Abstract
BACKGROUND: There is little evidence in the medical literature to guide empiric treatment of pediatric patients with long-term tracheostomies who present with signs and symptoms of a bacterial respiratory infection. The overall goal of this study was to describe the respiratory microbiology in this study population at our institution.
METHODS: This study was a retrospective chart review of all subjects with tracheostomies currently receiving care at the Arkansas Center for Respiratory Technology Dependent Children. Descriptive statistics were used to describe the respiratory microbiology of the full study group. Several subgroup analyses were conducted, including description of microbiology according to time with tracheostomy, mean time to isolation of specific organisms after the tracheostomy tube was placed, association between Pseudomonas aeruginosa or methicillin-resistant Staphylococcus aureus isolation and prescribed antibiotic courses, and description of microbiology according to level of chronic respiratory support. Available respiratory culture results up to July 2011 were collected for all eligible subjects. Descriptive statistics were used to describe subject characteristics, and chi-square analysis was used to analyze associations between categorical data. P < .05 was considered statistically significant.
RESULTS: A total of 93 subjects met inclusion criteria for the study. The median (interquartile range) age at time of tracheotomy was 0.84 (0.36–3.25) y, and the median (interquartile range) time with tracheostomy was 4.29 (2.77–9.49) y. The most common organism isolated was P. aeruginosa (90.3%), with Gram-negative organisms predominating. However, 55.9% of the study population had a respiratory culture positive for methicillin-resistant S. aureus. The first organism isolated after tracheostomy placement was Methiciliin-sensitive S. aureus was isolated the soonest after tracheostomy placement. Specific organisms were not related to level of chronic respiratory support or likelihood of receiving antibiotics.
CONCLUSIONS: This study provides an updated overview of the variety of potential pathogens isolated from respiratory cultures of pediatric subjects with long-term tracheostomies.
Introduction
The tracheobronchial tree of patients with long-term tracheostomies commonly becomes colonized with bacteria that may increase the likelihood of developing exacerbations of respiratory symptoms. There is not a recent description of the respiratory microbiology in children with long-term tracheostomies. Brook1 published a study in 1979 describing the respiratory microbiology of pediatric subjects with tracheostomy tubes in place for <1 y, concluding that Pseudomonas aeruginosa and other Gram-negative bacilli were the most commonly isolated bacteria. Niederman et al2 published a similar study in 1984, which included adults with tracheostomies, and found similar results, with Gram-negative bacilli the most commonly isolated organisms.
There is also little published data regarding the relationship between length of time with tracheostomy and first appearance of respiratory pathogens. Understanding this relationship may help to guide empiric therapy in a patient with a long-term tracheostomy presenting with signs and symptoms of a bacterial respiratory infection. Although controversy remains regarding the best approach to identifying and treating respiratory infections in pediatric patients with tracheostomies, having an understanding of which respiratory pathogens are most common may improve the empiric selection of antibiotic therapy until respiratory culture results are available. With this in mind, the primary aim of this study was to describe the respiratory microbiology of a group of pediatric subjects with long-term tracheostomies.
QUICK LOOK
Current knowledge
The most recent descriptions of the microorganisms isolated from respiratory cultures of subjects with long term tracheostomies describe primarily Gram-negative bacteria. However, over the past several decades, the epidemiology of infection in the United States has changed, with the number of Staphylococcus aureus infections increasing.
What this paper contributes to our knowledge
Pseudomonas aeruginosa was the most common organism isolated from respiratory cultures of children with long-term tracheostomies. However, S. aureus, both methicillin-susceptible and methicillin-resistant, was also isolated from approximately half of study subjects.
Methods
This study was a retrospective chart review of all subjects with tracheostomy tubes currently followed by the Arkansas Center for Respiratory Technology Dependent Children. It was reviewed and approved by the institutional review board. Because it was a retrospective chart review, the institutional review board waived the informed consent requirement. All patients with a tracheostomy tube in place for ≥1 y and currently being followed by the Arkansas Center for Respiratory Technology Dependent Children at the time of chart review in 2011 were considered eligible. For purposes of this study, we considered a tracheostomy tube in place for ≥1 y to be long-term.
Usual clinical practice at this center is at the physician's discretion. Patients are seen at least annually, with appointments every 3–6 months, depending on clinical status. Treatment is not based on guidelines because there are little data in the literature regarding best practices for care of children with long-term tracheostomy tubes in place. Depending on needs, our patient population utilizes several different techniques for airway clearance, including high-frequency chest wall oscillation, cough assist devices, intrapulmonary percussive ventilators, and manual chest physiotherapy. Patients generally receive heated humidification at night and heat and moisture exchangers during the day. Some may use a speaking valve during the day. The clinic does not have protocols in place for surveillance cultures or prophylactic antibiotics, and this is also at physician discretion.
Study Aims
The overall goal of this study was to describe the respiratory microbiology of a group of pediatric subjects with long-term tracheostomies. To further describe the relationship between clinical status and respiratory cultures, we also included several subgroup analyses. These included a description of the proportion of the study population with specific microorganisms on respiratory culture according to time with tracheostomy, mean time to isolation of specific microorganisms after the tracheostomy tube was placed, association between P. aeruginosa or methicillin-resistant Staphylococcus aureus (MRSA) isolation and prescribed antibiotic courses, and proportion of the study population with specific microorganisms according to level of chronic respiratory support.
Procedures
Data reviewed included general demographics, age at the time the tracheostomy tube was placed, primary diagnosis leading to the need for tracheostomy, respiratory culture results, and respiratory care regimen. Respiratory cultures reviewed for the study included cultures that were collected at routine out-patient clinic visits and during hospitalizations with a time period ranging from after the tracheostomy tube placement to the time of chart review. Cultures were obtained by suctioning with sterile technique, and saline was used. A positive culture was defined as growth of an organism that can cause infection.
Statistical Analysis
Statistical analyses for the full cohort and subgroups were conducted for the multiple aims, respectively. In total, 93 subjects were included in the study. P < .05 was considered statistically significant for all analyses. All means are presented with SD values. All medians are presented with interquartile ranges. SigmaStat 3.5 was used for all analyses (Aspire Software International, Point Richmond, California). Descriptive statistics, such as percentages, mean and SD, or median and interquartile range, were used to describe the cultured microorganisms in the full cohort of study subjects at any time since tracheostomy placement (N = 93).
For subgroup analysis 1, study subjects with a tracheostomy placed in 2007 or later were utilized (n = 45). This time period was chosen to allow several years of data that were still relatively recent and thus more clinically relevant. The purpose of this analysis was to explore how soon after the placement of a tracheostomy tube various microorganisms appeared on respiratory culture. For this group, the median time to the appearance of specific microorganisms was determined using a log-rank test for comparison of 2 survival distributions.
For subgroup analyses 2 and 3, study subjects with culture results from 2010 were utilized because this was the most recent full year of data (n = 82). The purpose of subgroup analysis 2 was to explore the relationship between time with the tracheostomy tube and respiratory culture results. For this analysis, subjects were divided into cohorts based on length of time with tracheostomy, and the percentage of subjects with certain microorganisms was determined. The purpose of subgroup analysis 3 was to explore the relationship between growth of certain microorganisms and need for antibiotics. For this analysis, the association between growth of P. aeruginosa or MRSA and antibiotic courses was explored by chi-square analysis.
For subgroup analysis 4, subjects with a clinic visit and corresponding respiratory culture in 2010 were utilized to explore the association between level of chronic respiratory support and growth of specific microorganisms (n = 66). Because the level of respiratory support fluctuates over time, for subjects who had more than one eligible clinic visit, data from only the first visit was included in the analysis. The classification of the level of chronic respiratory support was determined by using the Respiratory Management Score scale as previously described by Blucker et al.3 The Respiratory Management Score was determined for each subject from medical record documentation at pulmonary clinic visits in 2010. All children recruited for this study were levels 2–4. Briefly, level 2 includes children with a tracheostomy tube in place, level 3 includes children on a mechanical ventilator at night only, and level 4 includes children on a mechanical ventilator 24 h/d. The association was explored by chi-square analysis.
Results
A total of 109 charts were reviewed. Ninety-three subjects (N = 93) met initial inclusion criteria. Respiratory cultures that were reviewed spanned a time period from 1995 to 2011. The median follow up time was 4.3 (2.8–9.5) y. A total of 13 different pathogens appeared on respiratory cultures. The most common were P. aeruginosa, Stenotrophomonas maltophilia, Serratia marcescens, Moraxella catarrhalis, MRSA, methicillin-sensitive S. aureus, and Haemophilus influenza.
Table 1 gives characteristics of the total study population and subgroups, and Table 2 shows the primary diagnoses for the total study population. Table 3 shows the proportion of the full cohort of study subjects who have specific isolated microorganisms on respiratory culture at any time since tracheostomy placement.
Demographics of Study Population
Underlying Diagnoses That Led to Need for Respiratory Support (N = 93)
Proportion of Study Subjects With Specific Microorganism on Respiratory Culture at Any Time Since Tracheostomy Placement Between 1995 and 2011 (N = 93)
The mean times to isolation of microorganisms after tracheostomy tube placement are presented in Table 4. There were 45 subjects who had a tracheostomy tube placed in 2007 or later and were included in this analysis. The prevalence of various organisms among subjects included in Table 4 was similar to that in the overall study population. Methicillin-sensitive S. aureus had the shortest time to isolation at a median of 1.65 (interquartile range 0.5–2.4) months post-tracheostomy placement, followed by MRSA at a median of 4.1 (interquartile range 0.7–7.9) months. There was a longer time to isolation for Gram-negative organisms, such as P. aeruginosa.
Subgroup Analysis 1 (n = 45): Median Time to First Isolation of Microorganisms After Tracheostomy Tube Placement
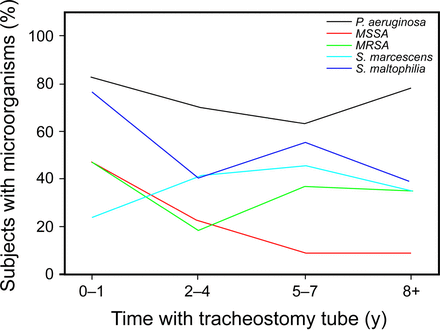
Figure 1 shows the percentage of the study population with certain microorganisms on respiratory culture in 2010 according to range of time with tracheostomy tube. A total of 82 subjects had culture results in 2010. The most abundant microorganism identified was P. aeruginosa (>50%) in all groups. Methicillin-sensitive S. aureus was most prevalent in the 0–1 y group and declined as the number of years after tracheostomy tube placement increased. The prevalence of MRSA increased as that of methicillin-sensitive S. aureus decreased. The relationship between the growth of P. aeruginosa or MRSA and antibiotic therapy in this group was also analyzed. There were 82 subjects with culture results in 2010, with 38 subjects receiving at least one course of antibiotics as an out-patient and 46 subjects as an in-patient in that same year. There were 18 subjects who did not receive any antibiotics in 2010. Of this group of 82, P. aeruginosa was isolated from 59 subjects, and MRSA was isolated from 24. There was not a statistically significant relationship between growth of P. aeruginosa or MRSA in 2010 and whether subjects received antibiotic therapy in that same year. Specifically, growth of P. aeruginosa was not associated with whether the subject received out-patient antibiotics (P = .68), in-patient antibiotics (P = .43), or any antibiotics (P = .36). Similar results were observed for MRSA (P = .85, P = .32, and P = .30, respectively).
Comparison of subjects with microrganisms and length of time with tracheostomy tube (n = 82). MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive S. aureus.
Figure 2 shows the prevalence of pathogens based on the level of chronic respiratory support required. A total of 66 subjects had pulmonary clinic visits with a concurrent respiratory culture in 2010. All 66 subjects had positive culture results (ie, an organism with the potential to cause infection was isolated). There were no statistically significant differences by chi-square analysis in the incidence of specific pathogens by Respiratory Management Score.
Comparison between microrganisms and Respiratory Management Score (RMS). MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive S. aureus.
Discussion
In this group of pediatric subjects with long-term tracheostomy, Gram-negative organisms were the most common on respiratory culture, with P. aeruginosa appearing within a year of the tracheostomy placement. This was consistent regardless of length of time with tracheostomy. Gram-negative organisms predominated and, in addition to P. aeruginosa, included S. maltophilia, S. marcescens, and M. catarrhalis. S. aureus was present in about half of the study population, with MRSA present in the majority of subjects and more common than methicillin-sensitive S. aureus over time. There was no relationship between the level of respiratory support, as indicated by the Respiratory Management Score, and specific organisms on respiratory culture. There was no association between growth of P. aeruginosa or MRSA and likelihood of receiving a course of antibiotics in the same year.
Collecting routine surveillance respiratory cultures in patients with long-term tracheostomy may not be helpful in predicting the causative organism in an individual with signs and symptoms of a respiratory infection,4 and practices vary regarding when to collect respiratory cultures and when to treat with antibiotics.5 To date, there are no published guidelines for the diagnosis and management of respiratory infections in pediatric patients with long-term tracheostomies. Although there is not an expert consensus on the signs and symptoms that indicate a bacterial respiratory infection in this population, when patients experience signs and symptoms that are not relieved by increased airway clearance, empiric antibiotics may be initiated in out-patient or in-patient settings. Utilizing overall patterns of microbiology cultures over time to inform empiric coverage may increase the likelihood of adequate antibiotic coverage before respiratory culture results are available. Based on our results, it would seem reasonable to consider empiric coverage for P. aeruginosa and MRSA with modification of therapy when culture results are available.
It has not been established whether the presence of organisms in the airway contributes to a decline in respiratory health in patients with a long-term tracheostomy tube in place. We did not find a relationship between type of organism and either the level of respiratory support required or the need for antibiotic therapy. A relationship between microorganisms and severity of lung disease would need to be further explored with more specific measures.
Several studies have established that subjects with long-term tracheostomies become chronically colonized with bacteria that may lead to serious illness and complications.1,2,6 However, there are no studies describing the relationship between the length of time with tracheostomy and the spectrum of bacterial growth. Understanding the spectrum of bacteria growth as it compares with time with tracheostomy may help to guide therapy when symptoms of bacterial infection are present.
In contrast to earlier studies describing the respiratory microbiology in subjects with tracheostomies, MRSA was isolated in over half of the study population. This may reflect changes in the general population, since S. aureus infections have increased in the United States over the past several decades.7 This study did demonstrate a trend over time for methicillin-sensitive S. aureus and MRSA. They were grown in similar proportions of subjects with a tracheostomy in place for ≤4 y. In contrast, in subjects with a tracheostomy in place ≥5 y, a higher proportion had grown MRSA than methicillin-sensitive S. aureus. Thus, when a patient with a tracheostomy presents with signs and symptoms of respiratory infection, suspicion of MRSA should be higher in those who have had a tracheostomy in place longer.
In our study, P. aeruginosa was isolated from respiratory cultures in most subjects with a tracheostomy tube. This is consistent with other studies that show predominance of Gram-negative organisms in this patient population.1,2,8 However, the results of this study suggest that growth of P. aeruginosa on respiratory culture in subjects with tracheostomies is not associated with a greater requirement for chronic respiratory support. This supports the current practice of only treating with antibiotics when patients are symptomatic and there is clear clinical evidence of bacterial infection. Furthermore, this study did not find a relationship between the growth of P. aeruginosa or MRSA and a need for antibiotics in that same year, also consistent with previous studies.1,4 This study reaffirms the findings of previous studies, which recognized P. aeruginosa and other Gram-negative organisms as common isolates in respiratory cultures, and indicates that MRSA coverage should also be considered when choosing an empiric antibiotic regimen for patients with a long-term tracheostomy.
There were limitations to this study. This was a single-center retrospective study with the associated limitations. Microbiology trends may vary by institution and geographical location. Microbiology techniques also change over time, and it is possible that the trends in overall prevalence of specific organisms observed in this study have been impacted by changes in this institution's microbiology laboratory. We did not separate surveillance cultures from those taken during an acute illness and therefore cannot draw conclusions about whether surveillance cultures are predictive of acute illness. We did not include an analysis of virology during acute illness. We also did not analyze culture results by underlying diagnosis. Finally, for the analysis based on level of respiratory support, we only used data from a single time point – the first clinic visit of 2010.
Conclusions
The children in our study with long-term tracheostomies were colonized most often with P. aeruginosa and other Gram-negative organisms, followed by methicillin-sensitive S. aureus and MRSA. This study provides an updated overview of the variety of potential pathogens isolated from the respiratory cultures of subjects with a long-term tracheostomy in place. When choosing empiric antibiotic therapy for symptoms of respiratory infection in this population, consideration may be given for coverage for P. aeruginosa and methicillin-sensitive S. aureus, with coverage for MRSA depending on time with tracheostomy and prevalence of MRSA in the region and the individual institution. Further studies are needed to draw conclusions about the relationship between respiratory microbiology and the severity of respiratory disease in this patient population.
Footnotes
- Correspondence: Catherine E O'Brien PharmD, Department of Pharmacy Practice, UAMS College of Pharmacy, 4301 West Markham Street, Slot 522, Little Rock, AR 72205. E-mail: obriencatherinee{at}uams.edu.
Dr McCaleb was supported by a student research fellowship funded by the University of Arkansas for Medical Sciences College of Pharmacy Summer Research Program. The authors have disclosed no conflicts of interest.
Dr McCaleb presented a version of this paper at the Southern Society for Pediatric Research Annual Meeting, held February 9–11, 2012, in New Orleans, Louisiana, and Dr O'Brien presented a version of this paper at the Enriching the Care of the Child with Complex Medical Needs Annual Meeting, held October 25, 2012, in Des Moines, Iowa.
- Copyright © 2016 by Daedalus Enterprises