Abstract
BACKGROUND: There has been a growing trend toward delivering aerosolized medications using high-flow nasal cannula (HFNC). In some cases, patients who do not require high-flow oxygen to maintain adequate oxygenation may benefit from aerosol delivery while receiving low-flow oxygen via HFNC. The objective of this study was to quantify and compare the relative pulmonary and systemic delivery of salbutamol, with 2 different nebulizers, in patients with COPD receiving low-flow oxygen therapy through an HFNC.
METHODS: Subjects were randomized to receive study doses of 5 mg salbutamol nebulized by either a jet nebulizer or a vibrating mesh nebulizer with a T-piece or spacer on days 1, 3, and 5 of admission. Subjects using the large spacer also received 2 puffs (100 μg each) of salbutamol via a pressurized metered-dose-inhaler prior to the nebulizer dose. Urinary salbutamol excretion 30 min post-inhalation and pooled samples of urinary salbutamol excretion up to 24 h post-inhalation were measured. On day 2, ex vivo studies were performed with salbutamol collected on filters placed between the HFNC and nebulizer, with drug eluted from filters and analyzed to determine inhaled dose.
RESULTS: Twelve subjects (6 females), age 51.3 ± 11.2 y, were included. The vibrating mesh nebulizer demonstrated higher urinary salbutamol excretion at 30 min and 24 h post-inhalation compared to a jet nebulizer (P = .001 and P = .02, respectively). No significant difference was found between the T-piece and large-spacer configurations, even though the spacer provided a significantly larger emitted aerosol dose at the opening of the HFNC (P = .002).
CONCLUSIONS: Aerosolized medication could be efficiently combined with low-flow oxygen, via HFNC, in COPD subjects without the need to interrupt the gas supply. The vibrating mesh nebulizer delivered larger doses to subjects compared to the jet nebulizer. However, there was no benefit of using the large spacer with HFNC in low-flow delivery, because the small inner diameter of the HFNC does not allow larger aerosol droplet sizes (preserved by the spacer) to reach the subject.
Introduction
Oxygen supplementation is one of the most commonly prescribed therapies in hospitals,1,2 and it is a mainstay of resuscitation in acutely ill patients.2 In hypoxic patients with COPD, long-term oxygen therapy together with pulmonary rehabilitation are crucial interventions that increase life expectancy and improve quality of life.3,4
In clinical practice, nasal cannulae are the most commonly used delivery method for supplemental oxygen.5–8 There are limitations associated with low-flow oxygen through cannulae, although these limitations usually do not have clinical consequences when the supplemental oxygen flow is adequate to correct hypoxemia5 and meet a patient's oxygen requirements at rest or with limited activity.6 However, increasing attention is being paid to humidified high-flow nasal cannula (HFNC) oxygen therapy.7 These nasal cannulae are designed to allow higher flows and prevent delivery of a gas jet directly onto mucosal surfaces.8
Moreover, devices used to deliver oxygen are classified into low-, intermediate- (eg, air-entrainment mask), and high-flow devices. The conventional low- and intermediate-flow devices have several disadvantages, including variable FIO29 and inadequately warmed or humidified gas, which lead to patient discomfort.3 Consequently, there is a clinical trend toward using high-flow devices that allow adjustment of FIO2 independently of gas flow to fall between 0.21–1.03,10,11 and, more importantly, that deliver a sufficiently heated and humidified medical gas.12
For patients suffering an exacerbation of COPD, respiratory support can be preceded by noninvasive ventilation (NIV) with a face mask or HFNC.10 However, HFNC is better tolerated than NIV and can deliver a wider range of FIO2 as required.10 In addition, HFNC offers additional physiological benefits, such as improved tissue oxygenation, improved functional residual capacity, decreased nasopharyngeal resistance, and flushing of pharyngeal dead space of CO2, which make HFNC an attractive alternative modality of respiratory support in COPD patients.1,9,13–15
To facilitate the delivery of inhaled medications during HFNC, there are 2 possible approaches. One approach is to abruptly remove the patient from the high-flow circuit and then administer the inhaled drug with either a nebulizer or a pressurized metered-dose inhaler (pMDI) with spacer. Although this approach potentially allows adequate drug delivery, it may contribute to worsening the patient's respiratory status due to the temporary removal of supplemental oxygen. Another approach is to deliver the inhaled medication through HFNC,16–19 thereby ensuring drug delivery without interrupting supplemental oxygen.20–22 In addition, nasal aerosol delivery is advantageous over oral inhalation because it may improve both subject comfort and compliance by using a cannula to administer doses, either frequently or over long durations.11,23
How then to combine aerosol delivery with the HFNC system while maximizing aerosol delivery to the lungs in COPD subjects? Previous in vitro studies that combined aerosol delivery with HFNC systems found that the delivered dose is inversely proportional to the gas flow and directly proportional to the cannula size.16,18,19,22–25
These findings were previously validated by our in vitro study,26 which found significantly improved delivered doses and aerodynamic aerosol characteristics for both vibrating mesh nebulizers and jet nebulizers in a HFNC circuit with adult nasal cannula with low oxygen flow (5 L/min), whereas pMDIs with spacers delivered negligible amounts of salbutamol. Our previous study also examined the performance of the large spacer, which facilitates delivery with both a pMDI and a vibrating mesh nebulizer.26 The manufacturer of this device recommends 1–2 prophylactic bronchodilator puffs from the pMDI before starting nebulizer delivery to improve dosing; we were unable to measure this as a possible benefit due to that study's limitations.
The present in vivo study used low oxygen flow (5 L/min) in a HFNC circuit using an adult nasal cannula to compare both the pulmonary and systemic bioavailability of jet nebulizer and vibrating mesh nebulizer delivery with their standard T-pieces, and pMDI and vibrating mesh nebulizer delivery via a large spacer in subjects with COPD.
QUICK LOOK
Current knowledge
There is a growing trend toward delivering aerosol medications via high-flow nasal cannula (HFNC). Previous bench studies that evaluated aerosol delivery via HFNC systems found that the delivered dose is inversely proportional to the gas flows and directly proportional to the cannula size.
What this paper contributes to our knowledge
Drug nebulization could be efficiently combined with HFNC therapy using low-flow oxygen in subjects with COPD with a history of exacerbations, without the need to temporarily halt the gas supply. A vibrating mesh nebulizer delivered a larger dose of drug to subjects compared to a jet nebulizer. There is no measurable benefit to using a spacer in HFNC-mediated aerosol delivery.
Methods
Participants
The study was approved by the Beni-Suef University Hospital Research Ethics Committee. Written informed consent was obtained from all subjects. A convenience sample of subjects, diagnosed with COPD and previously admitted to the respiratory unit with an exacerbation requiring respiratory support, were recruited into this study. All subjects were prescribed nebulized salbutamol and ipratropium bromide on alternating days upon admission. Subjects were excluded from this study if they had participated in any other research study during the previous 6 months, had a known hypersensitivity to salbutamol or ipratropium bromide, had a systolic blood pressure < 100 mm Hg, or had severe renal dysfunction defined as an estimated glomerular filtration rate < 20 mL/min.13
Circuit Description
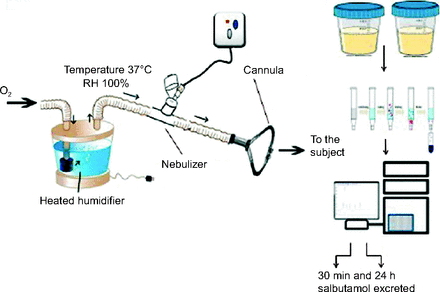
A heated humidifier (MR810, Fisher & Paykel Healthcare, Auckland, New Zealand) which can deliver gas flows of 1–60 L/min with adjusted FIO2 of 0.21–1.0014 while the gas is heated (37°C) and humidified (100%) was connected to an adult-sized nasal cannula (Jiaxing Sim Medical, Zhejiang, China) and then to the subject. The nebulizer with T-piece or spacer apparatus was positioned downstream of the humidifier, as shown in Figure 1, and the oxygen flow within the HFNC circuit was set to 5 L/min for all tests.
Schematic design of an in vivo setting showing the positions of the aerosol generator within the high-flow nasal cannula circuit and measurement of urinary salbutamol excretion. RH = relative humidity.
Aerosol Delivery Devices
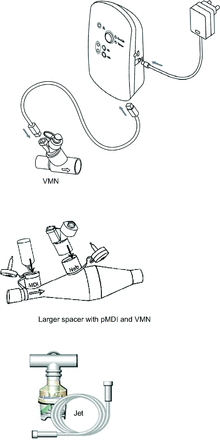
As shown in Figure 2, either a vibrating mesh nebulizer (Aerogen Solo, Aerogen, Galway, Ireland) or a jet nebulizer (Oxycare, Ceren Üretim A.S., Istanbul, Turkey) was tested with a large spacer (Combihaler Laboratoire Protec'Som, Valognes, France) in this study.26 Salbutamol (5 mg/mL; Farcolin Respirator solution, Pharco Pharmaceuticals, Alexandria, Egypt) was delivered using either the jet nebulizer or the vibrating mesh nebulizer connected to the HFNC system using the manufacturer-supplied T-pieces with an oxygen flow of 5 L/min (FIO2 = 1.0). The vibrating mesh nebulizer was also tested connected to the large spacer in combination with a pMDI (Ventolin Evohaler, GlaxoSmithKline, Cairo, Egypt) as shown in Figure 2. Two 100 μg puffs of salbutamol were delivered to the subject via pMDI, followed by 1 mL (5 mg) salbutamol using the vibrating mesh nebulizer per manufacturer recommendations. In all cases, nebulization continued until the aerosol was no longer visible emanating from the vibrating mesh nebulizer and to sputtering for the jet nebulizer.
Assembly of aerosol generators with its connections. VMN = vibrating mesh nebulizer; pMDI = pressurized metered-dose inhaler.
Study Design
Salbutamol administration was withheld before the beginning of the study for at least 12 h. During the study period, ipratropium bromide solution (250 μg/mL, Atrovent Inhalation Solution, Boehringer Ingelheim, Egypt) was prescribed to the subjects instead of their regular salbutamol. High-performance liquid chromatography (HPLC) analysis of the subject's urine was utilized to differentiate between ipratropium and salbutamol. Subjects were randomly selected to receive their usual salbutamol study dose either by vibrating mesh nebulizer, jet nebulizer, or vibrating mesh nebulizer with a large-spacer on days 1, 3, and 5 of the study. Approximately 15 min before each study dose, subjects were asked to void their urine. Two urine samples were then taken from each subject, one at 30 min after the start of dosing and the other consisting of all urine produced over the next 24 h. It was previously demonstrated that the concentration of salbutamol in urinary salbutamol excretion 30 min post-inhalation is a representative index of pulmonary salbutamol lung deposition, while the concentration found in urinary salbutamol excretion 24 h post-inhalation is an index of salbutamol systemic bioavailability.13,15
The volumes of urine (urinary salbutamol excretion 30 min post-inhalation and urinary salbutamol excretion 24 h post-inhalation) were recorded, and the urine was assayed, using HPLC, to determine salbutamol concentration. Bambuterol hydrochloride was added as the internal standard to the collected urine samples. Salbutamol and bambuterol were extracted from urine samples using solid phase extraction.16 As shown in Figure 1, the eluent was then injected into the HPLC system, which was composed of an ODS 5 μm (4.6 × 250 mm, ZORBAX Eclipse) C-18 HPLC column with a C-18 (ODS) guard column (4 mm × 3 mm, Agilent). The mobile phase, composed of acetonitrile solution in water containing 0.1% orthophosphoric acid (90:10, v/v), was pumped through the columns at a flow of 1 mL/min. A 25-photodiode array detector was set at 220 nm. The lower detection limit and lower quantification limit for salbutamol were 0.36 and 1.00 μg/mL, respectively.17
Ex Vivo Procedure
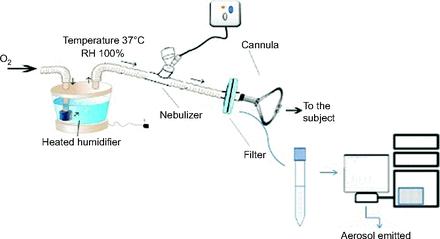
On day 2, subjects received study doses via the same equipment as previously, but this time with a breathing filter (Filta-Guard, Intersurgical, Wokingham, United Kingdom) placed between the nasal cannula and the nebulizer within the same HFNC circuit, as illustrated in Figure 3, to collect the entire dose delivered to the cannula outlet.13 Because this setup would not deliver any bronchodilator to the subjects, they resumed their prescribed dose of ipratropium bromide. Drug collected on the filter was eluted to determine the emitted dose. The amount of drug deposited within the circuit was recovered by rinsing with the mobile phase, and then recovered sample was assayed with HPLC as described previously.
Schematic design of ex vivo setting, showing the positions of the aerosol generator and the ex vivo filter within the high-flow nasal cannula circuit. RH = relative humidity.
Data Analysis
Data were analyzed with a 1-way ANOVA test with the application of least significant difference correction to determine any difference between aerosol generators in the urinary salbutamol excretion 30 min post-inhalation, urinary salbutamol excretion 24 h post-inhalation, and emitted dose. All other data were expressed as mean ± SD. Significance was defined as P < .05. All tests were performed using SPSS v17.0 (SPSS, Chicago, Illinois).
Results
Subject Characteristics
A convenience sample of 12 subjects completed the study (6 females). Their mean ± SD age was 51.3 ± 11.2 y, weight 83.5 ± 11.2 kg, and height 164.4 ± 9.0 cm.
Urinary Salbutamol Excretion
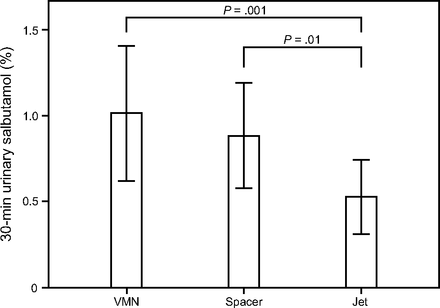
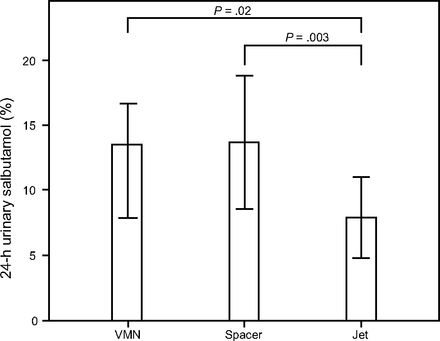
The mean ± SD salbutamol amount (μg) and salbutamol percentage of the nominal dose detected in both urinary salbutamol excretion 30 min post-inhalation and urinary salbutamol excretion 24 h post-inhalation for both vibrating mesh nebulizer and jet nebulizer are shown in Table 1, Figure 4, and Figure 5. The vibrating mesh nebulizer with T-piece and spacer resulted in a > 2-fold increase in the detected urinary salbutamol excretion percentage 30 min post-inhalation compared to the jet nebulizer (P = .001 and P = .012, respectively), whereas the vibrating mesh nebulizer with T-piece trended higher than the vibrating mesh nebulizer with the spacer (Fig. 4). Similarly, urinary salbutamol excretion percentage 24 h post-inhalation for the jet nebulizer was significantly lower than that for the vibrating mesh nebulizer with T-piece only or with the large spacer (P = .02 and P = .003, respectively). In contrast to urinary salbutamol excretion percentage 30 min post-inhalation, the vibrating mesh nebulizer with spacer resulted in higher urinary salbutamol excretion percentage 24 h post-inhalation than the vibrating mesh nebulizer with T-piece alone, but the difference was not significant (Fig. 5).
In Vivo and Ex Vivo Results of the Dose Delivered to Subjects Through HFNC
Percentage of the urinary salbutamol amount (mean ± SD) excreted 30 min after dosing by a different aerosol generator. VMN = vibrating mesh nebulizer.
Percentage of the urinary salbutamol amount (mean ± SD) pooled at 24 h after dosing by a different aerosol generator. VMN = vibrating mesh nebulizer.
Ex Vivo Filters
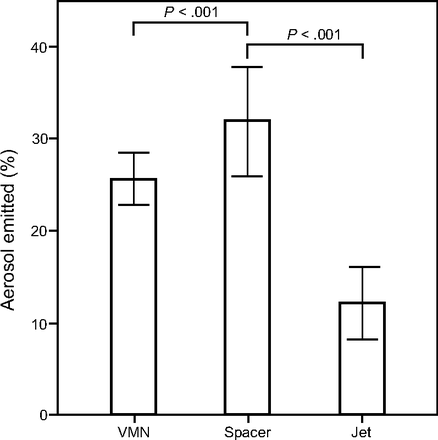
The fate of the aerosolized drug during the ex vivo study is illustrated in Table 1 and Figure 6. The amount of drug deposited on the filter provided important information on how much of the administered dose would be available to a patient at the cannula opening. The large spacer with pMDI and vibrating mesh nebulizer had the highest percentage, followed by the vibrating mesh nebulizer with the spacer and jet nebulizer (Fig. 6).
Percentage of the salbutamol amount (mean ± SD) deposited on the ex vivo filter (% aerosol emitted) by different aerosol generator. VMN = vibrating mesh nebulizer.
Discussion
Severe hypoxia is a harmful condition that can result in cardiorespiratory failure, irreversible organ damage, or even death if it persists for > 2–3 min.27 Many COPD patients with hypoxemia receive oxygen during exacerbations.18
This study examined aerosol delivery via either a vibrating mesh nebulizer, a pMDI/vibrating mesh nebulizer combined with a spacer, or a jet nebulizer via a HFNC breathing circuit in COPD subjects with a low oxygen flow (5 L/min). We found that the amount of aerosol delivered to the lungs (urinary salbutamol excretion percentage 30 min post-inhalation) was approximately the same, whether delivered by a vibrating mesh nebulizer connected by a T-piece with or without the spacer (∼1% nominal dose difference), whereas only half that amount (∼0.5%) was delivered to lungs by the jet nebulizer. This result is comparable to previously reported findings.16,28
Similarly, vibrating mesh nebulizer delivery resulted in approximately the same amount of the nominal dose of salbutamol absorbed systemically (urinary salbutamol excretion percentage 24 h post-inhalation) using a T-piece connection or a spacer setup (∼13%), whereas only about half that amount (∼8%) was absorbed systemically when delivered by jet nebulizer.
The actual differences between the vibrating mesh nebulizer and the jet nebulizer used in this study were similar to many different studies in different settings.29,30,31–42 Some studies recommended diluting the respiratory solution in the nebulization chamber of the jet nebulizer to overcome its low aerosol delivery. Even with dilution, however, the jet nebulizer still delivered a lower amount of aerosol compared to the vibrating mesh nebulizer.31,43,44
Our findings are lower but comparable to those observed by Réminiac et al,19 who evaluated aerosol delivery via vibrating mesh nebulizer with a T-piece at 3 different flows (2, 4, and 8 L/min) or a jet nebulizer at 8 L/min in both the macaque and Sophia anatomical infant nose-throat models, which represent a full-term neonate and a 9 month-old infant, respectively. They demonstrated using scintigraphic deposition that, at lower flows (ie, 2 and 4 L/min), the vibrating mesh nebulizer used resulted in higher lung deposition in both models compared to the jet nebulizer used at 8 L/min.19 One possible reason for the variation may be the difference in the methods of drug quantification used in both studies. The urinalysis method that we used indicates the effective lung dose (ie, the drug fraction cleared from lungs by absorption into the bloodstream), whereas the scintigraphic method determines this fraction in addition to that eliminated by mucociliary clearance in the lungs.20 Additionally, differences in target populations, nebulizer positions within the HFNC circuit, and experimental setups could result in such variation.
Rabea et al17 evaluated aerosol delivery with 3 types of vibrating mesh nebulizer (ie, Aerogen Pro, Aerogen Solo, and NIVO nebulizers) in subjects with COPD treated for exacerbation using NIV with standard recovery parameters. The authors demonstrated that 0.88%, 0.83%, and 0.94% of the nebulizer charge, respectively, were recovered from urinary salbutamol excretion 30 min post-inhalation, and 14.8%, 13.9%, and 14.7% were recovered from urinary salbutamol excretion 24 h post-inhalation, respectively. Hassan et al21 evaluated aerosol delivery with the Aerogen Pro vibrating mesh nebulizer and a sidestream jet nebulizer using NIV in subjects with COPD and observed that 1.1% and 0.7% of the nebulizer charge was recovered from urinary salbutamol excretion 30 min post-inhalation, respectively, and 13.7% and 8.2% from urinary salbutamol excretion 24 h post-inhalation, respectively. We obtained results similar to those reported by Rabea et al17 and Hassan et al,42 which highlights relative pulmonary delivery and systemic absorption, respectively.
Previously, no measurable difference in pulmonary dose delivery or systemic absorption was observed when the same inhalation devices were combined with either a HFNC system or NIV in subjects with COPD.34,39,42,45 Although those studies were done using the same urinary salbutamol pharmacokinetic technique, they were performed on different subjects, which adds more variables to those results. Hence, further comparisons between aerosol delivery in HFNC and NIV within the same patient populations are required.
Sarhan et al22 evaluated aerosol delivery with a vibrating mesh nebulizer in healthy subjects and observed that ∼2% of the nebulizer charge was recovered from urinary salbutamol excretion 30 min post-inhalation, and ∼18% was recovered from urinary salbutamol excretion 24 h post-inhalation. The lower delivery by the vibrating mesh nebulizer in subjects with COPD in our study, as well as the findings in Rabea et al and Hassan et al,34,42 compared to the findings in Sarhan et al22 in healthy subjects could be due to diminishing lung function in subjects with COPD compared to healthy subjects.
The results of our study are similar to our previous bench study,26 with the exception that the vibrating mesh nebulizer was used with a spacer, which demonstrated better dosing than how it was used in the in vitro study.26 This equipment combination resulted in approximately the same pulmonary deposition as the vibrating mesh nebulizer with a T-piece compared to the lower emitted dose in the in vitro study.26 The 2 preliminary pMDI puffs delivered when using the large spacer prior to vibrating mesh nebulizer delivery, as recommended by the manufacturer, could account for this difference because it probably causes an initial bronchodilator effect,40,47 increasing the lung deposition of salbutamol and consequently obtaining comparable results with a T-piece.24 However, the insignificant difference for urinary salbutamol excretion percentage 30 min post-inhalation and urinary salbutamol excretion percentage 24 h post-inhalation between the T-piece and the T-piece with spacer does not encourage the incorporation of this method as a new practice in nasal cannula–mediated aerosol delivery. However, the ex vivo element of this study showed that the vibrating mesh nebulizer with a spacer resulted in higher salbutamol deposition on the filter than both the vibrating mesh nebulizer with a T-piece and the jet nebulizer (P < .001). These results differed from the relative pulmonary deposition (urinary salbutamol excretion percentage 30 min post-inhalation) and systemic absorption (urinary salbutamol excretion percentage 24 h post-inhalation). This difference could be due to the position of the ex vivo filter, which was between the nebulizer and the opening of the nasal cannula, representing the amount of aerosol available to enter the respiratory system. The different dose between the in vivo and the ex vivo setups could be attributed to the small diameter of the cannula compared to the inserted spacer and the T-piece, which may preferentially allow only smaller particles to pass through it and reach the subject. If so, the use of the large spacer in a HFNC circuit would be of little benefit in conserving aerosol compared to the normal T-piece of the nebulizer.
When both a T-piece and a large spacer are utilized in either invasive ventilation or NIV, larger drug losses are reported during aerosol delivery, which results in a lower drug fraction delivered to the lungs (ie, 1–10% among adults in HFNC therapy).30,38,48 To reduce aerosol losses in HFNC circuits from 50–70% to < 20%, newer methods such as a condensational growth technique could be applied for the delivery of aerosols with submicrometer-sized droplets, which have proven high efficiency in delivering inhaled aerosols to the lungs within HFNC systems, rather than conventional micrometer-sized aerosols.25,29 However, validation of the condensational growth technique in animal and human models is needed before clinical use.30,35
In addition, HFNC improves heating and humidification of gas compared to NIV and can improve mucociliary clearance, decrease mucus viscosity, and thus facilitate expectoration in critically ill patients.27,34 Lung function and quality of life measurements could be improved, in addition to significant reduction in exacerbation frequencies and exacerbation days.36 Similarly, patient tolerance would likely be improved with continuous or prolonged nebulized aerosols.19 Nevertheless, long-term intranasal drug delivery and its effect on the mucosal lining anatomy and physiology, in addition to ciliary function, requires more scrutiny.28
Edwards et al31 compared the effect of oxygen or air as the driving gases in nebulizers on PaCO2 in subjects with COPD receiving salbutamol. The air-driven nebulizer tested did not affect PaCO2, whereas a significant rise in PaCO2 was noticed in subjects with baseline hypercapnia and not in the normocapnic COPD subjects. This effect was not investigated in our study, but such an increase was not expected because subjects involved in this study had no previous history of hypercapnia.
Overall, combining aerosol delivery with HFNC therapy is a desirable medical practice and is considered to be advantageous for patients and health care providers instead of the frequent treatments administered with a mouthpiece.3
In this study, salbutamol delivered at low oxygen flow through a HFNC circuit improved aerosol delivery to subjects compared to delivery data from previous bench studies.16,18,19 However, at low flows, some physiological benefits of using higher flows, such as nasopharyngeal dead-space washout and decreased work of breathing through inspiratory flow matching of the patient, will be diminished.27 However, titrating the flow back to higher levels after drug nebulization could overcome this problem.
Using low-flow oxygen is supported by Austin et al,32 who reported that subjects with COPD exacerbation given titrated oxygen in the pre-hospital setting rather than high flows to obtain PaO2 saturation falling in the range of 88–92% had a significant reduction in respiratory acidosis and mortality rates. In this way, hypoxemia could be corrected in COPD patients by the use of titrated oxygen while simultaneously avoiding unnecessarily high PaO2.33
Our study has several limitations. The lower significance difference found here in relative pulmonary deposition (urinary salbutamol excretion percentage 30 min post-inhalation) and systemic absorption (urinary salbutamol excretion percentage 24 h post-inhalation) compared to our previous in vitro result was expected because the coefficient of variation for urinary salbutamol was reported to be relatively high, as seen in our results. This finding and the expected 35% intra- and inter-patient variability contributed to the lack of statistical significance in our small patient population. However, our pilot data can now be used to appropriately size future studies.
Conclusion
Aerosol delivery by nebulizers can be efficiently combined with an adult HFNC circuit using low-flow oxygen in patients with COPD and a history of exacerbations. After treatment delivery, titrating the oxygen flow back to obtain the targeted arterial oxygen saturation may be a necessary recommendation, but this requires further investigation in clinical settings. The vibrating mesh nebulizer with both a T-piece and large spacer provided higher pulmonary drug delivery than the traditionally used jet nebulizer when combined with oxygen therapy within a HFNC circuit. However, no significant benefit of using the large spacer with the combined pMDI-nebulizer delivery was found.
Acknowledgments
Dr James B Fink provided his aerosol science expertise for the development of the manuscript. Professor Brendan Higgins lent his support in aerosol science expertise and English language editing for the development of the manuscript. Professor Sameh AbdelGhani proofread the manuscript.
Footnotes
- Correspondence: Mohamed EA Abdelrahim PhD, Faculty of Pharmacy, Beni-suef University, Salah Eldin street, Beni-suef, Egypt. E-mail: mohamedemem9{at}yahoo.com.
Dr Abdelrahim discloses a relationship with Aerogen. The other authors have disclosed no conflicts of interest.
- Copyright © 2019 by Daedalus Enterprises