Abstract
Noninvasive ventilation is an effective treatment for a significant proportion of patients with acute respiratory failure. The success of noninvasive ventilation, however, depends on several factors, a major one being the selection of the proper interface. The choice and application of the interface in patients with acute respiratory failure is a considerable challenge for any treatment team. This review discusses the different types of interfaces that can be used in patients with acute respiratory failure, the differences between nasal, oro-nasal, and total face masks and the helmet, as well as the effect of interface type on treatment success and upper airway patency, mask fitting, problems related to the interface, and the relationship between ventilator type and interface choice.
Introduction
Noninvasive ventilation (NIV) is a major treatment modality in patients with acute respiratory failure (ARF), as well as chronic respiratory failure and sleep-disordered breathing.1–3 Despite significant advances in NIV technology and the experience of medical staffs, the failure rate of NIV in the acute setting remains high, ranging between 18% and 40%.4 The success of NIV therapy depends on the underlying cause of ARF, patient cooperation, and staff experience. Nevertheless, therapy success depends, to a large extent, on the choice of the proper mask. Failure of mask-delivered NIV has been shown to increase hospital mortality.5
This review discusses the different types of masks that can be used in patients with ARF and the differences between nasal, oro-nasal, and total face masks, and the helmet. This review also discusses the effect of interface type on upper airway patency, mask fitting, interface-related problems, and the relationship between ventilator type and interface choice. For interface choice in patients with stable sleep-disordered breathing, readers are referred to a recently published review.6
Review Method
The literature search was carried out between February and May in 2017. The search key words included “interface,” “noninvasive ventilation,” “acute respiratory failure,” “mask,” “nasal pillows,” “helmet,” “air leak,” and “complications.” For the purpose of the literature search, PubMed, Google Scholar, and MEDLINE were used. The reference lists of retrieved articles were also searched for additional sources. Only English-language articles were taken into consideration. Publications were then filtered on the basis of whether they reported original findings, provided background information, or contained relevant theoretical speculation of the effect of different interfaces on the areas addressed in this review.
This project was partially funded by The Strategic Technologies Program of the National Plan for Sciences and Technology and Innovation in the Kingdom of Saudi Arabia (08-MED511-02).
Review of the Literature
Types of Interfaces for NIV
There are 6 types of interfaces that can be used during NIV therapy in the acute setting. Choosing the appropriate interface for patients with ARF involves consideration of patient preferences and tolerance, and determining the correct size and fit is essential to successful ventilation.
Although interfaces are constructed from a variety of materials, the most commonly used material is silicone, although gel masks are available from some manufacturers as well. The advantage of gel masks is that they adapt to the contours of the face. The availability of several types of interfaces makes the choice of appropriate interface for patients with ARF a significant challenge.
Noninvasive support in the form of CPAP, bi-level positive airway pressure, or other pressure- and volume-limited ventilatory modes is used in patients with ARF. Regardless of mode, however, a well-fit interface is essential for all forms of NIV.
Nasal Mask.
This mask covers the nose only and rests on the upper lip, the sides of the nose, and the nasal bridge (Fig. 1A).
Examples of different interfaces that can be used during noninvasive ventilation. A: nasal mask, B: oro-nasal mask, C: nasal pillows, D: oral mask, E: total face mask, and F: helmet photograph (from Reference 80, with permission).
Oro-Nasal Mask (also referred to as a face mask).
This mask covers the nose and mouth and rests on the chin, the sides of the nose and mouth, and the nasal bridge (Fig. 1B).
Nasal Pillow Mask.
This mask fits on the rim of the nostrils. This type of mask is usually recommended for individuals who find nasal or oro-nasal masks uncomfortable or experience skin breakdown on the nasal bridge. Nasal pillows are used mainly in stable patients with sleep-disordered breathing (Fig. 1C).
Oral Mask.
This mask fits inside the mouth between the teeth and lips and has a tongue guide to prevent the tongue from obstructing the airway passage. This type is not common in practice (Fig. 1D).
Helmet.
The helmet is a transparent hood that covers the entire head and face of the patient and has a rubber collar neck seal. It is used as an alternative to the oro-nasal mask in patients with acute hypoxemic respiratory failure or acute cardiogenic pulmonary edema in certain countries.8 It was developed to improve tolerability and reduce complications in patients with ARF on NIV.9 It is not commonly used in patients with acute hypercapnic respiratory failure (Fig. 1F).
Comparison of Different Types of Interfaces in the Acute Setting
In ARF, NIV efficacy is more important than patient comfort, yet appropriate mask fitting and care are essential to increase patient tolerance and, subsequently, to improve NIV outcome.4 Because there is no universally ideal NIV interface, choosing an interface requires a thorough evaluation of patient features, ventilatory modes, and respiratory failure type.10,11 The choice of proper interface is influenced by the shape of the patient's face, mouth, and nose; breathing pattern; preference; and the experience of the medical staff.
A limited number of studies have assessed the effects of different interfaces on the success of treatment in subjects with ARF and the improvements in respiratory parameters (ie, breathing frequency, dyspnea, and arterial blood gases). Table 1 presents a summary of randomized clinical trials that have compared the efficacy of different interfaces during NIV.
Summary of the Main Randomized Controlled Trials That Compared Interfaces During NIV in Subjects With ARF
Nasal Versus Oro-Nasal Masks.
Two randomized controlled trials that compared the oro-nasal mask with the nasal mask in subjects with ARF reported no evidence that one type of interface is consistently better than another in terms of clinical efficacy.12,13 In the first study, Kwok et al12 randomly assigned 70 subjects with ARF (most had acute cardiogenic pulmonary edema or COPD). Both masks performed equally with regard to improving vital signs, gas exchange, and avoiding intubation. However, the oro-nasal mask was better tolerated compared with nasal mask (4 subjects vs 12 subjects, respectively, P = .02). Additionally, the overall success was greater in the oro-nasal (65.7%) group than in the nasal group (48.6%), although the difference was not statistically significant.12 In the second study, Girault et al13 randomly assigned 90 subjects with hypercapnic ARF to NIV with an oro-nasal mask or a nasal mask. Both masks resulted in similar improvements in vital signs and respiratory parameters. However, mask failure occurred significantly more often in the nasal mask group (32 of 44 subjects vs 9 of 46 subjects, P < .001). In addition, air leaks were more prevalent among subjects who used the nasal mask (P < .05).13 However, from day 2 onward, NIV use, respiratory discomfort, and complications were more frequent in the group using an oro-nasal mask (P < .05). On the basis of these findings, the authors recommended the use of a face mask in the initial management of hypercapnic ARF with NIV, with a switch to a nasal mask if NIV therapy was projected to be prolonged, because the use of a nasal mask may improve comfort and reduce complications related to a face mask.13
A randomized study was conducted to compare nasal and full-face masks during NIV (N = 14 subjects with exacerbation of COPD).14 Apart from a greater decrease in breathing frequency in the group that used a total face mask, there were no other differences in blood gases or respiratory effort. NIV was well tolerated in both groups.14
Oro-Nasal Versus Total Face Mask.
A recent study randomly assigned 48 subjects with ARF into groups using an oro-nasal mask or a total face mask and monitored responses for 24 h.15 At 6 h, use of a total face mask was significantly more effective in reducing PaCO2 (P = .04). However, there was no difference between the 2 masks once subjects cleared the acute phase. Acceptance and comfort were similar in both groups.15
Because mouth breathing is prevalent in patients with ARF, a face mask (oro-nasal or total face mask) is considered to be the most suitable and effective interface, followed by a nasal mask and a mouthpiece.10,12,13 Therefore, the most commonly used interface in patients with ARF is the oro-nasal mask. This was revealed in a large Web-based survey conducted in North America and Europe, which showed that an oro-nasal mask was used with 70% of patients, followed by total face masks, nasal masks, and helmets.16 These findings are consistent with a more recent study from Brazil, which reported that total face and oro-nasal masks were the most commonly used interface in subjects with ARF (99%).17
Helmet Versus Other Interfaces.
In an attempt to improve comfort and reduce interface complications of NIV in patients with ARF, the helmet was developed, which has the advantage of avoiding skin contact and hence improving patient tolerance independent of face morphology.18,19 The helmet has some intrinsic advantages as it allows patients to freely drink, communicate, and expectorate. Moreover, it allows clearance of secretions and interaction with caregivers without removing the NIV interface. Studies have shown that the patient tolerance scale in the helmet group is significantly higher than with face masks.19–22
A recent systematic review and meta-analysis included 11 randomized and case-control studies (N = 621 subjects) that adopted the helmet as an NIV interface and compared it with another interface.19 The meta-analysis reported that the overall hospital mortality was 17.53% in the helmet NIV group compared to 30.67% in the control group. Additionally, the use of the helmet was associated with lower intubation rates (odds ratio 0.32, 95% CI 0.21–0.47, P < .001), hospital mortality (odds ratio 0.43, 95% CI 0.26–0.69, P < .001), and complications (odds ratio 0.6, 95% CI 0.4–0.92, P = .02). In a subgroup analysis, subjects were divided according to the type of ARF, and NIV with a helmet reduced mortality mainly in the group with hypoxemic ARF (odds ratio 0.38, 95% CI 0.22–0.65, P < .001). Moreover, NIV with a helmet decreased the intubation rate in both hypercapnic and hypoxemic ARF patients independent of the ventilatory mode (P < .05).19 A subgroup analysis of NIV interfaces in another recent systematic review and meta-analysis in subjects on NIV with hypoxemic nonhypercapnic ARF suggested that NIV by helmet could reduce intubation rate and hospital mortality compared to NIV with a face mask or a nasal mask.23
A recent randomized controlled trial assessed the effect of NIV delivered by helmet versus face mask on the rate of endotracheal intubation in subjects with ARDS (N = 83).24 The intubation rate was 61.5% for the face-mask group and 18.2% for the helmet group (P < .001). Moreover, the number of ventilator-free days was significantly higher in the helmet group (28 vs 12.5, P < .001). At 3 months, the mortality rate was 34% in the helmet group compared with 56% in the face-mask group (P = .02). An exciting finding of this study was that the helmet could reduce the mortality of subjects with hypoxemic ARF.24 Previous studies that assessed the effect of NIV through a mask in subjects with hypoxemic ARF had reported disappointing results.25–28 The helmet group had significantly higher levels of PEEP that were sustained throughout NIV therapy, which resulted in a significant reduction in the breathing frequency and similar oxygen saturation levels on a lower FIO2 compared with the face-mask group.24 The helmet has less air leakage due to the its lack of contact with the face and a better seal at the neck, which allows titration to higher positive airway pressures without substantial air leakage.24,29
There has been always a concern that a helmet may increase dead space and hence may increase PaCO2 as a result of the large internal volume of the helmet and its high compliance, which may lead to CO2 rebreathing. Therefore, patients managed with helmet NIV should have pressure-support levels set to ensure high inspiratory-flow levels (ie, > 100 L/min) and periodic arterial blood analysis.24,30,31 However, a meta-analysis showed that NIV particularly with pressure support ventilation via a helmet decreased PaCO2 in subjects with hypercapnic respiratory failure (P < .001) and did not increase PaCO2 in the overall group.19 Moreover, a few studies have compared face mask and helmet use in subjects with COPD.
A study evaluated the sequential use of a face mask and a standard helmet during NIV in 53 subjects with severe exacerbation of COPD.32 All subjects were ventilated for the first 2 h with NIV via a face mask. Subsequently, if gas exchange and clinical status improved, subjects were randomized to continue on NIV via a face mask or a helmet, and physiological parameters were measured at admission, after the first 2 h on NIV by face mask, at 4 h after randomization, and at discharge. In 40 subjects whose gas exchange and clinical parameters improved after 2 h and were randomized, PaCO2 was lower in the face mask group after 4 h. Duration of NIV and length of stay were lower in the face-mask group than in the helmet group.32 However, in a more recent multi-center, short-term, physiological, randomized trial in subjects with COPD who presented with hypercapnic ARF, the investigators compared the changes in arterial blood gases and tolerance score obtained using the helmet or oro-nasal mask.33 A new helmet was used that has less internal volume and a better rate of pressurization and triggering performance.34 Changes in blood gases and discomfort were similar in the 2 groups; however, dyspnea was significantly less while using the face mask. On the other hand, the rate of intubation and the need for interface change were not different between groups.33
These findings suggest that the helmet interface is a relatively novel and promising approach to NIV, particularly in patients who require high positive airway pressures and those who cannot tolerate masks. However, careful training of all physicians, respiratory therapists, and nurses is needed before applying this interface in patients with ARF.
Interface Type and Upper Airway Obstruction
As obese patients who present with ARF tend to have coexisting obstructive sleep apnea, similar to patients with obesity hypoventilation syndrome,35 it is important to discuss the effects of the interface used with NIV on upper airway patency. Therefore, in this context, it is important to understand the physiological effects of positive airway pressure applied through different interfaces on upper airway dynamics.6,36
To understand the effects of different interfaces on upper airway patency, we need to briefly review the difference between nose breathing and mouth breathing.37 When breathing occurs through the mouth, there is an increase in upper airway resistance and an increased tendency to develop upper airway obstruction. Moreover, open-mouth breathing while awake reduces the retropalatal and retroglossal cross-sectional area, and decreases the positive pharyngeal critical closing pressure during sleep.38
The oro-nasal mask applies pressure through the mouth and nose simultaneously, which may lead to collapse of the airway.39 It is proposed that oro-nasal positive airway pressure therapy applies equal positive pressure in both nasopharyngeal and oropharyngeal compartments, which eliminates the pressure gradient and allows gravity to displace the tongue and soft palate backward, resulting in airway obstruction.40
It is important for the therapist to understand that the differences in the efficacy of positive airway pressure applied via nasal and oro-nasal masks may influence the effectiveness of NIV therapy, particularly in patients with obesity hypoventilation syndrome who present with hypercapnic ARF. Therefore, oro-nasal masks or face masks are preferred in the acute settings; however, once the patient is stable, shifting to a nasal mask, if tolerated, is recommended.
Fitting the Interface
The choice of the proper interface is essential for the success of NIV therapy. Most interfaces are provided with a fitting gauge to help choose the correct size to improve tolerance and avoid complications. Securing the interface too tightly decreases patient tolerance and increases the risk of facial skin breakdown; therefore, when the headgear is fixed, it should be possible to permit 2 fingers beneath it (ie, the 2-finger rule).41 If the patient cannot tolerate the interface or if a significant leak is detected, a different interface should be used to avoid NIV failure. Nevertheless, when a different interface is used, trigger sensitivity, pressurization level, and compatibility with the circuitry must be verified.4 Once the patient's condition is stable, a nasal mask can be tried because it is less claustrophobic and is associated with a lower risk for skin problems.
Type of Ventilator and Interface Choice
NIV can be applied through a closed dual-limb circuit, an open single-limb circuit, or a closed single-tube circuit with an exhalation valve. A dual-limb circuit has one tube for inhalation and another for exhalation, and a built-in exhalation port or filter for CO2 removal. Therefore, a non-vented mask is used to maintain the closed circuit. On the other hand, a vented mask should be used in open single-limb circuits.
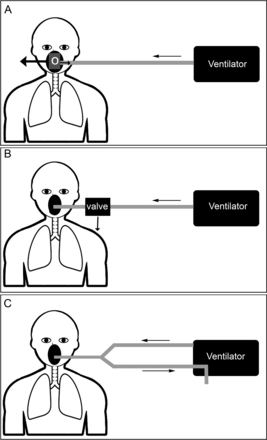
If a non-vented mask is used (open single-limb circuit), an additional exhalation valve in the circuit must be added to allow CO2 removal. Clinicians and respiratory therapists must be aware of this important difference between ventilators, because the use of a non-vented mask in an open single-tube system without an exhalation port in the system can be disastrous.4 Figure 2 shows the 3 examples of respiratory circuits.
A: Single-limb respiratory circuit with a vented mask. Exhalation occurs through the mask shell or in the swivel connector (O). B: Single-limb non-vented respiratory circuit with an exhalation valve incorporated into the ventilator circuit near the connection with the mask at the distal end of the inspiratory circuit. C: Double-limb respiratory circuit with inspiratory and expiratory limbs.
It is worth mentioning that the interface itself acts as a dead space, which theoretically may increase the risk of CO2 rebreathing and retention, particularly in hypercapnic ARF. It is thought that the dead space is related to the internal volume of the interface.42 Nevertheless, an in vivo study showed that the internal volume of the masks had no apparent short-term dead-space effect on gas exchange, minute ventilation, or patient effort.42 Additionally, another study that used computational fluid dynamics to describe pressure, flow, and gas composition during ventilation with the different interfaces revealed that the effective dead space is not related to the internal gas volume of the interface, which indicates that the internal volume is not a limiting factor for the interface efficacy during NIV.43 This suggests that the interfaces may be interchangeable in clinical practice with the exception of the helmet.44
Problems Related to the Interface
Several interface-related problems may appear during NIV. Table 2 shows a summary of the common problems and the proposed solutions.
Common Interface-Related Problems During NIV and Proposed Solutions
Air Leaks.
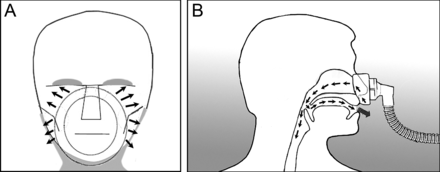
Air leaks are very common during NIV. There are 2 kinds of air leaks: intentional and unintentional leaks. Intentional leaks are deliberately generated during NIV when a single-limb circuit without an expiratory valve is used. An intentional leak aims to avoid rebreathing by having holes in the mask or in the circuit to allow a leak proportional to their size and the set inspiratory pressure or mean inspiratory flow.45 On the other hand, an unintentional leak can occur between the mask and the skin (Fig. 3A), through the open mouth with nasal mask (Fig. 3B), or through the nose with mouthpiece ventilation. Air leaks depend on sealing features of interfaces; a leak is proportionately greater with a smaller facial mask than with a larger mask or a helmet.46 Large air leaks have detrimental effects on the success of NIV, as leaks decrease the FIO2 and arterial oxygen saturation and increase ventilator auto-triggering, hence increasing patient–ventilator asynchrony, which increase the risk of NIV failure.46 Additionally, air leaks may cause mouth and throat dryness, conjunctivitis, or sleep disturbances.46 In general, nasal masks tend to have more air leakage than face masks.47 Moreover, the use of an oro-nasal mask reduces the changes in the relative humidity related to mouth leaks.48 Air leakage is negligible when a proper interface for NIV is chosen and fitted.49 Tight fitting of the interface may partially improve air leakage and patient–ventilator asynchrony; however, it should be done cautiously because it increases the risk of facial skin discomfort and ulceration.50 Adjusting the ventilator mode may affect air leakage. Pressure controlled ventilation causes less air leakage compared with volume controlled ventilation as it delivers a similar tidal volume at a lower peak inspiratory pressure.49 Similarly, a reduction in peak inspiratory pressure and tidal volume may reduce air leakage.51
Schematic drawing of an unintentional leak, A: between the mask and the face, and B: through the open mouth when using a nasal mask.
Clinicians and therapists should be aware of the differences between ventilators when applying leak compensation during invasive ventilation versus NIV.52 A study has shown that leak compensation in invasive and noninvasive modes has varies widely between ventilators.52 In a bench study of 8 ICU ventilators featuring an NIV mode, Vignaux et al53 found that, in most of the tested ventilators, leaks led to an increase in trigger delay and a decrease in ability to reach the pressure target and delayed cycling. Moreover, some investigators have reported that dedicated NIV ventilators could produce better synchronization than ICU ventilators in the presence of a leak.53–55 Additionally, it is important to know that masks have various leak levels.56 Therefore, trigger sensitivity, pressure level, and rebreathing must be checked when switching to a mask with a different degree of leakage.
Nasal or Oral Dryness and Nasal Congestion.
Nasal or oral dryness and nasal congestion are common during NIV. These side effects can be related to air leaks and the interface used. Previous studies have shown that during NIV, nasal or oral dryness and nasal congestion affect 10–20% and 20–50% of subjects, respectively, particularly when a nasal mask is used.46,57,58 Nasal or oral dryness usually indicates air leakage through the mouth, which results in the loss of the nasal mucosal capacity to heat and to humidify inspired air.47 Progressive nasal mucosal dryness releases inflammation mediators that increase nasal congestion and hence nasal resistance, which in turn reduces tidal volume and patient comfort.47,59 Strategies to decrease airway dryness and congestion during NIV mainly focus on decreasing air leak (Table 2).47
Interface and Patient–Ventilator Synchrony.
Interfaces used for NIV have been shown to affect patient–ventilator interaction. Of all interfaces, studies reported the helmet has more problems with patient–ventilator synchrony and ventilator cycling due to its soft compliant wall, upward displacement, and elevated internal compressible volume. This, in turn, increases work of breathing. A bench study in healthy volunteers evaluated patient–ventilator interaction during NIV with an oro-nasal mask or helmet.60 The helmet resulted in more asynchrony, which was attributed to a longer trigger delay and a shorter time of synchrony between ventilatory support and patient inspiration.60 Other studies in healthy volunteers and subjects with stable COPD revealed that helmet was less efficient in decreasing inspiratory effort compared with oro-nasal masks, resulting in triggering delay and patient–ventilator asynchrony.61,62 In a prospective crossover study of 11 subjects at risk for respiratory failure after extubation, Vargas et al63 demonstrated that, compared with the face mask, the helmet with the same settings caused longer triggering and cycling delays, which worsened patient–ventilator synchrony. Increasing both the pressure support level and PEEP and using the highest pressurization rate achieved the same effects of NIV delivered by a helmet or facial mask.63
To overcome the upward displacement of the old designs of helmets and to reduce the discomfort associated with the armpit braces, new helmet models have been designed to attenuate these limitations of the standard helmet in both bench study and healthy volunteers.34,64,65 A newly introduced helmet design is characterized by an annular openable ring located underneath an inflatable cushion that fixes the helmet without the need for armpit braces, as opposed to the standard helmet.64 Additionally, the new helmet is more effective in delivering NIV by reducing the upward displacement of the helmet during inflation.64 A recent study tested the new helmet design in 14 ICU subjects who underwent 30-min trials in pressure support during invasive ventilation; subjects then used a standard helmet or the new helmet in random order.65 The new helmet provided more comfort and faster responses to effort than the standard helmet, but an endotracheal tube enabled the most rapid responses.65
Interfaces and Ventilatory Mode.
Several studies have shown that neurally adjusted ventilatory assist (NAVA) via masks or helmet improves patient–ventilator interaction and reduces asynchrony compared to pneumatically triggered and cycled pressure support, which is the most commonly used mode of NIV delivery.66–71 NAVA quantifies the patient's neural output with the electrical activity of the crural diaphragm to optimize synchrony between ventilator inspiratory cycle and neural respiratory drive, and to eliminate the problems of respiratory drive due to intrinsic PEEP and impaired respiratory drive.72 In general, NAVA results in better patient–ventilator interaction and synchrony compared with pressure support ventilation, as reflected by the significantly longer time of synchrony between diaphragm contraction and ventilator assistance and by a lower asynchrony index, with no differences in gas exchange, breathing frequency, and neural drive, and timing.
Facial Skin Lesions.
With extended use of NIV, nasal skin lesions such as erythema and ulcers may appear at the site of mask contact.46,73,74 Nasal erythema or ulcers account for a significant portion of interface complications during NIV, reported to occur in 5–30% of patients and increasing to 50% of patients after a few hours; skin lesions may occur in almost 100% of patients after 48 h of NIV with a mask.46 Although the nasal bridge is the most sensitive area, skin lesions can also appear on other facial areas, in particular over the zygomatic bone. There are different types of skin lesions, ranging from slight redness over the nasal bridge to open ulcers. The development of skin abrasions or necrosis is an important factor that limits the tolerance and duration of NIV.46 Progressive tightening of the straps may increase pressure on the nasal bridge and cause nasal pressure lesions.74 Different dressings have been evaluated for their ability to prevent nasal bridge abrasion. The fit and comfort of the mask can be improved by including mask cushions and seal/support rings.75 Regardless of the interface selected, proper fit is essential to optimize patient comfort and tolerance. Sizing gauges should be used to choose the proper size and minimize strap tightening. Using the correct interface design and size, not tightening the headgear excessively, and using wound-care tape on the bridge of the nose with oro-nasal or nasal masks may help avoid facial skin breakdown.76 Techniques to reduce facial and nasal skin erythema and abrasions are shown in Table 2.
Oxygen Supply and Interface
When using an ICU ventilator that can control the FIO2, FIO2 can be accurately administered. However, some portable ventilators that are used outside the ICU setting do not have oxygen control and, thus, supplemental oxygen is bled into the interface or the ventilator circuit. Low-flow oxygen is usually connected to the circuit, which results in variably delivered FIO2. Additionally, portable devices for NIV operate with one tube only, in contrast to ICU ventilators that have an inhalation and exhalation tube. Therefore, an additional exhalation port is added to the circuit. When oxygen is bled into the circuit or interface, the actual level of inspired FIO2 can vary under the influence of several factors, such as intentional and unintentional leak through the interface or circuit, inspiratory and expiratory pressure settings of NIV, the interface, oxygen flow, and the oxygen administration site.77–79 Oxygen supply is frequently administered via an oxygen port already built into the frame of the interface. The site of oxygen delivery into the circuit is the most important factor in determining inspired FIO2.77 Administering oxygen into interface results in exhaustion of oxygen out of the exhalation port.79 Furthermore, oxygen may leak unintentionally between the interface and the face, resulting in low FIO2 and, hence, low arterial PaO2 and oxygen saturation. Consequently, when oxygen is administered directly into an interface with a leak port, patients may receive very low oxygen concentrations, which may result in low oxygen saturation in the blood.78,79 Therefore, during NIV of patients with ARF, using a ventilator in which FIO2 can be precisely controlled is recommended.
Summary
Based on the available evidence, oro-nasal and face masks are preferred in patients with ARF in the acute setting. Experience with fitting and prevention of interface-related problems such as air leaks, skin irritation, and pressure ulcers are essential for successful NIV therapy. Once stable, patients may be shifted to nasal masks if tolerated. Nevertheless, it is important to let the patient try different types of interfaces and choose the one that is most comfortable. The helmet is a promising alternative to masks during NIV in patients with ARF, particularly in patients who cannot tolerate masks and those requiring high positive airway pressures. However, further studies are needed to identify the ideal patient population for NIV with the helmet.
Footnotes
- Correspondence: Ahmed S Bahammam, Professor of Medicine, Director, Sleep Disorders Center, College of Medicine, King Saud University, Box 225503, Riyadh 11324, Saudi Arabia. E-mail: ashammam{at}ksu.edu.sa
Dr Singh is an employee of Philips Respironics India. The other authors have disclosed no conflicts of interest.
- Copyright © 2018 by Daedalus Enterprises
References
- 1.↵
- 2.
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.
- 21.
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.
- 27.
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.
- 68.
- 69.
- 70.
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵