Abstract
BACKGROUND: The use of shared ventilation, or the simultaneous support of multiple patients connected in parallel to a single mechanical ventilator, is receiving considerable interest for addressing the severe shortage of mechanical ventilators available during the novel coronavirus pandemic (COVID-19). In this paper we highlight the potentially disastrous consequences of naïve shared ventilation, in which patients are simply connected in parallel to a ventilator without any regard to their individual ventilatory requirements. We then examine possible approaches for individualization of mechanical ventilation, using modifications to the breathing circuit that may enable tuning of individual tidal volumes and driving pressures during either volume-controlled ventilation (VCV) or pressure-controlled ventilation (PCV).
METHODS: Breathing circuit modifications included a PEEP valve on each expiratory limb for both VCV and PCV, an adjustable constriction and one-way valve on the inspiratory limb for VCV, and a pressure-relief valve for peak inspiratory pressure reduction on the inspiratory limb for PCV. The ability to regulate individual tidal volumes using these breathing circuit modifications was tested both theoretically in computer simulations as well as experimentally in mechanical test lungs.
RESULTS: In both the simulations and experimental measurements, naïve shared ventilation resulted in large imbalances across individual tidal volume delivery, dependent on imbalances across patient mechanical properties. The proposed breathing circuit modifications for shared VCV and shared PCV enabled optimization of tidal volume distributions. Individual tidal volume for one patient during shared VCV was sensitive to changes in the mechanical properties of other patients. By contrast, shared PCV enabled independent control of individual patient-received ventilation.
CONCLUSIONS: Of the shared ventilation strategies considered, shared PCV, with the inclusion of in-line pressure-relief valves in the individual inspiratory and expiratory limbs, offers the greatest degree of safety and lowest risk of catastrophic mechanical interactions between multiple patients connected to a single ventilator.
- COVID-19
- coronavirus
- surge capacity
- mechanical ventilation
- shared ventilation
- differential lung ventilation
Introduction
Amidst the novel coronavirus (COVID-19) pandemic, there has been a surge of demand for mechanical ventilators to support patients with acute respiratory failure. New open-source, low-cost, and rapidly produced ventilators pose unknown risks to patients due to the lack of field experience, regulatory review, and post-market surveillance. Alternative respiratory devices such as anesthesia machines, transport ventilators, and noninvasive ventilators (ie, CPAP and bi-level positive airway pressure devices) may offer additional options for ventilatory support during contingencies in which the availability of ICU-grade ventilators is lacking.1 In the United States, the Food and Drug Administration has authorized the use of modified ventilator devices and breathing circuits to increase surge capacity (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-ventilators-and-accessories-and-other-respiratory-devices-during-coronavirus, Accessed April 7, 2020). Thus, one strategy that has been proposed to augment scarce ventilation resources is shared or “parallel” ventilation in which a single mechanical ventilator supports multiple patients simultaneously (Fig. 1).2,3
Naïve approach to shared ventilation. All patients are connected in parallel to a single ventilator, using separate inspiratory and expiratory limbs for each patient, but without any modifications to individualize the mechanical ventilation parameters (eg, peak inspiratory pressure, inspiratory flow). Differences in patient mechanics may adversely affect the ventilation received by one or more connected patients. The lack of one-way valves in the inspiratory and expiratory limbs of each breathing circuit also presents an infection-control hazard and allows pendelluft flow between patients.
The 2017 mass shooting incident in Las Vegas provided anecdotal proof-of-concept as to how shared ventilation may be implemented to support a sudden overflow of trauma patients with acute respiratory failure.4 In this scenario, the short-term feasibility of shared ventilation may have been possible due to the relatively normal and well-matched respiratory mechanical properties of this adult patient cohort, as well as the relatively short time frame in which it was used. However, neither a case report nor outcome data were reported in the peer-reviewed literature. The evidence is simply that it was done, with no evaluation of safety or efficacy. Previous animal studies limited the duration of shared ventilation to 12 h, expecting this to be the delay before ventilators from the national stockpile could be deployed to an affected area.5 By contrast, the current COVID-19 pandemic presents a syndrome that profoundly affects mechanical properties of the respiratory system to varying degrees of severity, along with concomitant impairments in gas exchange and ventilation-to-perfusion matching.6 Patients with COVID-19 (as well as any chronic underlying respiratory condition) exhibit wide ranges of respiratory resistance and compliance, and therefore may present vastly different requirements for ventilatory support. Additionally, it is highly unlikely that 2 well-matched patients at initiation will remain matched through progression of their disease.
The naïve approach to shared ventilation exposes all connected patients directly to the same inspiratory outlet from a single mechanical ventilator, with diversion of their collective exhaled gases to a common expiratory port. This approach was implemented in New York in response to the surge of COVID-19 patients.7 Such an arrangement mandates the delivery of controlled mechanical ventilation, in which each patient sharing the ventilator is subjected to the same breathing frequency and the same level of PEEP and 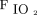 . By its very nature, shared ventilation precludes patient-triggered assisted modes, such as pressure-support ventilation (PSV) or synchronized intermittent mandatory ventilation. Shared controlled ventilation thus presents the clinician with a number of unique and challenging safety concerns that must be addressed differently for volume-controlled ventilation (VCV) versus pressure-controlled ventilation (PCV).8
. By its very nature, shared ventilation precludes patient-triggered assisted modes, such as pressure-support ventilation (PSV) or synchronized intermittent mandatory ventilation. Shared controlled ventilation thus presents the clinician with a number of unique and challenging safety concerns that must be addressed differently for volume-controlled ventilation (VCV) versus pressure-controlled ventilation (PCV).8
In this paper, we address the major concerns of shared controlled ventilation, with specific emphasis on the impact of mismatched respiratory mechanical properties. We propose potential solutions for overcoming such challenges to respiratory support, which may enable the individualization of ventilation parameters for patients sharing a common mechanical ventilator. Mathematical modeling is used to illustrate the pitfalls of the naïve approach, as well as the potential possibilities of the proposed techniques for safe ventilator sharing.
QUICK LOOK
Current Knowledge
A single mechanical ventilator can support multiple patients connected in parallel as a last resort to increase surge capacity. Patients sharing ventilation must be well-matched in terms of mechanical properties and ventilatory requirements, otherwise there may be large imbalances in received ventilation.
What This Paper Contributes to Our Knowledge
Breathing circuits were modified using pressure-relief valves and/or adjustable constrictions to enable control of individual tidal volumes in computer simulations and mechanical test lungs. A shared pressure-controlled ventilation approach with a modified breathing circuit limited adverse interactions between mechanically mismatched simulated patients connected in parallel.
Theory
Limitations of Shared VCV
In VCV, the tidal volume set on the ventilator is most often delivered with a constant inspiratory flow. At any instant in time during a shared inspiration, the distribution of flow to each patient depends on the effective resistance in each parallel pathway and the pressure difference between the ventilator inspiratory output and the effective alveolar compartment for each patient. Thus, relatively small differences in the respiratory mechanics (ie, resistance and compliance) across individual patients may produce large differences in their corresponding delivered flows and tidal volumes.8 Monitoring the individual tidal volumes received by each patient is therefore essential. Moreover, the redistribution of gas volume delivered from the ventilator may occur insidiously or suddenly across all patients due to gradual evolution of the disease process or sudden decompensation (or improvement), respectively, in a single patient. Thus, any substantial alteration of the mechanical properties of one patient’s respiratory system (whether better or worse) could be catastrophic for all connected patients. It is for this reason primarily that several professional societies have issued a joint statement against the use of parallel ventilation in patients with COVID-19.9
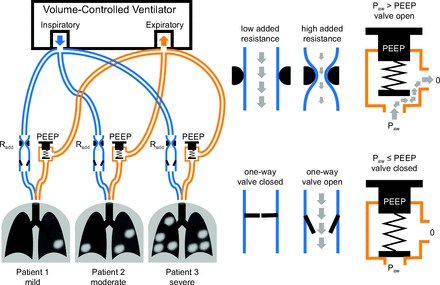
Another major concern with shared VCV is the selection of appropriate PEEP and 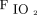 . These settings are typically increased to enhance lung recruitment and oxygenation in hypoxic patients. Adjustments to ventilator settings usually follow a stepwise protocol designed to manage hypoxia for ARDS.10 Given recent interest in the protocolization of PEEP titration for patient-specific optimization,11,12 it is no surprise that current techniques for shared ventilation, with the same PEEP applied to each patient, may pose substantial risk for ventilator-induced lung injury. For example, aggressive settings to manage hypoxia in one patient may endanger simultaneously those with compliant lungs or lower oxygen requirements. However, individualization of PEEP in shared ventilation may be possible through the use of separate PEEP valves in the expiratory limb for each individual patient’s breathing circuit. Because most mechanical ventilators require the exhaled gas to be returned to an exhalation port rather than be vented to ambient, such an arrangement would require that the individual PEEP valves are specially configured for in-line placement, with their exhaust gases feeding into a common manifold connected to the ventilator, rather than venting to atmosphere (which will also result in the release of contaminated aerosols).
. These settings are typically increased to enhance lung recruitment and oxygenation in hypoxic patients. Adjustments to ventilator settings usually follow a stepwise protocol designed to manage hypoxia for ARDS.10 Given recent interest in the protocolization of PEEP titration for patient-specific optimization,11,12 it is no surprise that current techniques for shared ventilation, with the same PEEP applied to each patient, may pose substantial risk for ventilator-induced lung injury. For example, aggressive settings to manage hypoxia in one patient may endanger simultaneously those with compliant lungs or lower oxygen requirements. However, individualization of PEEP in shared ventilation may be possible through the use of separate PEEP valves in the expiratory limb for each individual patient’s breathing circuit. Because most mechanical ventilators require the exhaled gas to be returned to an exhalation port rather than be vented to ambient, such an arrangement would require that the individual PEEP valves are specially configured for in-line placement, with their exhaust gases feeding into a common manifold connected to the ventilator, rather than venting to atmosphere (which will also result in the release of contaminated aerosols).
An additional problem for shared VCV is shunting of gas volume delivered from the ventilator into distensible breathing circuits. Sharing ventilation among patients who are mechanically in parallel requires a considerable increase in the total breathing circuit volume presented to the ventilator because each patient requires their own individual inspiratory and expiratory limbs. As such, during VCV, a sizeable portion of the flow delivered from the ventilator will be lost into distending the compliant walls of the breathing circuit and compressing the volume of gas therein. Most modern mechanical ventilators have firmware that can compensate for the resistive and compliant losses of the breathing circuit, as well as minor circuit leaks, using numerical calibration routines when the device is powered on. Such numerical routines may be subject to considerable inaccuracies, especially when there are large variations in the mechanical load presented by the patient.13 In some cases, the additional volume of circuits defeats the preoperational verification routines of ventilators essential to accurate volume delivery and monitoring.14-16 Moreover with shared VCV, the additional inspiratory and expiratory limbs for each patient requires further recalibration to reduce such gas flow shunting. Thus, there is still potential for large discrepancies between the expected and delivered tidal volumes for each patient during shared VCV, with errors expected to be compounded for multiple patients and breathing circuits even after recalibration.
Limitations of Shared PCV
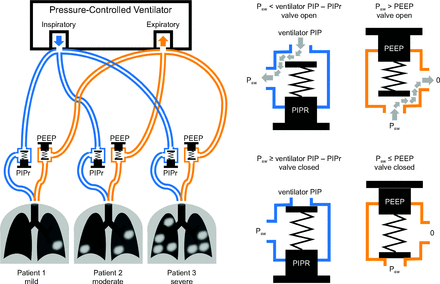
Shared ventilation in PCV avoids many of the problems associated with shared VCV, particularly shunted flow losses in the breathing circuit and the maldistribution of gas volume to patients with different respiratory mechanical properties. With PCV, each patient is exposed to a constant inspiratory pressure and will receive a tidal volume commensurate with their own respiratory compliance. Individual tidal volumes for a given patient will thus be independent of the other patients. Moreover, alterations in one patient’s respiratory mechanics will not adversely affect the volume of gas delivered to the other patients sharing the ventilator. For this reason alone, PCV presents a much more appealing alternative to VCV for shared ventilation. However, a major challenge with the shared PCV mode is the inability to individualize the peak inspiratory pressure (PIP) for each patient. In addition, like VCV, shared PCV is complicated by the challenges of individualized PEEP and  management. For example, when 2 or more patients are exposed to the same level of PEEP, their individual end-expiratory lung volumes will be determined by their respective compliances. Moreover, the presence of variable mechanical time constants in the parallel array of breathing circuits may result in variable airway flow profiles for each patient, with the possibility of dynamic hyperinflation and breath-stacking.
management. For example, when 2 or more patients are exposed to the same level of PEEP, their individual end-expiratory lung volumes will be determined by their respective compliances. Moreover, the presence of variable mechanical time constants in the parallel array of breathing circuits may result in variable airway flow profiles for each patient, with the possibility of dynamic hyperinflation and breath-stacking.
Proposed Approaches for Individualization
To overcome the limitations of shared controlled ventilation as noted above, we propose some simple modifications to the breathing circuit for each patient that have the potential to allow clinicians to individualize mechanical ventilation for multiple patients sharing a common ventilator. During either VCV or PCV, an in-line PEEP valve can be added to the expiratory limb of each individual breathing circuit (Fig. 2, Fig. 3). Given the potential for release of contaminated aerosol into the atmosphere, such a PEEP valve needs to be exhausted into a common manifold that will feed back into the expiratory port of the ventilator. Because each patient will have their own PEEP set with an external PEEP valve, the ventilator itself may be set to a low PEEP (eg, 0 cm H2O). Such an arrangement of expiratory limbs would allow for the individualization of PEEP.
Proposed modifications to breathing circuits during shared volume-controlled ventilation. A variable resistance (Radd such as an adjustable constriction), and a one-way valve are added to the inspiratory limb of the breathing circuit, and a pressure-relief valve (or PEEP valve) is added to each expiratory limb. One-way valves and PEEP valves prevent flow reversal and flow between patients. PEEP valves allow individualization of end-expiratory pressure level for each patient. Radd allows the optimization of inspired volume distribution. For example, Radd may be increased for a patient with more compliant lungs to limit the inspiratory flow and tidal volume delivered to that patient, redistributing this flow to the other connected patients. For this approach, the ventilator setting for PEEP is zero. Paw = pressure at the patient airway.
Proposed modifications to breathing circuits during shared pressure-controlled ventilation. Adjustable pressure-relief valves are added to both the inspiratory and expiratory limbs of each breathing circuit. The pressure-relief valve in the expiratory limb provides individualized PEEP, whereas the pressure-relief valve in the inspiratory limb provides individualized peak inspiratory pressure reduction (PIPr). Note that the PIPr valve is oriented with the intake toward the ventilator, whereas the PEEP valve is oriented with intake toward the patient. For this approach, the ventilator setting for PEEP is zero. Paw = pressure at the patient airway.
Customization of the inspiratory flow or pressure profile for each patient can also be achieved to a limited degree with shared ventilation. For example, with shared VCV, an adjustable resistance and one-way valve may be added to each individual inspiratory limb, allowing for some individualization of tidal volume delivery, as illustrated in Figure 2 (see also https://www.differentialmultivent.org, Accessed April 15, 2020). The same concept has been implemented for differential ventilation of 2 lungs in a single patient.17,18 Such an arrangement allows for better control of the distribution of the total volume being delivered from the ventilator to each patient. A managing clinician may increase the serial flow resistance within the inspiratory-limb of a patient with higher lung compliance in comparison to the other patients. Such an increase in this inspiratory- limb resistance would reduce the delivered volume for the patient with higher compliance, thus allowing for increased volume delivery to the other patients. The one-way valves prevent the flow of gas from one patient to another, which may occur due to coughing or spontaneous effort, discrepant mechanical time constants (ie, pendelluft flow),19 or differences in individual PEEP. The one-way valve therefore serves 2 purposes: limiting the adverse alterations in tidal volume due to mechanical interactions between connected patients, as well as promoting infection control and preventing the spread of contaminated aerosols through the breathing circuits.
With shared PCV, a spring-loaded pressure-relief valve (similar to a PEEP valve) may be added to the inspiratory line to allow for customization of PIP for each patient, as illustrated in Figure 3 (see also https://galwayventshare.com, Accessed April 7, 2020). With this arrangement, the intake of the valve is oriented toward the ventilator and the exhaust is oriented toward the patient. This valve does not provide PEEP but acts as a PIP-reduction (PIPr) valve. By way of example, if the ventilator PIP is set at 35 cm H2O for shared PCV, a patient with an inspiratory PIPr valve with a relief pressure of 20 cm H2O will have an individual PIP of only 15 cm H2O; in other words, individual PIP = ventilator PIP – PIPr.
Methods
Computer Simulations and Modeling
We used a computational model of shared ventilation to demonstrate the potential pitfalls and dangers of connecting patients in parallel to a ventilator without regard to individualization of PIP, tidal volume, or PEEP. The model can also serve to elucidate the mechanisms by which the proposed circuit alterations enable safe individualization of ventilator sharing. The methods for numerical solution of these models is provided in the online supplementary materials (http://www.rcjournal.com).
In our simplified model, the respiratory mechanics for each patient are represented by a single resistance and compliance. Breathing-circuit compliance per unit length is assumed to be 1.2 mL/(cm H2O × m) on the inspiratory limb only (volume shunting is considered irrelevant on the expiratory limb), accounting for the total length of all connected breathing circuits. Breathing-circuit resistance per unit length is conservatively assumed to be 1 (cm H2O × s)/(L × m) on each patient’s inspiratory and expiratory pathways. Inspiratory and expiratory limbs of breathing circuits were assumed to be 3 m in length. A PEEP valve was positioned in-line on each patient’s expiratory pathway to achieve individualized PEEP, with the exhaust directed back to the ventilator. The shared ventilator PEEP was set at 0 cm H2O. Breathing frequency was 12 breaths/min, with an inspiratory-expiratory time ratio of 1:2.
During VCV, the flow from the ventilator was controlled at a constant rate during inspiration, determined by the sum of individual tidal volume requirements divided by the inspiratory time. As an example, for 3 patients with desired tidal volumes of 500 mL each, the ventilator flow was set to 0.9 L/s, calculated from 1,500 mL total volume divided by an inspiratory time of 1.67 s. Note that this calculation does not account for compressible volume losses in the breathing circuit. Finally, a one-way valve and an adjustable resistance (eg, a variable constriction of a flexible tube) were added to the inspiratory pathway for each patient.
During PCV, the pressure from the ventilator was controlled at a constant value during inspiration. The ventilator PIP was set at the highest required value among all patients. For example, if the maximum individual PIP was 35 cm H2O, then the ventilator PIP was set at 35 cm H2O. A second PEEP valve was positioned in-line on each patient’s inspiratory pathway to achieved individualized PIPr by closing the inspiratory pathway once the difference between patient airway pressure and ventilator PIP increased to the individual PIPr. For example, if the desired individual PIP for a patient was 15 cm H2O but ventilator PIP was 35 cm H2O, the individual PIPr was set at 20 cm H2O.
Experimental Measurements
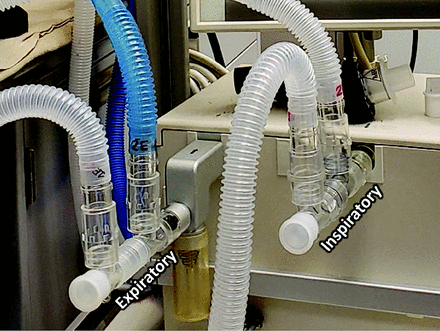
To demonstrate proof-of-concept for the computer simulations detailed above, we made in vitro measurements in mechanical test lungs using a Servo Ventilator 300 (Siemens, Munich, Germany) in both VCV and PCV modes. Breathing frequency was 15 breaths/min, with an inspiratory-expiratory ratio set at 1:2.5 throughout. Manifolds for the inspiratory and the expiratory limbs were made using T-piece adapters for each load, and fitted over the inspiratory and expiratory ports of the ventilator (Fig. 4). The inspiratory and expiratory limbs for each simulated patient consisted of standard corrugated tubing 183 cm (6 ft) in length (22 mm inner diameter), with low-resistance one-way valves (Hudson RCI 1665, Teleflex Medical, Wayne, Pennsylvania) inserted in each limb at the patient Y-piece to reduce cross-contamination risk. Airway flow and pressure were measured at the Y-piece for each simulated patient using a hotwire anemometer and the strain gauge transducer of a Florian monitor (ACUTRONIC Medical Systems, Zurich, Switzerland), with resistance, compliance, and expiratory volume calculated using the device firmware. Two mechanical test loads were placed in parallel for these experiments. Hereafter in the experimental modeling, we will use the term “patient” when referring to simulated patients for convenience. Patient 1 consisted of an Adult/Pediatric Demo Lung (IngMar Medical, Pittsburgh, Pennsylvania) with adjustable resistances and spring-loaded bellows, and Patient 2 consisted of an adjustable orifice resistor (Parabolic Resistor Ring, IngMar Medical, Pittsburgh, Pennsylvania) placed in series with various parallel combinations of anesthesia reservoir bags (1-3L, Vital Signs, Vyaire Medical, Chicago, Illinois) to achieve desired compliance values. We adjusted both loads to yield resistance values of R1 = R2 = 20 cm H2O × s/L and compliance values of C1 = C2 = 50 mL/cm H2O for the baseline conditions.
Picture of a T-based manifold connected to inspiratory and expiratory ports of the ventilator for naïve shared ventilation.
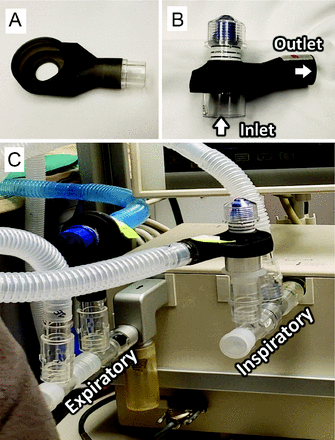
A collective expiratory PEEP manifold was developed using adjustable spring-loaded PEEP valves (0–20 cm H2O; CareFusion, San Diego, California, or AMBU, Ballerup, Denmark) modified with exhaust sleeves that were produced on a 3-dimensional printer to fit over the base of the PEEP valve, to collect and return vented gases to the expiratory manifold (Fig. 5). Design, rapid prototyping process, 3-dimensional printing, and assembly of the components was conducted at the University of Iowa’s medical device prototyping hub, Protostudios (Iowa City, Iowa). This PEEP valve was inserted into the inspiratory limb (PIPr), in which the base of the valve was connected to the manifold and the collected exhaust was connected to the patient circuit. Another PEEP valve in the expiratory limb was oriented in the opposite direction with the exhaust connected to the manifold.
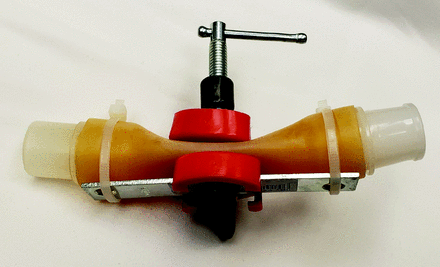
Picture of (A) a sleeve produced on a three-dimensional printer; (B) the sleeve from (A) fitted over a PEEP valve; (C) orientation of pressure-relief valves in inspiratory and expiratory limbs.
For the shared VCV protocol, a variable resistor was added to one inspiratory limb by squeezing a length of latex tubing in a C-clamp (Fig. 6). For the shared PCV protocol, an additional PEEP valve was placed in one inspiratory limb as described above to reduce the set PIP of the ventilator to one of the loads. The data collected during the protocol included peak inspiratory and end-expiratory pressures recorded by the ventilator, the exhaled tidal volume from each simulated patient, and the total tidal volume delivered by the ventilator during the inspiratory phase. For the shared PCV protocol, we also calculated the effective driving pressure to each simulated patient as ΔP = ventilator PIP – individual PIPr – max (ventilator PEEP, individual PEEP). Data were collected under conditions for which the patient models were simultaneously ventilated, with the Florian anemometer and pressure transducer alternately moved from the Y-piece of one simulated patient to the other.
Variable constriction using a C-clamp around a flexible length of tubing for implementing adjustable inspiratory resistance in the modified volume-controlled ventilation strategy.
Results
Computer Modeling and Simulations
Table 1 shows a summary of the parameters for 3 simulated patients, including resistances, compliances, and individual PEEP settings. Table 1 also provides the VCV and PCV breathing circuit modifications required to achieve uniform tidal volumes across all 3 patients. When the circuits were not modified, discrepancies in the tidal volume distribution during both VCV and PCV were enormous (Table 1), representing extreme mismatching of patients with mechanically disparate lungs. During VCV, tidal volume was > 5 times higher in the most compliant patient compared to the least compliant patient before optimization. After optimization of individual inspiratory resistance added to each inspiratory limb (Radd), tidal volumes were uniformly distributed. Note that despite 1,500 mL delivered by the ventilator, each patient received only 436.5 mL of actual tidal volume, reflecting losses in the breathing circuit. The optimal values for individual Radd were higher for simulated patients with higher compliance (Table 1). Figure 7 shows a simulation of VCV with and without the optimized inspiratory resistance applied. The simulated patients had different mechanical time constants (defined as the product of resistance and compliance), such that volume equilibration during expiration occurred at different time scales in each patient. Note that individual inspiratory flows were not constant, despite constant inspiratory flow at the ventilator. Adjusting Radd values in each inspiratory limb results in uniform distribution of tidal volumes, but each patient experienced different inflation rates throughout the inspiratory period. Also note that ventilator PIP during VCV reflects only the highest individual PIP.
Parameters of Simulated Patients Before and After Optimization*
Mathematical model results for shared volume-controlled ventilation (VCV) using the proposed modifications to the breathing circuits shown in Figure 2. Patients 1, 2, and 3 are shown in blue, red, and yellow, respectively. The ventilator waveforms are shown as black dashed lines. A: Before optimization, the added inspiratory resistance (Radd) for each patient is set to zero, representing the worst case of tidal volume maldistribution in these mismatched patients. B: After optimization, the Radd values are set as shown in Table 1, with tidal volumes distributed uniformly. Note that not all patients received the targeted 500 mL of tidal volume, despite the ventilator setting at 1,500 mL total, due to losses in breathing circuit compliance and gas compression. Also note that individual inspiratory flows were not constant despite constant inspiratory flow at the ventilator.
During PCV in the same simulated patients, tidal volume was > 4 times higher in the most compliant patient compared to the least compliant patient before optimization (Table 1). Adjusting the PIP reduction valve in each inspiratory limb resulted in uniform distribution of tidal volumes (Table 1). Figure 8 shows a simulation of PCV with and without the optimized PIP reduction applied. Note that mechanical time constants influenced the flow profiles during both inspiration and expiration during PCV. Also note that changes in PIPr for 2 of the simulated patients had no effect on the flow profile, tidal volume, or airway pressure of the third connected patient (Fig. 8).
Mathematical model results for shared pressure-controlled ventilation (PCV) using the proposed modifications to the breathing circuits shown in Figure 3. Patients 1, 2, and 3 are shown in blue, red, and yellow, respectively. The ventilator waveforms are shown as black dashed lines. A: Before optimization, the peak inspiratory pressure reduction valve (PIPr) for each patient is set to zero, representing the worst case of tidal volume maldistribution in these mismatched patients. B: After optimization, the PIPr values are set as shown in Table 1, with tidal volumes distributed uniformly. Note that all patients received the targeted 500 mL of tidal volume.
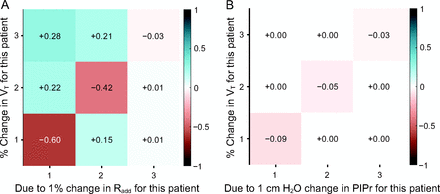
Figure 9 shows sensitivity analyses during VCV and PCV using the optimized circuits. Note that changes in one patient’s Radd affected all other patients’ tidal volumes during shared VCV, whereas during shared PCV, changes in the individual PIPr for one patient affected only that patient’s tidal volume.
Sensitivity analyses for shared volume-controlled ventilation (VCV, A) and shared pressure-controlled ventilation (PCV, B). The row of each array corresponds to the affected patient, and the column corresponds to the patient whose circuit is modified. Note that during shared VCV, a change to the added inspiratory resistance (Radd) of any patient’s breathing circuit affects all other connected patients. By contrast, during PCV each patient’s received ventilation is insensitive to changes in the peak inspiratory pressure reduction (PIPr) of any other patient’s breathing circuit.
Experimental Measurements
Table 2 presents the results from the shared VCV protocol. Under baseline conditions when the compliant loads were well-matched (ie, R1 = R2 = 20 cm H2O × s/L and C1 = C2 = 50 mL/cm H2O), the delivered volumes to each patient were each about 50% of the total ventilator VT. However when the compliance was reduced by 56% of baseline in Patient 2, the delivered VT in Patient 1 (VT1) was increased by 23% (592 mL), whereas the VT in Patient 2 (VT2) decreased by 30% (337 mL). Under these conditions, the addition of a clamp resistance of 24 cm H2O × s/L to the inspiratory limb of Patient 1 rebalanced the delivered ventilation such that VT1 = 453 mL and VT2 = 459 mL. When the compliance was reduced by 85% of its baseline in Patient 2, the delivered VT in Patient 1 increased by 52% (735 mL), while the delivered VT in Patient 2 decreased by 67% (159 mL). Under these conditions, the addition of a clamp resistance of 71 cm H2O × s/L to the inspiratory limb of Patient 1 improved the imbalance in ventilation, such that VT1 = 476 mL and VT2 = 368 mL.
Results of Shared Volume-Controlled Ventilation Protocol for Simulated Patients 1 and 2
Table 3 shows the results from the shared PCV protocol. Under baseline conditions when the compliant loads were well-matched (ie, R1 = R2 = 20 cm H2O × s/L and C1 = C2 = 50 mL/cm H2O), the delivered volumes to each patient were close in response to the same driving pressure of 10 cm H2O (ventilator PIP = 15 cm H2O, ventilator PEEP = 5 cm H2O). However when the compliance was reduced by 56% of its baseline in Patient 2, the delivered VT in Patient 1 was maintained (460 mL), while the delivered VT in Patient 2 was reduced by 59% (196 mL). In response to the change in Patient 2, the driving pressure was increased to 15 cm H2O (ventilator PIP = 20 cm H2O, ventilator PEEP = 5 cm H2O), resulting in the delivered VT in Patient 1 increasing by 47% (655 mL) while the delivered VT in Patient 2 decreased by 21% (375 mL) relative to baseline. Further increases in driving pressure to 20 cm H2O (ventilator PIP = 25 cm H2O, ventilator PEEP = 5 cm H2O) resulted in the delivered VT in Patient 1 increasing by 74% (775 mL) while the delivered VT in Patient 2 was maintained (475 mL). The addition of a PIPr valve to the inspiratory limb of Patient 1 resulted in an effective driving pressure of 10 cm H2O for Patient 1 (ventilator PIP = 25 cm H2O, Patient 1 PIPr = 10 cm H2O, ventilator PEEP = 5 cm H2O) resulting in a baseline VT of 488 mL. Simultaneously, Patient 2 had a driving pressure of 20 cm H2O (ventilator PIP = 25 cm H2O, ventilator PEEP = 5 cm H2O) resulting in baseline ventilation (488 mL). Similarly, as the compliance of Patient 2 decreased toward its baseline value (only 30% from baseline), the driving pressure for Patient 2 could be reduced from 20 cm H2O to 15 cm H2O (ventilator PIP = 20 cm H2O, ventilator PEEP = 5 cm H2O) resulting in a VT of 423 mL, while the Patient 1 PIPr was reduced by 5 cm H2O to maintain preferred driving pressure of 10 cm H2O (ventilator PIP = 25 cm H2O, Patient 1 PIPr = 5 cm H2O, ventilator PEEP = 5 cm H2O) and baseline a VT of 453 mL. Conversely, if Patient 2 compliance increased by 30% from baseline, the PIPr valve was introduced into Patient 2’s limb, such that Patient 1 received the preferred driving pressure of 10 cm H2O (ventilator PIP = 15 cm H2O, ventilator PEEP = 5 cm H2O), resulting in VT of 498 mL and Patient 2 receiving a driving pressure of 5 cm H2O (ventilator PIP = 15 cm H2O, Patient 2 PIPr = 5 cm H2O, ventilator PEEP = 5 cm H2O) and receiving a VT of 423 mL.
Results of Shared Pressure-Controlled Ventilation Protocol for Simulated Patients 1 and 2
Discussion
This study demonstrates the critical dangers associated with parallel ventilation in mechanically disparate patients. We proposed 2 approaches to individualize ventilation during shared VCV or PCV using simple modifications to each patient’s breathing circuit. Both approaches were able to achieve desired individualized ventilation across a wide range of connected patient mechanics. Based on our simulations, only shared PCV enables independent and insensitive control of individual ventilation.
During shared VCV, adding variable resistance and one-way valves to the inspiratory pathway enables control of the distribution of individual tidal volumes.17,18 The distribution of flow is commonly considered to follow the path of least resistance. However, such an assumption is applicable only to scenarios in which the same pressure differential is applied across all resistive paths. In the shared-ventilation scenario, the pressure of the gas entering the inspiratory limb of each breathing circuit may be the same, but each patient may exhibit different alveolar pressures due to differences in compliance and individualized PEEP, such that the flow of gas to each patient will be determined not only by relative differences in the resistance of each pathway but also by the alveolar pressure at the end of each pathway. Thus, respiratory resistance alone does not determine flow distribution. Nevertheless, incorporating carefully tuned resistances into the inspiratory limb for each patient can alter the dynamics of flow distribution during inspiration, such that individual inspired volumes for each patient can be appropriately measured and titrated. Unfortunately, there is no obvious heuristic approach for tuning the resistances, especially at the bedside with limited information about patient mechanics. Any supposedly optimum value for an inspiratory limb resistance during shared VCV might be determined empirically or by iteratively adjusting all patients’ added resistances until the tidal volume distribution is acceptable, assuming that the delivered tidal volume to each patient can actually be measured. In an event resulting in shortages of mechanical ventilators, it is unlikely that volume-monitoring devices would be readily available. However, such an iterative adjustment may be prohibitively tedious and is not guaranteed to be successful, especially because patient mechanics may vary over short time intervals. The tidal volume distribution with the shared VCV approach may also be sensitive to small fluctuations in patient mechanics. Therefore, implementation of shared VCV is likely to be impractical.
During shared PCV, adding a pressure-relief valve to each inspiratory pathway to act as a PIPr valve enables control of the individual PIP for each patient. Because tidal volume is determined by the product of driving pressure and lung compliance, each patient may be ventilated individually and independently using this approach.20 Fluctuations in resistance or compliance of one patient do not in theory affect the ventilation received by any other patient. Furthermore, no optimization or iterative adjustment is required for individualization of the shared ventilation. The ventilator PIP is set at the highest level required for any individual patient, and then the proposed PIPr valve is directly adjusted to achieve the lower desired individual PIP. Additionally, PEEP valves in the expiratory limb allow for individualizing the PEEP setting for each patient independent of any other patient. Thus, implementation of shared PCV appears to be more practical in comparison to shared VCV.
Despite the best efforts to match patients when initiating shared ventilation, it is inevitable that each patient will change over time, resulting in poor matches. When naïve shared ventilation is implemented, the clinician must decide when common ventilator settings are no longer beneficial or when a patient should be matched with a different partner (or partners) to improve ventilation. In the experimental portion of our study, we investigated how mechanical imbalances between patients could be addressed by interventions in both the VCV mode and the PCV mode. With shared VCV, even small changes in one patient can directly affect the other. An intervention requiring fine-tuning of a mechanical resistor in the inspiratory limb of a patient receiving a disproportionate share of the total tidal volume can rebalance the distribution of flow, albeit at the expense of individual flow or volume measurement. Shared PCV appears to be safer than shared VCV, with each patient being unaffected by changes in the mechanical properties of the other patient. For both the shared VCV and shared PCV experimental protocols, the total volume delivered by the ventilator was higher than the sum of the volumes for each patient measured separately. In the case of naïve shared ventilation, the difference was usually < 100 mL. However when the corrective interventions were implemented, the delivered volume was higher than the sum of the volume delivered to each patient, with a discrepancy of 200–300 mL in some cases. This suggests that there may have been gas leaking from our PEEP valve sleeves. Despite this, changes in delivered volume over time can alert the clinician to a change in one of the patients. Plans for the production of connectors by 3-dimensional printers to handle multiple patient circuits are available worldwide, including VESper (Prisma Health, Columbia, South Carolina), which received emergency-use authorization by the FDA in March 2020. However, it does not appear that any of these designs include or recommend one-way valves to maintain even basic separation between the patient circuits.
One might infer that changes in the overall volume delivered by the ventilator indicate that at least one of the patients has experienced a change in mechanical properties. However, it may be possible to monitor individual tidal volumes and respiratory mechanics without additional instrumentation during shared PCV. If all patients but one are temporarily occluded (eg, by clamping the endotracheal tubes), the delivered flow and tidal volume reported by the ventilator will reflect only the ventilation and mechanics of the remaining nonoccluded patient. Note that occluding all other connected patients will have virtually no impact on the tidal volume delivered to the nonoccluded patient during shared PCV. Although tedious for the clinician, and potentially hazardous for occluded patients (due to apnea and risk of acute hypoxia), this procedure may enable intermittent assessment of patient status despite a lack of instrumentation available for individual monitoring.
Limitations
As with standard implementation of unidirectional flow control valves, extreme caution must be used when constructing and connecting the modified breathing circuit. Incorrect orientation of valves may be immediately detrimental to patient safety. Even when correctly oriented, there is still some risk associated with unidirectional valves. Due to the configuration of valves in both the VCV mode and the PCV mode, there is no ability for a patient to exhale or cough during inspiration, nor to inhale during expiration. Any cough, spontaneous effort, or other asynchrony could create potentially harmful pressure excursions. Thus, pharmacologic suppression of respiratory drive, with possible neuromuscular blockade, may be necessary. Depending on the availability of components, such as adjustable PEEP valves, it may not be possible to adjust the individual PEEP, PIPr, and Radd without temporarily dismantling the breathing circuit and risking exposure to contaminated aerosols. In this study, we used rapid prototyping capabilities (Protostudios, Iowa City, Iowa) to create adaptors for common PEEP valves that allowed adjustment of pressure-relief thresholds without dismantling the breathing circuit. Appropriate infection-control protocols will need to be considered on a per-institution basis.
In addition, the ability to implement the proposed breathing-circuit modifications may be dependent on the specific manufacturer and ventilator model being used. Despite the ability to titrate airway pressures and flows for individual patients, the actual transduced pressures and flows as measured within the ventilator itself may be considerably different from its internal programing or its expectations, which may exceed its internal safety thresholds and trigger alarms. Such a scenario may be encountered if the ventilator PEEP is set to 0 cm H2O but the actual transduced PEEP is higher in the presence of the external adjustable PEEP valves. This can occur depending on whether the ventilator transduces pressure at its inspiratory outlet, at the patient Y-piece, or at the expiratory inlet. A recent non-peer-reviewed report using PCV with pressure-relief valves also implemented a bias flow with a one-way valve to prevent occlusion alarms on the ventilator.21
Continuous monitoring of arterial oxygen saturation is also desirable in individual patients sharing a single ventilator, for appropriate titration of PEEP,  , and detection of acute hypoxic events. Continuous flow monitoring in all connected patients is only necessary for individualization of delivered volume during shared VCV, during which the distribution of volume from the ventilator may suddenly change due to fluctuations in the mechanics of any single patient. Individual flow monitoring during shared PCV, although helpful for assessing minute ventilation and lung compliance, may not necessarily be required for individualization of pressure-controlled parameters, although its use should still be considered if available.
, and detection of acute hypoxic events. Continuous flow monitoring in all connected patients is only necessary for individualization of delivered volume during shared VCV, during which the distribution of volume from the ventilator may suddenly change due to fluctuations in the mechanics of any single patient. Individual flow monitoring during shared PCV, although helpful for assessing minute ventilation and lung compliance, may not necessarily be required for individualization of pressure-controlled parameters, although its use should still be considered if available.
Our computational model simulated a breathing frequency of 12 breaths/min, which may be considerably less than that used for patients with severe ARDS (eg, up to 30 breaths/min), for whom low tidal volumes or driving pressures are desirable. With high breathing frequencies, inspiratory times may be < 1 s and equilibration of patient lung volume will not be guaranteed. High expiratory resistance or flow limitation may also result in adverse effects on mechanical ventilation such as intrinsic PEEP. Because it is not possible to individualize breathing frequency during shared controlled ventilation, we did not explore the consequences of these dynamics at high breathing frequencies. Nonetheless, it is important to consider mechanical time constants (ie, the product of resistance and compliance) when determining which patients would be suitable partners for sharing a single ventilator.
Finally, the individualization of  was not considered. Theoretically, this may be achieved by including another breathing circuit component on the inspiratory pathway. For example, it may be possible to utilize a fluid dynamics phenomenon such as jet-mixing or the Venturi effect to entrain gas from ambient room air, with a one-way valve to prevent reverse flow through the entrainment device. However, such an arrangement would also augment the tidal volume delivered to each patient and mandate the use of additional sensors and monitoring of the flows and O2 content of each individual breathing circuit.
was not considered. Theoretically, this may be achieved by including another breathing circuit component on the inspiratory pathway. For example, it may be possible to utilize a fluid dynamics phenomenon such as jet-mixing or the Venturi effect to entrain gas from ambient room air, with a one-way valve to prevent reverse flow through the entrainment device. However, such an arrangement would also augment the tidal volume delivered to each patient and mandate the use of additional sensors and monitoring of the flows and O2 content of each individual breathing circuit.
Conclusions
The naïve approach to shared ventilation, without efforts to individualize the inspiratory and expiratory pressures and flows, is particularly dangerous for patients with disparate respiratory mechanical properties. Such mechanical disparity may occur among patients with COVID-19. However, given the scarcity of medical resources in certain environments, shared ventilation may be considered under extreme circumstances, after a thoughtful risk/benefit analysis. Individualization during VCV is possible via adjustable inspiratory resistances and flow monitoring, although this method is sensitive to mechanical interactions and is vulnerable to the compression of gas volume within the breathing circuit. VCV sharing, even with such inspiratory resistive compensation, should be used as a last resort because changes in the mechanics of one patient could be catastrophic for other connected patients. In contrast, individualization during PCV may be possible via inspiratory pressure-relief valves with minimal concern for mechanical interactions or pendelluft flow, and without a need for volume monitoring. PCV sharing, with inspiratory and expiratory pressure compensation and appropriate separation of patients via one-way valves, could be considered when the ability to provide ventilatory support with independent devices cannot be achieved. However, the suggested modifications described herein greatly affect the complexity of the ventilator circuit and the possibility of disconnection or inadvertent adjustments to the shared ventilatory parameters.
ACKNOWLEDGMENTS
The authors acknowledge the support of NIH STTR R41 HL140640, the Department of Anesthesia at the University of Iowa and the support of the Chief Innovation Officer’s team, which includes Protostudios, UI Ventures, and the Translational Research Incubator at the University of Iowa.
Footnotes
- Correspondence: David W Kaczka MD PhD, The University of Iowa Hospital and Clinics, Department of Anesthesia, 200 Hawkins Drive, Iowa City, IA 52242. E-mail: david-kaczka{at}uiowa.edu
Dr Kaczka has disclosed relationships with Zoll Medical Corporation and Monitor Mask. Mr Branson has disclosed relationships with Mallinckrodt, Ventec Life Systems, and Zoll Medical Corporation. Drs Herrmann and Kaczka are co-founders and shareholders of OscillaVent, and Dr Hawley is an employee of OscillaVent. The other authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
SEE THE RELATED EDITORIAL ON PAGE 1059
- Copyright © 2020 by Daedalus Enterprises