, in some aspect, differ from those of medical patients. We recently developed and validated the score for prediction of postoperative respiratory complications.31 This score predicts the risk of extubation failure following surgery based on the patient's comorbidities and the acuity of disease leading to surgery, including the American Society of Anesthesiologists physical status, emergency surgery, high-risk surgical service, history of congestive heart failure and chronic pulmonary disease. The score ranges from 0 to 11, with a higher score predicting a higher likelihood for postoperative re-intubation. Nevertheless, the predictors for re-intubation in the critically ill surgical population have not been studied in detail. We therefore aimed to determine the risk factors for re-intubation in the surgical ICU population. We hypothesized that preoperative comorbidities, acquired muscular weakness, and renal dysfunction would be predictors of re-intubation in the surgical ICU population. Additionally, we sought to develop a score predicting re-intubation in this patient population.
QUICK LOOK
Current knowledge
The incidence of extubation failure reported in the literature varies from 6 to 40%. Re-intubation in nonsurgical critically ill patients is associated with increased mortality and morbidity. In medical patients, the most common reasons for re-intubation are respiratory distress due to either primary respiratory failure or secondary causes such as congestive heart failure, hemodynamic instability, airway obstruction, inability to clear airway secretions, or altered mental status.
What this paper contributes to our knowledge
In a surgical ICU population, 8.5% of subjects required re-intubation. Independent risk factors of re-intubation were elevated blood urea nitrogen level, low hemoglobin, and decreased muscle strength. These parameters have been included in a prediction score for re-intubation.
Methods
Setting
This prospective observational study was approved by the institutional review board of Massachusetts General Hospital (Boston, Massachusetts). The study was conducted between December 1, 2012, and January 31, 2014, in 2 surgical ICUs at Massachusetts General Hospital, which is a university-affiliated, tertiary care referral and level-1 trauma center.
Patient Population
All adult patients admitted to these 2 surgical ICUs were eligible for enrollment if they were intubated and required mechanical ventilation during their surgical ICU stay. The patient care plan as well as the decision to liberate from mechanical ventilation and to extubate were directed by the surgical ICU team. Per standardized protocol,3 spontaneous awakening and spontaneous breathing trials were performed in each patient. The surgical ICU team then decided whether to extubate the patient. Once the patients were extubated, they were included in the study and were followed for up to 30 d or until discharge from the hospital. Patients with terminal extubation (extubation with comfort measure orders in place), those who died without an extubation attempt, and those with tracheostomy placement without an extubation attempt were excluded from the study.
Data Collection
After a review of the literature, we held multidisciplinary focus groups with surgical and medical intensivists, respiratory therapists, physical therapists, and surgical ICU nurses to pre-identify potential predictors for respiratory failure after extubation, which included demographics, underlying and acute illness, and subjects' clinical profiles assessed during the 24 h before extubation.
Demographic data and acute illness included age, gender, body mass index (BMI), past medical history, type of surgical ICU admission (whether following elective or emergency surgery or no surgery), APACHE II (Acute Physiology and Chronic Health Evaluation) score, laboratory values at surgical ICU admission, the development of acute kidney injury defined as the RIFLE (risk, injury, failure, loss, and end-stage kidney) classification of 1 or higher,32 and duration of mechanical ventilation. The score for prediction of postoperative respiratory complications,31 the American Society of Anesthesiologists physical status, and sites of surgery were additionally recorded in subjects admitted to the surgical ICU following surgery. For non-postoperative subjects, reasons for intubation were determined and recorded.
Subjects' clinical profiles obtained during the 24 h before extubation included the mechanical ventilation settings before the spontaneous breathing trial, oxygen saturation, PaO2/FIO2, results of the spontaneous breathing trial and cuff leak test performed by the bedside respiratory therapists, number of tracheal suctions needed, muscle strength before extubation, fluid balance, and medications administered. In our surgical ICUs, the muscle strength of the extremities was routinely assessed by the bedside nurses and scored ranging from 0 to 4 as no visible movement of limb, inability to overcome gravity but ability to move in the plane of the supported limb, ability to overcome gravity but not resistance, ability to overcome both gravity and resistance, and normal motor power, respectively. The average muscle strength over 4 extremities was recorded. This scale was tested for inter-rater agreement with the Medical Research Council (MRC) sum score33 in 50 randomly selected subjects by a well-trained physical therapist (KW) who was blinded from the study. The MRC sum score has been widely used for assessing muscle strength. In brief, the score is the sum of the muscle strength grading scale comprehensively tested on 6 muscle groups on both limbs, including deltoid, biceps, wrist extensor, iliopsoas, quadriceps femoris, and tibialis anterior. The scale ranges from 0 (paralysis) to 5 (normal strength); therefore, the sum score ranges from 0 to 60.33 The medications recorded included neuromuscular blocking agents, neostigmine, opioids, hypnotics, steroids, and vasopressors.
Outcomes
As per the protocol of the surgical ICU, subjects were started on face mask oxygen after extubation and were then weaned to nasal cannula if the oxygenation remained above the threshold prescribed by the clinicians. The primary end point for this study was re-intubation within 72 h after extubation. Subjects who required intubation within this time frame for a surgical procedure were not considered as receiving re-intubation. Reasons for re-intubation were also determined and recorded. The secondary end points were LOS in the surgical ICU and in the hospital as well as in-hospital mortality. We also recorded respiratory status following extubation, including FIO2 requirement, PaO2/FIO2, and oxygen saturation; the use of noninvasive ventilation (NIV); either bi-level positive airway pressure or CPAP for either prevention or treatment of extubation failure; and tracheostomy placement. At our hospital, there were 24/7 in-house surgical ICU physicians. The strategy for management of failing subjects was based on the protocol of our surgical ICU. For subjects with hypercarbic respiratory failure, NIV was the first line treatment. If no significant improvement was observed, subjects were endotracheally intubated. Subjects with hypoxic respiratory failure were directly re-intubated. Nevertheless, the final decision regarding initiation of NIV as well as the timing of tracheostomy placement was made by the ICU attending.
Statistical Analysis
Subjects were categorized into 2 groups based on whether they required re-intubation or not. Categorical variables were presented as a number with proportion and were compared between groups using the Pearson chi-square or the Fisher exact test when appropriate. Continuous variables were presented as median with interquartile range (IQR) and were compared between groups using the Mann-Whitney U test. The inter-rater agreement between the muscle strength scale assessed by the bedside nurse and the MRC sum score evaluated by the physical therapist was tested using the Cohen kappa coefficient. To determine the independent risk factors of re-intubation, the predefined variables, including age, sex, chronic pulmonary and cardiovascular diseases, severity of illness, and duration on mechanical ventilation, as well as significant variables from the univariate analysis were further analyzed using multivariate logistic regression analysis in a backward stepwise fashion. The final model included only variables with a P value of <.05. If continuous variables remained in the model, optimal cut-off points based on the receiver operating characteristic curves were specified using the Youden index. A point value was assigned to each variable proportional to the estimates from the multivariate logistic regression model. For this, the β estimate of each predictor was divided by the smallest estimate. These results were rounded to the nearest whole number to define the score point values. The predictive value of the score for re-intubation was assessed using analysis of the area under the receiver operating characteristic curve. The probabilities for re-intubation depending on the score values were also determined. All statistical tests were performed using SAS 9.2 (SAS Institute, Cary, NC). A 2-sided P value of <.05 was considered statistically significant.
Results
Study Cohort and Patients' Characteristics
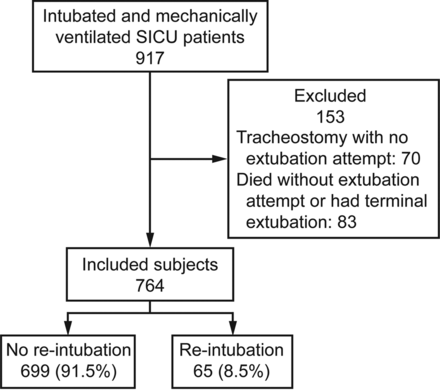
During the study period, 917 consecutive patients were eligible for enrollment (Fig. 1). Of these, 153 patients were excluded (70 patients underwent tracheostomy placement without extubation attempt, and 83 patients died without an extubation attempt), leaving 764 subjects included in the study. Overall, 65 of 764 subjects (8.5%) required re-intubation.
Flow chart. SICU = surgical intensive care unit.
Table 1 shows the demographic data and acute illness of subjects with and without re-intubation. There was no significant difference in the baseline demographic data between groups. There was a higher proportion of non-postoperative surgical ICU admission (32.3% vs 21.5%, P = .045) but a lower proportion of surgical ICU admission after elective surgery (32.3% vs 48.8%, P = .01) in the re-intubation group compared with that in the no re-intubation group. Subjects requiring re-intubation had higher APACHE II scores (median 17 [IQR 13–22] vs 15 [11–19], P = .003), lower hemoglobin level (72 [69–81] g/L vs 81 [72–96] g/L, P < .001), lower serum albumin (30 [24–37] g/L vs 33 [27–40] g/L, P = .03), higher blood urea nitrogen level (BUN) (12.5 [8.2–22.1] mmol/L vs 7.1 [4.6–13.6] mmol/L, P < .001), and higher serum creatinine (129.1 [79.6–229.8] μmol/L vs 93.7 [66.3–155.6] μmol/L, P = .002) compared with those without re-intubation. In addition, re-intubated subjects more frequently carried the diagnosis of acute kidney injury (53.8% vs 34.6%, P = .002) and had longer duration of mechanical ventilation (median 73 [IQR 30–146] h vs 31 [18–68] h, P < .001) compared with those who were not re-intubated.
Demographics and Underlying and Acute Illness of Subjects With and Without Re-Intubation
Among 593 subjects admitted to the surgical ICU after either elective or emergency surgery, there were 44 subjects (7.4%) who required re-intubation (Table 1). When compared with subjects without re-intubation, those who required re-intubation had more frequent vascular site of surgery (29.5% vs 15.8%, P = .02), more frequent American Society of Anesthesiologists physical status of ≥3 (92.3% vs 79.1%, P = .01), and a higher score for prediction of postoperative respiratory complications (median 7 [IQR 5–8] vs 5 [3–7], P < .001). Meanwhile, there were 21 (12.3%) of 171 non-postoperative subjects who required re-intubation (Table 1). Within this subgroup, re-intubated subjects had a higher frequency of respiratory failure as a reason for intubation when compared with those without re-intubation (57.1% vs 32.0%, P = .03).
Subjects' Clinical Profiles Assessed During 24 h Before Extubation
Table 2 shows the subjects' profiles assessed during the 24 h before extubation. There was no difference in mechanical ventilation settings before the spontaneous breathing trials between groups. When compared with subjects without re-intubation, re-intubated subjects had lower oxygen saturation before extubation (median 98 [IQR 97–99]% vs 99 [98–100]%, P = .006). However, there was no significant difference in PaO2/FIO2 between groups. There was also no significant difference in the rate of failed spontaneous breathing trial and negative cuff leak test between groups. Re-intubated subjects had lower muscle strength when compared with those without re-intubation (median 3 [IQR 2–3] vs 3 [3–4], P < .001). The average muscle strength assessed by the bedside nurses had a 46.0% inter-rater agreement and a 0.25 Cohen kappa coefficient with the MRC sum score (validated in 50 randomly selected subjects). Regarding the medication administered during 24 h before extubation, re-intubated subjects more frequently received sedative medications (haloperidol, 38.5% vs 16.9%, P < .001; dexmedetomidine, 30.8% vs 14.9%, P = .001; quetiapine, 12.3% vs 4.7%, P = .18) but less frequently received neuromuscular blocking agents (29.2% vs 41.8%, P = .049). However, there was no significant difference in the frequency of reversal with neostigmine between groups (4.6% in re-intubation group vs 1.1% in no re-intubation group, P = .059).
Clinical Profiles Assessed During 24 h Prior to Extubation of Subjects With and Without Re-Intubation
Predictors for Re-Intubation and Prediction Scores
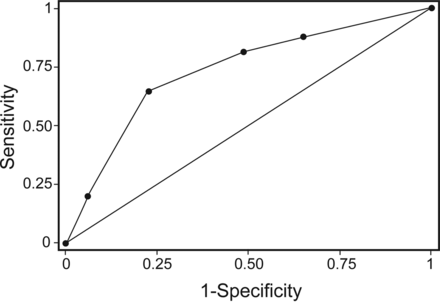
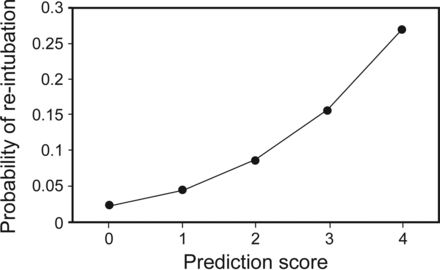
Using a stepwise regression model, 3 variables were identified as independent predictors for re-intubation, and the optimal cutoff points were specified for these predictors. Independent risk factors of re-intubation were BUN of >8.2 mmol/L (OR 3.66, 95% CI 1.97–6.80), hemoglobin level of <75 g/L (OR 2.10, 95% CI 1.23–3.61), and muscle strength of ≤3 (OR 2.03, 95% CI 1.16–3.55) (Table 3). A point value was then assigned to each variable proportional to the estimates from the logistic regression model: BUN of >8.2 mmol/L, 2 points; hemoglobin level of <75 g/L, 1 point; and muscle strength of ≤3, 1 point (Table 3). The summed point values of the developed score ranged from 0 to 4. The score led to an area under the receiver operating characteristic curve of 0.73 (Fig. 2), and a value of 0, 1, 2, 3, and 4 was associated with an estimated probability for re-intubation of 2.3, 4.5, 8.5, 15.6, and 26.8%, respectively (Fig. 3).
Variables Independently Associated With Re-Intubation and Prediction Scores
Receiver operating characteristic curve of the developed prediction score for re-intubation. Area under the curve = 0.73.
Estimated probabilities for re-intubation according to different prediction scores.
Subjects' Status Following 72-h Postextubation and Clinical Outcomes
While requiring higher FIO2 after extubation (median 0.36 [IQR 0.24–0.42] vs 0.21 [0.21–0.24], P < .001), re-intubated subjects had lower PaO2/FIO2 compared with those without re-intubation (169 [109–228[ mm Hg vs 279 [203–371] mm Hg, P < .001). These subjects also had more frequent oxygen desaturation after extubation (72.3% vs 28.5%, P < .001), and NIV was more frequently applied (40.0% vs 9.2%, P < .001). When compared with subjects without re-intubation, those with re-intubation had a higher rate of tracheostomy placement (60.0% vs 2.7%, P < .001), longer LOS in the surgical ICU (median 16 [12–26] d vs 6 [4–9] d, P < .001) and in the hospital (31 [22–44] d vs 16 [9–27] d, P < .001), and higher hospital mortality (20.0% vs 5.7%, P < .001).
There were a total of 90 (11.8%) subjects who required NIV after extubation. Of these 90 subjects, 26 (28.9%) required re-intubation. Subjects who required re-intubation following an NIV attempt, when compared with those who did not require re-intubation, had a higher rate of tracheostomy (61.5% vs 4.7%, P < .001), longer surgical ICU LOS (median 18 [IQR 11–24] d vs 8 [5–15] d, P < .001), longer hospital LOS (30 [23–45] d vs 18, 11–37 d, P = .002), and a trend toward higher mortality (26.9% vs 14.1%, P = .22). Among 65 re-intubated subjects, the median time (IRQ) to re-intubation was 21.3 (7.3–43.0) h after extubation. Of these, 21 (32.3%) and 36 (55.4%) were re-intubated within 12 and 24 h, respectively. The reasons for re-intubation were classified as respiratory distress (70.8%), secretion-related problems (18.5%), and airway-related problems (10.8%).
Discussion
The most important results in our prospective study were that high BUN, low hemoglobin level, and muscle weakness were identified as independent predictors for re-intubation in a large population of noncardiac surgical ICU subjects. The presence of all 3 parameters was associated with a 26.8% probability of re-intubation, a 10-fold higher probability compared with those without any predictor. In studies in medical, neurological, or mixed ICUs, many variables have been identified as risk factors for extubation failure and re-intubation, such as age and underlying diseases,14 severity of acute illness,11,12,19 impairment of oxygenation,6,18 the amount of secretions,7,8,10,19 cough strength,7,8,12 or mental status.6,8,10,15 These variables were included in our model. However, they were not independent predictors of re-intubation in our study. This might be explained, in part, by the difference in patient population. We included only mechanically ventilated subjects in the surgical ICUs, whereas most other studies concentrated either on medical7,8,14,18 or neurological ICU subjects.6,11
In our study, increased BUN level was identified as an independent predictor for re-intubation. Elevation of BUN levels is associated with increased long-term mortality.34,35 In most critically ill patients, such abnormal values are often from renal dysfunction, mainly acute kidney injury. Both acute kidney injury and elevated BUN have been identified as independent risk factors for prolonged mechanical ventilation and weaning failure.27–30 The results of these earlier studies support our findings that increased BUN was an independent risk factor for re-intubation.
More than one-third of critically ill subjects develop anemia during their hospitalization, and this has been considered as a marker of severity of illness.36–38 In addition, hemoglobin levels of <80 g/L have been reported as a risk factor for weaning failure and re-intubation.7,39 However, current practice guidelines on liberation from mechanical ventilation have not recommended the optimal hemoglobin levels for weaning and extubation.3,4 Nowadays, there is growing evidence demonstrating that a restrictive transfusion strategy with a lower hemoglobin transfusion trigger is not associated with increased morbidity and mortality in adult subjects or that it is even associated with better outcome in some subjects.40–43 Nevertheless, we cannot conclude from our study that a hemoglobin level of <75 g/L should be a trigger for blood transfusion in mechanically ventilated patients undergoing weaning from mechanical ventilation, but rather that it was an independent predictor for re-intubation.
Muscle weakness was one of the independent predictors for re-intubation in our study. The incidence of ICU-acquired weakness ranges from 25 to 50%, depending on the patient population and measures used for diagnosis.22,44,45 Multiple predisposing factors can potentiate ICU-acquired weakness in critically ill patients, such as inflammation, infection, consequence of drug effects, immobilization, and malnutrition.46 ICU-acquired weakness was associated with prolonged weaning from mechanical ventilation24 and weaning failure.22 Weakness in respiratory muscles, as indicated by low maximal inspiratory and expiratory pressure or diaphragmatic dysfunction on ultrasonography, is associated with delayed successful extubation as well as weaning failure.25,26
The MRC sum score33 has been used to comprehensively assess muscle strength47,48 as well as to predict morbidity and mortality in critically ill subjects.45 In our study, we used the muscle strength assessed by bedside nurses, and we validated this easily applicable scale with the MRC sum score in our cohort and found a fair agreement (46.0% agreement and 0.25 Cohen kappa coefficient). Nevertheless, we did not test for the inter-observer reliability for the muscle strength scale assessed by the bedside nurses. Therefore, we could not completely exclude observer bias. Despite this, this scale has been implemented in our units for >10 y, and all nurses in our units are well trained and familiar with this task as part of their daily routine. The MRC sum score, on the other hand, might have some limitations when performed in critically ill patients, polytrauma patients, or those with altered mental status.45,49 In addition, the MRC sum score cannot be applied simply in daily practice, since the dedicated clinical exam is time-consuming and requires an expert level of experience.45,49,50 The interesting point is that muscle weakness is difficult to reliably quantify: Small differences in the clinical testing procedure lead to meaningful differences in results. Moreover, when talking about a weak patient, health-care providers of different professions may not always have the same clinical picture in mind and draw different conclusions about what to do about their patient's weakness based on nosological misunderstandings. Interestingly, our data indicated that nursing assessment of muscle weakness, which could be performed easily and as an alternative to the MRC sum score, predicted the success of tracheal extubation.
Nowadays, NIV is increasingly used to facilitate early extubation as well as to prevent and manage failed extubation.51,52 In our study, the rate of NIV used after extubation was 12%, and of these, 29% required re-intubation. Our study demonstrated that subjects who required re-intubation following a failed NIV attempt had a higher rate of morbidity and mortality than those who did not require re-intubation. One important concern is that the clinicians should have some experience in using NIV, especially when used for management of extubation failure. The patient's respiratory status should be closely monitored, and, if required, re-intubation should not be delayed.
As demonstrated in this present study and others,5,9,13,14,16–18,20 extubation failure and re-intubation is strongly associated with increased morbidity and mortality. On the other hand, delayed extubation is associated with worse outcomes, including pneumonia, LOS in ICU and in hospital, and mortality.53 It is therefore important not to delay extubation in patients who meet validated criteria of extubation readiness, such as a successful completion of a spontaneous breathing trial. We strongly recommend clinicians to weigh risks versus benefits between delayed extubation and premature extubation and subsequent re-intubation. Our prediction scores for re-intubation, which incorporated parameters that were assessed daily in our subjects, should aid clinicians in identifying those patients with a high risk of extubation failure and in preparing any measures or interventions toward prevention, or treatment if it occurs.
Our study had several limitations. It was an observational study in surgical ICUs of a single large academic medical center. It is unclear whether the results are applicable to different settings, such as a medical ICU. Unlike medical ICU patients, surgical ICU patients usually require a shorter duration of mechanical ventilation support. It was therefore the purpose of the present study to determine the risk factors for re-intubation in the surgical ICU population. Although we included many patient and treatment factors in our analysis, our final model consisted of only 3 factors that explain up to 27% of re-intubations. There might be significant factors that influence re-intubation but have not been included in our model. The relatively low incidence of re-intubation (8.5%) in our study may result in an inadequate power for our prediction scores, and we did not validate in an external cohort. The muscle strength, which was included in our prediction scores, was considered as a subjective test. Nevertheless, unlike the MRC sum score, the muscle strength assessment by bedside nurses can be easily performed in our daily routine care. We included all tracheally intubated and mechanically ventilated subjects who proceeded to extubation regardless of the weaning time (eg, simple, difficult, and prolonged weaning). If we had included only patients with difficult and prolonged weaning and complicated postoperative patients, the results might have been different. Finally, at the time of the study performed, high flow nasal oxygen had not been implemented in the protocol following extubation. High flow nasal oxygen has been shown to prevent re-intubation in several patient populations, including cardiac surgical and medical patients.54,55 This might also hold true for a general surgical patient population.
Conclusions
We identified high BUN, low hemoglobin level, and muscle weakness as independent risk factors for re-intubation in surgical ICU subjects. We also provided simple prediction scores for re-intubation, which incorporated parameters that are routinely assessed in ICU patients. The presence of these risk factors can potentially aid clinicians in the decision process around extubation.
Footnotes
- Correspondence: Ulrich H Schmidt MD PhD MBA, 200 West Arbor Drive #8770 San Diego, CA 92103. E-mail: uschmidt{at}ucsd.edu.
Dr Williams presented some parts of this work as a poster presentation at the 43rd Critical Care Congress of the Society of Critical Care Medicine, held January 10, 2014, in San Francisco, California.
The authors have disclosed no conflicts of interest.
- Copyright © 2016 by Daedalus Enterprises