Abstract
BACKGROUND: The overwhelming demand for mechanical ventilators due to COVID-19 has stimulated interest in using one ventilator for multiple patients (ie, multiplex ventilation). Despite a plethora of information on the internet, there is little supporting evidence and no human studies. The risk of multiplex ventilation is that ventilation and PEEP effects are largely uncontrollable and depend on the difference between patients’ resistance and compliance. It is not clear whether volume control ventilation or pressure control ventilation is safer or more effective. We designed a simulation-based study to allow complete control over the relevant variables to determine the effects of various degrees of resistance-compliance imbalance on tidal volume (VT), end-expiratory lung volume (EELV), and imputed pH.
METHODS: Two separate breathing simulators were ventilated with a ventilator using pressure control and volume control ventilation modes. Evidence-based lung models simulated a range of differences in resistance and compliance (6 pairs of simulated patients). Differences in VT, EELV, and imputed pH were recorded.
RESULTS: Depending on differences in resistance and compliance, differences in VT ranged from 1% (with equal resistance and compliance) to 79%. Differences in EELV ranged from 2% to 109%, whereas differences in pH ranged from 0% to 5%. Failure due to excessive VT (ie, > 8 mL/kg) did not occur, but failure due to excessive EELV difference (ie, > 10%) was evident in 50% of patient pairs. There was no difference in failure rate between volume control and pressure control ventilation modes.
CONCLUSIONS: These experiments confirmed the potential for markedly different ventilation and oxygenation for patients with uneven respiratory system impedances during multiplex ventilation. Three critical problems must be solved to minimize risk: (1) partitioning of inspiratory flow from the ventilator individually between the 2 patients, (2) measurement of VT delivered to each patient, and (3) provision for individual PEEP. We provide suggestions for solving these problems.
Striving to better, oft we mar what is well. —William Shakespeare
Introduction
The notion that a single ventilator could be used to ventilate more than one patient was suggested in 2006. We refer to this technique as multiplex ventilation because the word “multiplex” is defined as a system or signal involving simultaneous transmission of several messages along a single channel of communication (analogous to transmitting gas destined to be more than one tidal volume [VT] from a single source). The original paper by Neyman and Irvin1 reported ventilation of 4 simple test lungs using a single ventilator and 4 separate circuits. No scientific measurements were attempted. That work was followed by a study by Paladino et al,2 who used 4 sheep to demonstrate that hourly blood gases were required to maintain adequate gas exchange in animals with normal lungs. The authors reported that normal animals had both hypercarbia and hypoxemia related to maldistribution of volumes. These studies included severe limitations, as noted by Branson and colleagues,3,4 yet the idea has been resurrected by the recent COVID-19 pandemic due to the possibility of a shortage of mechanical ventilators. Indeed, social media has given this scheme a life of its own, with one source claiming to allow ventilation of 9 patients at once (https://interestingengineering.com/canadian-doctor-rigs-ventilator-to-treat-nine-patients-instead-of-just-one, Accessed March, 23, 2020)!
To date, there are no published studies of the use of multiplex ventilation in humans and only anecdotal short-term use in traumatic injury. The previously mentioned media report failed to yield any meaningful data. There are several theoretical complications that must be considered before such use should be attempted. The 2 primary problems with any mode of ventilation are setting safe and effective values for VT and PEEP. These problems are exacerbated with multiplex ventilation because VT and PEEP are not adjustable with the systems described in the literature.
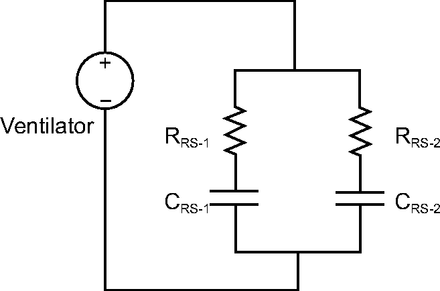
When performing multiplex ventilation with 2 patients, they share the VT provided by the ventilator. Those shares are determined by the mechanical properties of their respective respiratory systems. Each patient gets the same PEEP (set on the ventilator), but the effect on end-expiratory lung volume (EELV) depends on the individual’s respiratory system compliance. Each respiratory system can be represented as a flow resistance (representing the natural and artificial airways) connected in series with a compliance (representing the lungs and chest wall), what we will call a resistance-compliance circuit (the product of resistance and compliance also represents the respiratory system time constant) (Fig. 1). These 2 resistance-compliance circuits (and their individual time constants) are connected in parallel to the ventilator (ie, they share the same pressure driving flow). The flow (and hence VT) received by each patient depends on the relative flow impedances of their resistance-compliance circuits; the higher the impedance, the lower the flow and VT. Given that both patients share the same ventilatory frequency (ie, the breathing frequency set on the ventilator), each patient receives a minute ventilation (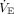 ) determined by their relative mechanical properties. Hence, as a rule of thumb, the patient with the lowest impedance will receive the largest VT and the highest
) determined by their relative mechanical properties. Hence, as a rule of thumb, the patient with the lowest impedance will receive the largest VT and the highest  . This could pose a risk of volutrauma to that patient and a risk of hypoventilation to the other patient.
. This could pose a risk of volutrauma to that patient and a risk of hypoventilation to the other patient.
Electrical circuit equivalent of multiplex ventilator circuit. RRS = resistance of respiratory system; CRS = compliance of respiratory system.
PEEP is intended to increase EELV, decrease intrapulmonary shunt, and improve oxygenation, but excessive PEEP (ie, intrathoracic pressure) has negative consequences. The risks of volutrauma and hemodynamic compromise appear to exceed the risk of atelectrauma.5 At end expiration, if flow is zero, each patient is exposed to the same level of PEEP. Hence, their EELVs will be determined by respiratory system compliance (ie, volume = pressure × compliance). When patients have different compliances, they will experience different risks of both poor oxygenation (ie, PEEP too low) and hemodynamic compromise (ie, PEEP too high).
Finally, for multiplex ventilation to work at all, patients must be chemically paralyzed, otherwise random trigger efforts will invoke chaos in the ventilatory pattern, leading to alarms and ventilatory compromise. Clearly one patient should not be allowed to determine the 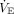 of the other by means of a higher ventilatory drive and trigger rate. It is also theoretically possible that the increased compliance of the parallel ventilator circuits may defeat triggering efforts and gas may move between circuits (ie, pendelluft flow), risking cross infection. Note that parallel compliances are additive, and this increased circuit compliance may be rejected by the ventilator during a pre-use operational verification procedure. If this is true, then compensation for the patient circuit compressible volume during volume control ventilation (VCV) must be performed manually.
of the other by means of a higher ventilatory drive and trigger rate. It is also theoretically possible that the increased compliance of the parallel ventilator circuits may defeat triggering efforts and gas may move between circuits (ie, pendelluft flow), risking cross infection. Note that parallel compliances are additive, and this increased circuit compliance may be rejected by the ventilator during a pre-use operational verification procedure. If this is true, then compensation for the patient circuit compressible volume during volume control ventilation (VCV) must be performed manually.
Although these risks of multiplex ventilation are patently obvious, their magnitudes (as functions of respiratory system resistance and compliance) are not. Nor is it clear whether VCV (ie, preset tidal volume and inspiratory flow) or pressure control ventilation (PCV; ie, preset inspiratory waveform or inspiratory pressure proportional to inspiratory effort) would be preferable.6 Therefore, we designed a simulation-based study to allow complete control over the relevant variables. Specifically, we sought to determine the effects of various degrees of resistance-compliance imbalance on resultant imbalance in VT,  , EELV, and imputed pH.
, EELV, and imputed pH.
QUICK LOOK
Current knowledge
The use of a single ventilator to ventilate more than one patient was suggested in 2006. To date, there are no published studies of actual use of multiplex ventilation in humans and only anecdotal short-term use in traumatic injury. There are several theoretical complications that must be considered before such use should be attempted.
What this paper contributes to our knowledge
This simulation-based study confirmed the potential for markedly different ventilation and oxygenation for patients with very different respiratory system impedances during multiplex ventilation. Three critical problems must be solved to improve clinical management and minimize risk: (1) partitioning of inspiratory flow from the ventilator between the 2 patients for individualized VT, (2) a means of measuring the VT delivered to each patient, and (3) provision for individual PEEP, with the possibility of one patient having PEEP higher than the value set on the ventilator.
Methods
We restricted this study to the case of ventilating 2 simulated patients with a single ventilator for simplicity. The 2 simulated patients were connected in parallel in 2 configurations. The first configuration was identical to that originally described by Neyman and Irvin (Fig. 2).1 However, in this configuration, when the impedance of Patient 1 is higher than that of Patient 2—imagine complete obstruction for clarity—gas is shunted from the Y-adapter of Patient 1 through the exhalation limb (containing CO2 from the last exhalation) and into Patient 2.
Schematic of multiplex ventilation with 2 patients connected in parallel, no one-way valves. Potential CO2 rebreathing due to increased impedance of Patient 1 relative to Patient 2.
On the other hand, to individualize VT delivery, it is possible to balance the flows to the 2 patients by increasing the resistance of the inspiratory circuit of 1 patient. For example, if the need is to decrease the VT of Patient 1, then increasing impedance in that circuit can accomplish that; however, without one-way valves, this will cause some amount of CO2 rebreathing for Patient 2 (Fig. 3). Therefore, one-way valves should be placed in the expiratory limbs for both patients (Fig. 4). Note that, for some ventilators, such valves may interfere with patient triggering of inspiration because the pressure sensor for triggering is in the expiratory portion of the ventilator. However, as mentioned above, paralysis is required, so patient initiated triggering is not an issue.
Schematic of multiplex ventilation with 2 patients connected in parallel, no one-way valves. Potential CO2 rebreathing due to increased impedance of the inspiratory limb of Patient 1.
Schematic of multiplex ventilation with 2 patients connected in parallel, with a one-way valve added to prevent rebreathing.
Mechanical ventilation was implemented with a Servo-i (Getinge Medical, Rastatt, Germany) using either VCV with set-point targeting or PCV with set-point targeting.6 Settings are shown in Table 1. The ventilator was connected to 2 ASL 5000 breathing simulators (software version 3.6, IngMar Medical, Pittsburgh, Pennsylvania). Simulation models created with this device are composed of a lung model (respiratory system resistance and compliance) and an effort model (muscle pressure as a function of time). Lung model parameters are shown in Table 2. The effort model was set to simulate a paralyzed patient (ie, maximum muscle pressure = 0, representing no inspiratory effort).
Ventilator Settings
Experimental Lung Models
The design of the lung models (Table 2) was based on several considerations. First, the values for resistance and compliance had to be realistic.7 This was assured by using values from the study by Arnal et al8 and by making the values similar to those from a small number of patients ventilated for COVID-19 at Cleveland Clinic in Cleveland, Ohio. Second, we wanted to evaluate the effects of resistance-compliance imbalance for resistance separately from compliance to determine which might have a larger effect on the distribution of VT and  . Third, we wanted to define extreme cases to roughly characterize the performance envelope of multiplex ventilation.
. Third, we wanted to define extreme cases to roughly characterize the performance envelope of multiplex ventilation.
Outcome Variables
The effects of unbalanced lung mechanics were represented by 3 variables: VT (in both mL and mL/kg, assuming a 70 kg ideal body weight for both patients), 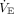 , EELV, and imputed values for
, EELV, and imputed values for  and pH. Tidal volume was reported by the simulator (as measured, meaning uncorrected for temperature or humidity) and obtained from recordings using the Multi-Parameter Trend option of the Post-Run Analysis feature of the ASL 5000 software.
and pH. Tidal volume was reported by the simulator (as measured, meaning uncorrected for temperature or humidity) and obtained from recordings using the Multi-Parameter Trend option of the Post-Run Analysis feature of the ASL 5000 software.  was calculated as the product of measured average VT and breathing frequency set on the ventilator. EELV was obtained from lung volume waveform recordings using the Multi-Parameter Waveforms option of the Post-Run Analysis feature of the ASL 5000 software (EELV = 0 for PEEP = 0). Imputed
was calculated as the product of measured average VT and breathing frequency set on the ventilator. EELV was obtained from lung volume waveform recordings using the Multi-Parameter Waveforms option of the Post-Run Analysis feature of the ASL 5000 software (EELV = 0 for PEEP = 0). Imputed 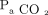 was calculated as (0.863 ×
was calculated as (0.863 × 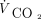 )/(
)/( × [1 – VD/VT]), where 0.863 is the factor to reconcile measurement units,
× [1 – VD/VT]), where 0.863 is the factor to reconcile measurement units, 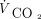 is carbon dioxide output (assumed to be 200 mL/min), and VD/VT is the dead space fraction (assumed to be 0.5, an average of our COVID-19 patients).9 Imputed pH was calculated as 6.1 + log(24/[0.03 ×
is carbon dioxide output (assumed to be 200 mL/min), and VD/VT is the dead space fraction (assumed to be 0.5, an average of our COVID-19 patients).9 Imputed pH was calculated as 6.1 + log(24/[0.03 ×  ]), where 24 is a normal bicarbonate concentration.9
]), where 24 is a normal bicarbonate concentration.9
Data Analysis
All data are reported as the mean of at least 10 breaths. The difference between simulated patients for any variable was calculated as the absolute difference of the 2 values for that variable divided by the average of the 2 values. Standard deviations are not given because they are very small with this type of simulation (eg, the coefficient of variation for VT was approximately 0.05%). Hence, any statistical test for difference between means (eg, a t test) will almost always yield significant results for differences that are not clinically important. For example, in the case of VCV, where both patients have identical VT, the measured mean (SD) VT values were 396.18 (0.206) mL and 392.88 (0.194) mL. A t test for difference between the means yields a P value < .001. However, the difference amounts to only 1%, which is much less than the error of the ventilator’s VT control system. Hence we deemed this a priori as not clinically important.
To interpret these data, we assumed the following arbitrary safe limits: acceptable VT delivery = 4–8 mL/kg, acceptable difference in EELV = 10%, and acceptable pH = 7.20–7.45. The lower limit on VT is, in practice, determined by VD, which was 200 mL in this simulation.
Results
VCV Circuits With No One-Way Valves
Experimental data for VCV are shown in Table 3. When the lung mechanics of the 2 simulated patients were identical, there was no important difference in outcome variables. However, with unequal compliance, the distribution of ventilation was markedly different. Comparing a simulated patient with mild ARDS with one who has severe ARDS, there was a 32% difference in VT (lower compliance resulted in lower VT). The difference in compliance produced a 2% difference in pH and a 76% difference in EELV, which exceeded the safe limits for EELV. Extreme differences in compliance further exacerbated differences in the distribution of VT. Comparing a simulated normal patient (eg, early in the progression of COVD-19) with one who has severe ARDS, there was a 54% difference in VT (ie, much lower compliance resulted VT below safe limit). The difference in compliance produced an 86% difference in EELV and a 3% difference in pH, both of which exceeded safe limits.
Experimental Data for Volume Control Ventilation Without One-Way Valves
The impact of resistance differences was not as severe as changes in compliance. The inequality in resistance of the simulated patient was modeled using the resistance of a patient with a heated humidifier (ie, lower resistance) compared to one who has a heat and moisture exchanger. We would probably only use a heat and moisture exchanger in the practice of multiplex ventilation, and we would not mix the 2 types of humidification. There was a 23% difference in VT (ie, higher resistance resulted in lower VT). The difference in resistance produced a 2% difference in EELV and a 1% difference in pH, neither of which exceeded safe limits. Comparing a simulated patient with mild ARDS to a simulated patient with both asthma and ARDS, there was a 79% difference in VT (ie, much higher resistance resulted in VT below safe limit). The difference in resistance produced a 7% difference in EELV and a 5% difference in pH, and this difference in pH exceeded the predefined safe limit.
The impact of extreme differences in time constant (τ) produced large changes in EELV. Comparing a simulated patient with severe ARDS to a patient with COPD (early in the progression of COVD-19), there was a 6% difference in VT (ie, the longer time constant of the patient with COPD resulted in lower VT). The difference in resistance produced a 0% difference in pH and a 108% difference in EELV, which far exceeded the safe limit for EELV.
PCV Circuits With No One-Way Valves
As with VCV, we assumed the following arbitrary limits for delivering safe mechanical ventilation to patients with ARDS to interpret the data: acceptable VT delivery = 4–8 mL/kg, acceptable difference in EELV = 10%, and acceptable pH = 7.20–7.45. The lower limit on VT is, in practice, determined by VD, which was 200 mL in this simulation. Experimental data for PCV are shown in Table 4.
Experimental Data for Pressure Control Ventilation Without One-Way Valves
When the lung mechanics of the 2 simulated patients were identical, there was no important difference in outcome variables. Unequal compliance resulted in changes in VT distribution as predicted. Comparing a simulated patient with mild ARDS with one who has severe ARDS, there was a 59% difference in VT (ie, lower compliance resulted VT below safe limit). The difference in compliance produced a 76% difference in EELV and a 4% difference in pH, with EELV exceeding the safe limit. Extreme differences in compliance created greater differences. Comparing a simulated normal patient (eg, early in the progression of COVD-19) with one who has severe ARDS, there was a 64% difference in VT (ie, much lower compliance resulted in VT below safe limit). The difference in compliance produced an 86% difference in EELV and a 4% difference in pH, and these differences exceeded safe limits.
Changes in resistance resulted in less severe distribution of volumes. In the case of mildly different resistances, there was a 21% difference in VT (ie, higher resistance resulted in lower VT). The difference in resistance produced a 2% difference in EELV and a 1% difference in pH, neither of which exceeded the safe threshold.
Comparing a simulated patient with mild ARDS with a patient with both asthma and ARDS (ie, extreme resistance differences), there was a 69% difference in VT (ie, much higher resistance resulted in VT below safe limit). The difference in resistance produced a 7% difference in EELV and a 4% difference in pH, both of which exceeded safe limits.
Extreme differences in τ produced large differences in EELV. Comparing a simulated patient with severe ARDS to a patient with COPD (early in the progression of COVD-19), there was a 9% difference in VT (ie, the longer time constant of COPD resulted in VT below safe limit). The difference in resistance produced a 1% difference in pH and a 106% difference in EELV, which far exceeded the safe limit for EELV.
VCV Versus PCV
Circuits With No One-Way Valves.
When the lung mechanics of the 2 simulated patients were identical, there were no important differences between PCV and VCV with respect to delivered VT or VEE. When compliance was unbalanced, PCV produced greater differences in VT than VCV (59–64% vs 32–54%, respectively). When resistance was unbalanced, PCV produced smaller differences in VT than VCV (21–69% vs 23–79%, respectively). Extreme differences in τ resulted in similar differences in volume distribution. Comparing a simulated patient with severe ARDS to a patient with COPD, PCV produced a slightly greater difference in VT than VCV (9% vs 6%).
Circuits With One-Way Valves Added.
Experimental data for VCV are shown in Table 5, and experimental data for PCV are shown in Table 6. In comparison with the data for multiplex ventilation without one-way valves (Table 3, Table 4), several differences can be seen.
Experimental Data for Volume Control Ventilation With One-Way Valves
Experimental Data for Pressure Control Ventilation With One-Way Valves
For VCV, the use of one-way valves increased the VT differences in the case of unequal compliance (32% without valves vs 50% with valves). This effect was not seen in the case of extreme inequality in compliance. The use of one-way valves decreased the VT differences in the case of unequal resistance (23% without valves vs 24% with valves), and this was similar in the case of extreme inequality in resistance (79% without valves vs 72% with valves). The use of one-way valves increased the VT difference in the case of inequality in τ (6% without valves vs 8% with valves).
For PCV, the use of one-way valves had virtually no effect on the VT differences in the case of unequal compliance (59% without valves vs 58% with valves). This was similar to the case of extreme inequality in compliance (64% without valves vs 63% with valves). This held true in the case of inequality in resistance (21% without valves vs 22% with valves) and extreme inequality in resistance (69% without valves vs 69% with valves), as well as in the case of extreme inequality in τ (9% without valves vs 8% with valves).
VCV With Ramp Flow Versus Constant Flow
We repeated the VCV experiments with one-way valves using a descending ramp flow instead of constant flow because the latter is very popular among respiratory therapists in the United States. We kept the tidal volume and inspiratory time the same as for constant flow (Table 7). The overall pattern of failure (ie, values in bold) was the same as for VCV with constant flow and for PCV.
Experimental Data for Control Ventilation With Ramp Flow With One-Way Valves
When the lung mechanics of the 2 simulated patients were identical, there were no differences in delivered VT or VEE between constant flow and ramp flow (see above). When compliances were unbalanced, ramp flow produced a greater difference in VT than constant flow (59–70% vs 50–54%, respectively). Ramp flow results were similar to PCV results in this regard.
When resistances were unbalanced, ramp flow produced less difference in VT than constant flow (19–62% vs 24–72%, respectively). Ramp flow results were similar to PCV results in this regard. Extreme difference in τ had a minor impact on volume distribution. Comparing a simulated patient with severe ARDS to a patient with COPD, ramp flow produced a slightly lower difference in VT than constant flow (7% vs 8%).
Discussion
This study confirms that during multiplex ventilation with 2 patients, major outcome variables for each patient, such as  (determinant of
(determinant of 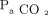 ) and EELV (determinant of
) and EELV (determinant of  in ARDS), are dependent on the distribution of lung mechanics (ie, resistance and compliance) between patients. Lower compliance and higher resistance for one patient will decrease VT,
in ARDS), are dependent on the distribution of lung mechanics (ie, resistance and compliance) between patients. Lower compliance and higher resistance for one patient will decrease VT,  , and pH for that patient compared to the other patient. The patient with the highest compliance will get the largest VT and greatest effect of PEEP (ie, largest EELV). For the case of extreme inequality in τ, (ie, the simulated patient with ARDS vs the simulated patient with both ARDS and COPD), the decrease in VT due to increased resistance was partially balanced by the effect of increasing VT by the higher compliance. Our comparison between normal mechanics and ARDS was done for theoretical and illustrative purposes only. However, we emphasize the importance of thorough screening to avoid pairing patients with comorbidities that complicate matching such as asthma or COPD. Importantly, matching patients simply by height or predicted body weight, as suggested by others,1 is unwise without knowledge of lung mechanics.
, and pH for that patient compared to the other patient. The patient with the highest compliance will get the largest VT and greatest effect of PEEP (ie, largest EELV). For the case of extreme inequality in τ, (ie, the simulated patient with ARDS vs the simulated patient with both ARDS and COPD), the decrease in VT due to increased resistance was partially balanced by the effect of increasing VT by the higher compliance. Our comparison between normal mechanics and ARDS was done for theoretical and illustrative purposes only. However, we emphasize the importance of thorough screening to avoid pairing patients with comorbidities that complicate matching such as asthma or COPD. Importantly, matching patients simply by height or predicted body weight, as suggested by others,1 is unwise without knowledge of lung mechanics.
VCV provides a more equal distribution of VT than PCV in the case of unequal compliance values, but distribution is less equal with differing resistances. These observed differences between VCV and PCV are supported by a previous theoretical study that compared 2 hypothetical lung units with different impedances (analogous to 2 patients with different respiratory system impedances) during VCV (ie, constant inspiratory flow) and PCV (ie, constant inspiratory pressure).10 The results suggest that for patients with equal impedance, both PCV and VCV result in equal distribution of volume between the patients. For patients with different compliance values but equal resistance, VCV yields more uniform VT distribution than PCV and possibly lower risk of either hypoventilation or volutrauma for one of the patients. For patients with different resistance values but equal compliance, PCV provides more uniform VT distribution than VCV.10 Failure of ventilation (ie, imputed pH < 7.20) occurred during VCV for the cases of extremely unequal compliance and extremely unequal resistance. Failure of ventilation occurred during PCV for the same cases.
Failure of PEEP can be inferred from EELV results. For VCV, the set PEEP would be too low for one of the patients in the case of unequal compliance but not unequal resistance. On the other hand, set PEEP would be too high for one of the patients in the case of extremely unequal τ (ARDS vs ARDS + asthma). For PCV, the PEEP effects were the same.
Finally, although not mentioned in the original studies of multiplex ventilation, from a theoretical standpoint (Fig. 4) it appears that one-way valves are a necessary addition to the exhalation limbs of the circuits. Addition of one-way valves had minor effects on volume distribution in VCV but not in PCV. We cannot infer effects of rebreathing (ie, inhaling previously exhaled CO2) because we did not test this hypothesis. Use of chemical paralysis is required for multiplex ventilation to avoid the effects on 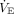 of one patient triggering at a different rate than the other. Use of one-way valves may interfere with pressure monitoring, depending on the design of the ventilator.
of one patient triggering at a different rate than the other. Use of one-way valves may interfere with pressure monitoring, depending on the design of the ventilator.
Problems and Directions for Further Research
As this study implies, matching subjects for both resistance and compliance at initiation is prudent. However, as the disease evolves in each patient, disparities are almost certain to arise. Catastrophic failure in one patient (eg, pneumothorax or a plugged endotracheal tube) may result in injury to the contralateral patient. It follows that the extent of this injury may be mitigated by use of PCV. The complexities of this technique require that it only be done with ethics board approval and family consent to treatment. Only experts in mechanical ventilation should attempt this technique under extreme duress of ventilator supply and demand.
Our data indicate that the use of multiplex ventilation without modification may be temporarily successful if patients are adequately matched for lung mechanics at initiation of ventilation. The question of how closely they must match remains. For example when the differences in compliance were great enough, (normal vs ARDS-severe) VT and pH were outside safe limits. But this was not true when the differences in compliance were 86% and both VT and pH were outside safe limits. This supports the need for continuous VT monitoring. Multiplex ventilation will fail to adequately support at least one patient as disease progresses and lung mechanics begin to differ to a great enough extent. Indeed, it appears that there are 2 major reasons for failure: low VT and either low or high PEEP.
In addition to using one-way valves, we suggest that PCV is preferable when performing multiplex ventilation. The vastly increased patient circuit compliance due to multiple parallel patients most likely will not allow the ventilator to pass a pre-use operation verification procedure. We found this to be a problem with only 2 patient circuits in parallel. Furthermore, VCV is not recommended because a sudden increase in flow impedance of one patient (eg, tube kink, mucous plug, or tube advancement into the right main-stem bronchus) will create a sudden increase in VT to the other patient, possibly to dangerous levels. The increase in total impedance as seen by the ventilator will register as a sudden increase in airway pressure. If this exceeds the high-pressure alarm setting, then inspiration will be cycled off and both patients will fail to be ventilated as long as the alarm condition remains. This sequence of events cannot occur with PCV. On the contrary, the patient whose flow impedance remains unchanged will continue to receive ventilation so long as the pressure waveform remains undisturbed. It is important to note that it is incorrect to assume that alterations in one patient’s respiratory mechanics will not affect the volume delivered to the other patient sharing the ventilator. For example, as shown in Table 6, when simulated Patient 1 with mild ARDS was paired with another patient with mild ARDS, Patient 1 received VT = 5.3 mL/kg. However, when the same simulated Patient 1 was paired with a patient who had severe ARDS, Patient 1 then received inadequate VT (3.9 mL/kg). The same effect was observed when Patient 1 was paired with a simulated patient with ARDS and asthma.
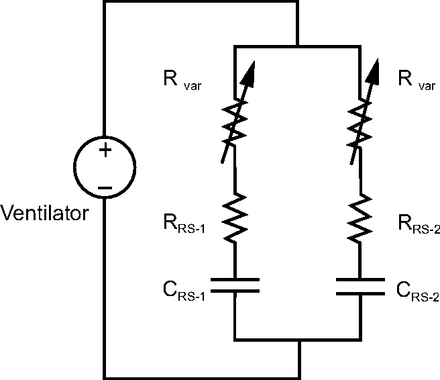
Three issues must be addressed to make multiplex ventilation more manageable at the bedside and maximize safety. The first important issue is that, because VT distribution between patients depends on the distribution of respiratory system mechanics, multiplex ventilation will fail if their impedance values differ beyond some critical threshold due to the different time courses of disease (eg, extreme imbalance in resistance or compliance, see values in bold in Tables 3–7). Hence, some means of diverting flow from one patient to the other is important to extend the time that multiplex ventilation remains effective for both patients. Multiple sources have suggested placing flow-restrictor valves in the patient circuit (Figure 5). Our experience suggests that this is not as simple as described. For some ventilators, the pressure waveform is controlled by a signal generated by a pressure sensor in the exhalation manifold. This means that any obstruction to flow in the patient circuit between the flow outlet of the ventilator and the exhalation manifold (eg, by placing adjustable valves) may alter the shape of the pressure waveform and thus alter volume distribution. A better solution is shown in Figure 6.
Electrical circuit equivalent of multiplex ventilator circuit with adjustable resistors in each inspiratory limb. RRS = resistance of respiratory system; CRS = compliance of respiratory system; Rvar = variable resistance (eg, pneumatic globe valve).
Electrical circuit equivalent of multiplex ventilator circuit with adjustable resistors in each inspiratory limb. The variable resistors are coupled such that increasing resistance in one decreases resistance in the other, hence proportioning flow to the 2 patients, ideally while maintaining the same total resistance to avoid altering the pressure waveform during pressure control ventilation. RRS = resistance of respiratory system; CRS = compliance of respiratory system.
Second, given the first issue, there is a need to monitor each patient’s VT. Alternatively, preliminary results from our related research indicate that there is a way to configure the patient circuit such that one patient exhales to atmosphere while the other exhales through the ventilator’s exhalation manifold. If this can be accomplished, then the exhaled tidal volume of one patient is displayed on the ventilator and the other is simply the difference between the displayed inhaled and exhaled values. More research in this area is imperative.
Third, our data indicate that there is a large difference in the effect of PEEP (ie, EELV) due to even a modest difference in compliance between the patients (see values in bold in Tables 3–7). When this difference reaches some critical threshold, there may be a situation where, even after careful adjustment of the PEEP setting on the ventilator, adverse oxygenation or hemodynamic consequences remain for one patient. Again, solutions have been presented on the Internet, but without any supporting evidence of effective performance. We believe we have a solution for independent PEEP control using standard patient circuit parts, but it is still in testing.
The point of explaining these 3 issues is that, by identifying the problem, an effective crowd-sourced solution may emerge in a timely fashion. What we hope to avoid is failed attempts to improve multiplex ventilation due to a misunderstanding of the basic theory. Failure may come to light, perhaps catastrophically, only when used on patients if appropriate simulation-based research is not conducted first.
Multiplex Ventilation of > 2 Patients
If you believe YouTube, ventilating 4, 9, or even 10 patients with one ventilator is just as easy as ventilating 2 patients. It might be reasonable to presume that the responses of, say, 4 patients would fall along the spectrum of extreme cases shown in this study. However, the practical problems with monitoring VT and optimizing PEEP increase not linearly, but exponentially. We do not recommend multiplex ventilation unless some means of flow balancing, VT monitoring, and customizing PEEP have been incorporated into the procedure and sufficient skill has been acquired in their use. These social networking demonstrations suggest a simple solution, one that, in fact, is fraught with peril. It is clear the presenters have not thought beyond the simplest concepts of the physical connection of tubing. These ill-advised and academically inadequate demonstrations encourage a laissez faire approach to a serious challenge and should be taken down.
Limitations
The main limitations of this study are the same for any simulation-based research. We chose only a small set of mechanical lung parameters among an infinite variety that may be experienced in clinical practice. Although we chose evidence-based values, there are as yet no published data on respiratory system mechanics for patients with COVID-19. Our results regarding use of one-way valves test the hypothesis that these valves affect the distribution of tidal volume between 2 patients. It does not test any hypothesis about rebreathing, which would have required a breathing simulator that exhaled carbon dioxide. Clinical experience and formal research of multiplex ventilation are necessary before this approach can be adequately evaluated.
Conclusions
These experiments confirmed the potential for markedly different ventilation and oxygenation for patients with uneven respiratory system impedance values during multiplex ventilation. Therefore, ventilation of just 2 patients with as single ventilator presents substantial practical problems that may preclude its use in randomly selected pairs of patients. Even if patients are matched in terms of resistance and compliance, the initial values are likely to diverge as the disease progresses (for better or worse) to the point that one patient may fail and thus endanger the other patient.
Results of this simulation-based study suggest that 3 critical problems must be solved to minimize risk: (1) partitioning of inspiratory flow from the ventilator between the 2 patients to individualize VT, (2) some means of measuring the VT delivered to each patient, and (3) provision for individual PEEP, with the possibility of one patient having PEEP higher than the value set on the ventilator. These problems are ripe for innovative solutions.
Footnotes
- Correspondence: Robert L Chatburn MHHS RRT RRT-NPS FAARC. E-mail: chatbur{at}ccf.org
Mr Chatburn has disclosed relationships with IngMar Medical, and Vyaire Medical. Mr Branson has disclosed relationships with Mallinckrodt, Ventec Life Systems, and Zoll Medical Corporation. Dr Hatipoğlu has disclosed no conflicts of interest.
SEE THE RELATED EDITORIAL ON PAGE 1059
- Copyright © 2020 by Daedalus Enterprises