Abstract
BACKGROUND: High-frequency jet ventilation (HFJV) is primarily used in premature neonates; however, its use in pediatric patients with acute respiratory failure has been reported. The objective of this study was to evaluate HFJV use in the pediatric critical care setting. We hypothesized that HFJV would be associated with improvements in oxygenation and ventilation.
METHODS: Medical records of all patients who received HFJV in the pediatric ICU of a quaternary care center between 2014 and 2018 were retrospectively reviewed. Premature infants who had not been discharged home were excluded, as were those in whom HFJV was started while on extracorporeal membrane oxygenation. Data on demographics, pulmonary mechanics, gas exchange, and outcomes were extracted and analyzed using chi-square testing for categorical variables, nonparametric testing for continuous variables, and a linear effects model to evaluate gas exchange over time.
RESULTS: A total of 35 subjects (median age = 2.9 months, median weight = 5.2 kg) were included. Prior to HFJV initiation, median (interquartile range) oxygenation index (OI) was 11.3 (7.2–16.9), 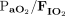 = 133 (91.3–190.0), pH = 7.18 (7.11–7.27),
= 133 (91.3–190.0), pH = 7.18 (7.11–7.27), 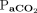 = 64 (52–87) mm Hg, and
= 64 (52–87) mm Hg, and 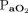 = 74 (64–125) mm Hg. For subjects still on HFJV (n = 25), there was no significant change in OI,
= 74 (64–125) mm Hg. For subjects still on HFJV (n = 25), there was no significant change in OI,  , or
, or  at 4–6 h after initiation, whereas pH increased (P = .001) and
at 4–6 h after initiation, whereas pH increased (P = .001) and  decreased (P = .001). For those remaining on HFJV for > 72 h (n = 12), the linear effects model revealed no differences over 72 h for OI,
decreased (P = .001). For those remaining on HFJV for > 72 h (n = 12), the linear effects model revealed no differences over 72 h for OI, 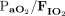 ,
, 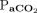 , or mean airway pressure, but there was a decrease in
, or mean airway pressure, but there was a decrease in 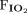 while pH and
while pH and 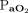 increased. There were 9 (26%) subjects who did not survive, and nonsurvivors had higher Pediatric Index of Mortality 2 scores (P = .01), were more likely to be immunocompromised (P = .01), were less likely to have a documented infection (P = .02), and had lower airway resistance (P = .02).
increased. There were 9 (26%) subjects who did not survive, and nonsurvivors had higher Pediatric Index of Mortality 2 scores (P = .01), were more likely to be immunocompromised (P = .01), were less likely to have a documented infection (P = .02), and had lower airway resistance (P = .02).
CONCLUSIONS: HFJV was associated with improved ventilation among subjects able to remain on HFJV but had no significant effect on oxygenation.
- pediatric respiratory failure
- high-frequency ventilation
- jet ventilation
- gas exchange
- pediatric ARDS
- mechanical ventilation
- children
- oxygenation
- ventilation
Introduction
Respiratory illnesses are the most common reason for admission to a pediatric ICU.1 A large, international, multi-center point prevalence study reported that 53% of subjects required invasive or noninvasive ventilation.2 The vast majority of children with acute respiratory failure requiring intubation and invasive support can be managed with conventional mechanical ventilation. Those with more severe or refractory respiratory failure, and those in whom a lung-protective strategy cannot be achieve through conventional ventilation, may be treated with high-frequency ventilation or extracorporeal life support.3 High-frequency modalities used within the pediatric ICU include high-frequency oscillatory ventilation, high-frequency percussive ventilation, and high-frequency jet ventilation (HFJV), although strong evidence supporting their use is lacking.4 In the absence of guidance from high-quality data, individual centers currently elect to utilize high-frequency ventilation based on clinician preference, experience using each modality, and the patient’s underlying physiology.
HFJV delivers high-velocity inspiratory gas into the trachea through a jet injector. Inspiratory pulses are generated by flow stream interruption at a rate range of 240–660 cycles/min with an inspiratory time of 0.02–0.03 s. This results in attenuation of the set peak inspiratory pressure (PIP) with a delivered tidal volume that is less than anatomic dead space and theoretically avoids cyclic shearing stress observed during conventional ventilation.5,6 PIP during HFJV is controlled by the flow interrupter, but unlike high-frequency oscillatory ventilation, exhalation is passive.
HFJV is currently approved by the U.S. Food and Drug Administration for use in neonates up to 28 d old, and it is used predominantly to treat perinatal respiratory failure in premature and term neonates admitted to a neonatal ICU.7-9 There are limited data evaluating HFJV use outside of the neonatal ICU setting. Single-center case series have reported successful use of HFJV in pediatric patients with air leak related to ARDS,10 critical bronchiolitis due to respiratory syncytial virus,11 congenital diaphragmatic hernia,12 and pediatric ARDS,13 although some of these studies were conducted in the early 1990s and predate the widespread adoption of lung-protective ventilation. In addition, HFJV has been used in pediatric patients with congenital heart disease, with improved carbon dioxide clearance but minimal effect on oxygenation.14,15 Thus, there is a need for additional data evaluating HFJV in larger sample sizes and mixed patient populations.
In our pediatric ICU, HFJV is used in the setting of inadequate gas exchange, refractory air leak, pulmonary interstitial emphysema, or inability to achieve lung-protective settings as defined by the Pediatric Acute Lung Injury Consensus Conference (PALICC) via a conventional ventilator.4,16 HFJV is generally our first choice of high-frequency ventilation in infants with oxygenation and ventilation failure, while high-frequency oscillatory ventilation is used in patients with more severe oxygenation and ventilation failure or in larger patients in whom HFJV would likely be ineffective, although clinical practice varies depending upon each patient’s pathophysiology. We conducted this study to demonstrate the feasibility of HFJV use in a cohort of critically ill infants with acute respiratory failure treated in the pediatric ICU. We hypothesized that HFJV would result in improvements in oxygenation and ventilation.
Quick Look
Current Knowledge
Children with severe respiratory failure are currently treated with high-frequency ventilation. High-frequency jet ventilation (HFJV) is primarily used in premature neonates; however, its use in pediatric patients with acute respiratory failure has been reported in small single-center case series. In our pediatric ICU, HFJV is used in the setting of inadequate gas exchange, refractory air leak, pulmonary interstitial emphysema, or inability to achieve lung-protective settings via conventional ventilator.
What This Paper Contributes to Our Knowledge
HFJV in pediatric acute respiratory failure was feasible in severe pediatric respiratory failure resulting from multiple etiologies. In subjects with a median (interquartile range) weight of 5.2 (3.8–6.9) kg, there were short-term improvements in  and pH in 71% of subjects and ventilation remained stable over 72 h. There were no significant changes in oxygenation at 4–6 h after initiation or over 72 h. A total of 43% of subjects required transition to other high-frequency modalities or extracorporeal membrane oxygenation and overall survival was 74%.
and pH in 71% of subjects and ventilation remained stable over 72 h. There were no significant changes in oxygenation at 4–6 h after initiation or over 72 h. A total of 43% of subjects required transition to other high-frequency modalities or extracorporeal membrane oxygenation and overall survival was 74%.
Methods
Following Institutional Review Board approval with waiver of informed consent, we reviewed the medical records of all patients > 14 d old who received HFJV in our pediatric ICU between July 2013 and December 2018 (Fig. 1). Patients were excluded if HFJV was started while the subject was on extracorporeal membrane oxygenation (ECMO) or in the neonatal ICU. Subjects were identified through a search of the electronic medical records. We collected demographic data, pertinent medical history, documented infection, surgical history, pre-HFJV ventilator settings, pre-HFJV arterial blood gas measurements, initial HFJV settings, dynamic compliance, airway resistance, volume of exhaled carbon dioxide, need for extracorporeal life support or nitric oxide use, duration of HFJV support, and survival.
Flow chart. HFJV = high-frequency jet ventilation, HFOV = high-frequency oscillatory ventilation, HFPV = high-frequency percussive ventilation, ECMO = extracorporeal membrane oxygenation.
Dynamic compliance, airway resistance, and volume of exhaled carbon dioxide measurements just prior to transition to HFJV were measured with an NM3 monitor (Phillips, Andover, Massachusetts). Oxygenation index (OI), 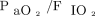 , and ventilation index were calculated from pre-HFJV values. The pre-HFJV mean airway pressure (
, and ventilation index were calculated from pre-HFJV values. The pre-HFJV mean airway pressure (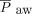 ) used for calculations was the measured
) used for calculations was the measured 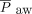 from the conventional ventilator; after HFJV initiation, the documented
from the conventional ventilator; after HFJV initiation, the documented 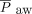 was measured by the HFJV ventilator. During HFJV, a conventional ventilator operating in tandem with the HFJV ventilator was used to apply PEEP. The desired PEEP value was set on the conventional ventilator and was measured continuously with the HFJV ventilator. Pediatric Index of Mortality 2 (PIM2) score was calculated from pediatric ICU admission values. Ventilator and gas exchange data were extracted (when available) prior to HFJV initiation, at 4–6 h, 24 h, 48 h, and 72 h post-HFJV initiation, and when subjects were transitioned back to conventional mechanical ventilation.
was measured by the HFJV ventilator. During HFJV, a conventional ventilator operating in tandem with the HFJV ventilator was used to apply PEEP. The desired PEEP value was set on the conventional ventilator and was measured continuously with the HFJV ventilator. Pediatric Index of Mortality 2 (PIM2) score was calculated from pediatric ICU admission values. Ventilator and gas exchange data were extracted (when available) prior to HFJV initiation, at 4–6 h, 24 h, 48 h, and 72 h post-HFJV initiation, and when subjects were transitioned back to conventional mechanical ventilation.
Subjects were managed via a respiratory therapist-driven protocol for both conventional mechanical ventilation and HFJV. The primary conventional ventilator mode used in this protocol is pressure-synchronized intermittent mandatory ventilation. The protocol targets a tidal volume of 6–8 mL/kg for machine-triggered breaths and 4–8 mL/kg for spontaneous breaths. PEEP is managed via a PEEP: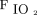 table, whereas PIP is maintained ≤ 30 cm H2O; goal pH is ≥ 7.25. Inadequate gas exchange was defined in our conventional ventilator protocol as a pH < 7.25, inadequate oxygenation as per clinical discretion based on the subject’s pathophysiology, or inability to maintain a lung-protective strategy with PIP ≤ 30 cm H2O. For the HFJV protocol,
table, whereas PIP is maintained ≤ 30 cm H2O; goal pH is ≥ 7.25. Inadequate gas exchange was defined in our conventional ventilator protocol as a pH < 7.25, inadequate oxygenation as per clinical discretion based on the subject’s pathophysiology, or inability to maintain a lung-protective strategy with PIP ≤ 30 cm H2O. For the HFJV protocol,  was titrated to optimal lung inflation and oxygenation; the HFJV rate was adjusted to minimize air-trapping as measured by the difference in set PEEP and PEEP measured with HFJV; the conventional ventilator rate was set at 0–5 breaths/min; and goal pH was > 7.25. Optimal lung inflation was defined as 8–9 ribs of expansion on bedside chest radiography. When initiating HFJV,
was titrated to optimal lung inflation and oxygenation; the HFJV rate was adjusted to minimize air-trapping as measured by the difference in set PEEP and PEEP measured with HFJV; the conventional ventilator rate was set at 0–5 breaths/min; and goal pH was > 7.25. Optimal lung inflation was defined as 8–9 ribs of expansion on bedside chest radiography. When initiating HFJV, 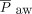 is usually set equal to the
is usually set equal to the 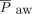 on conventional ventilation. Details of both protocols are included in the supplemental materials (available at http://www.rcjournal.com). HFJV was conducted with a Bunnell LifePulse ventilator (Bunnell, Salt Lake City, Utah) in tandem with an Avea ventilator (Vyaire, Yorba Linda, California).
on conventional ventilation. Details of both protocols are included in the supplemental materials (available at http://www.rcjournal.com). HFJV was conducted with a Bunnell LifePulse ventilator (Bunnell, Salt Lake City, Utah) in tandem with an Avea ventilator (Vyaire, Yorba Linda, California).
The primary physiologic outcome was change in oxygenation as defined by OI. Secondary outcomes included change in ventilation as measured by increase in pH with a decrease in  , duration of mechanical ventilation, duration of HFJV support, need for ECMO or inhaled nitric oxide, survival, and need for oxygen supplementation at discharge. HFJV failure was defined as transition to another high-frequency mode or ECMO, at which time data collection was discontinued. We chose not to include adverse events such as barotrauma because it was not possible to attribute adverse events to HFJV in a retrospective chart review.
, duration of mechanical ventilation, duration of HFJV support, need for ECMO or inhaled nitric oxide, survival, and need for oxygen supplementation at discharge. HFJV failure was defined as transition to another high-frequency mode or ECMO, at which time data collection was discontinued. We chose not to include adverse events such as barotrauma because it was not possible to attribute adverse events to HFJV in a retrospective chart review.
Data were extracted from the medical records by trained respiratory therapists and entered into a REDCap database. OI was calculated as (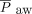 ×
×  × 100)/
× 100)/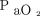 . Ventilation index was calculated as (breathing frequency × (PIP – PEEP) ×
. Ventilation index was calculated as (breathing frequency × (PIP – PEEP) ×  )/1,000.
)/1,000.
Continuous data are presented as medians with IQRs, and categorical variables are presented as counts and percentages. We performed paired nonparametric analysis to evaluate changes in pH, 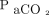 ,
, 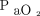 , HCO3–, OI, and
, HCO3–, OI, and  between pre-HFJV values and values at 4–6 h post-HFJV initiation. The Wilcoxon signed ranked test was performed to compare changes in blood gas parameters, OI, and
between pre-HFJV values and values at 4–6 h post-HFJV initiation. The Wilcoxon signed ranked test was performed to compare changes in blood gas parameters, OI, and 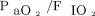 at 4–6 h post-HFJV initiation. To compare blood gas results, OI,
at 4–6 h post-HFJV initiation. To compare blood gas results, OI,  , and HFJV settings over time, separate linear mixed-effects models using exchangeable covariance structure were constructed. The following transformations for normal distribution were performed prior to model construction: OI,
, and HFJV settings over time, separate linear mixed-effects models using exchangeable covariance structure were constructed. The following transformations for normal distribution were performed prior to model construction: OI, 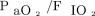 , PEEP, and
, PEEP, and 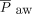 by logarithmic function;
by logarithmic function; 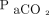 ,
,  , and HCO3– by inverse square root function; rate by square function; and
, and HCO3– by inverse square root function; rate by square function; and 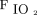 by inverse function. For each model, subjects were included as random effects. Normality, homoscedasticity, and linearity assumptions were assessed by visual inspection and plots of residuals against fitted values. Subgroup analysis of only those remaining on HFJV for > 72 h was performed to evaluate the effect of survivor bias. For survivors versus nonsurvivors, continuous variables were compared using an independent sample Mann-Whitney U test, while categorical variables were compared using the chi-square test. Statistical significance was defined as P < .05, and data were analyzed with SPSS 24 (IBM, Armonk, New York) and Stata 16.1 (StataCorp, College Station, Texas).
by inverse function. For each model, subjects were included as random effects. Normality, homoscedasticity, and linearity assumptions were assessed by visual inspection and plots of residuals against fitted values. Subgroup analysis of only those remaining on HFJV for > 72 h was performed to evaluate the effect of survivor bias. For survivors versus nonsurvivors, continuous variables were compared using an independent sample Mann-Whitney U test, while categorical variables were compared using the chi-square test. Statistical significance was defined as P < .05, and data were analyzed with SPSS 24 (IBM, Armonk, New York) and Stata 16.1 (StataCorp, College Station, Texas).
Results
Forty patients were placed on HFJV in the pediatric ICU during the time frame studied. Five patients were excluded due to HFJV being initiated while on ECMO, leaving 35 subjects to be included in the study with a median (IQR) age of 2.9 (1.6–7.9) months and median (IQR) weight of 5.2 (3.8–6.9) kg. Overall, 9 (26%) subjects died. Subjects were on conventional ventilation for a median (IQR) of 0.6 (0.1–2.9) d prior to HFJV. A total of 9 (26%) required ECMO, and 7 (77%) of those receiving ECMO survived. Respiratory failure was the primary indication for mechanical ventilation in 34 (97%) subjects, with 1 subject receiving HFJV due to postoperative sepsis. Infection was documented in 26 (74%) of subjects, with 22 (63%) having a viral infection. The most common viral infections noted were respiratory syncytial virus in 14 (63%) subjects and rhinovirus in 8 (36%) subjects (Table 1).
Subject Characteristics
Blood gas analysis prior to HFJV initiation was not available in 5 subjects. Pre-HFJV measurements for the remaining 30 subjects included a median (IQR) pH of 7.18 (7.11–7.27), 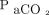 64 (52–87) mm Hg,
64 (52–87) mm Hg, 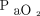 74 (64–125) mm Hg, HCO3– 26 (22–32) mEq/L, and base deficit of –2 (–8 to 3) mmol/L. Complete data were available for calculated values in 27 subjects with a median (IQR) OI of 11.3 (7.2–16.9),
74 (64–125) mm Hg, HCO3– 26 (22–32) mEq/L, and base deficit of –2 (–8 to 3) mmol/L. Complete data were available for calculated values in 27 subjects with a median (IQR) OI of 11.3 (7.2–16.9),  of 133 (91.3–190.0), and ventilatory index of 47 (35–64). Conventional ventilator settings prior to initiation of HFJV, available for 30 subjects, were a set median (IQR) breathing frequency of 30 (28–34) breaths/min, PIP 30 (29–31) cm H2O, set inspiratory pressure 22 (20–24) cm H2O, set PEEP 8 (7–9) cm H2O,
of 133 (91.3–190.0), and ventilatory index of 47 (35–64). Conventional ventilator settings prior to initiation of HFJV, available for 30 subjects, were a set median (IQR) breathing frequency of 30 (28–34) breaths/min, PIP 30 (29–31) cm H2O, set inspiratory pressure 22 (20–24) cm H2O, set PEEP 8 (7–9) cm H2O, 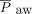 14 (11–16) cm H2O, and
14 (11–16) cm H2O, and 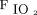 0.70 (0.50–1.00). Median (IQR) tidal volume was 5.6 (4.5–6.8) mL/kg, airway resistance was 103 (78–156) cm H2O/L/s, and lung compliance was 2.1 (1.6–3.0) mL/cm H2O.
0.70 (0.50–1.00). Median (IQR) tidal volume was 5.6 (4.5–6.8) mL/kg, airway resistance was 103 (78–156) cm H2O/L/s, and lung compliance was 2.1 (1.6–3.0) mL/cm H2O.
Ten of 35 (29%) subjects did not have a blood gas at 4–6 h after HFJV initiation. Of these, 3 subjects were placed on ECMO, 3 were transitioned to high-frequency oscillatory ventilation, 3 were transitioned back to conventional mechanical ventilation, and 1 subject was placed on high-frequency percussive ventilation. For the 25 subjects with complete data at 4–6 h after HFJV initiation, paired nonparametric analysis demonstrated no significant differences in OI (11.4 vs 10.0, P = .85), 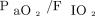 (133 vs 112.0, P = .63), or
(133 vs 112.0, P = .63), or  (74 vs 79 mm Hg, P = .39); however, pH increased (7.18 vs 7.39, P = .001),
(74 vs 79 mm Hg, P = .39); however, pH increased (7.18 vs 7.39, P = .001), 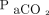 decreased (64 mm Hg vs 51 mm Hg, P = .001), and HCO3– increased (26 vs 29 mEq/L, P = .007). A linear effects model revealed that, in subjects who remained on HFJV for 72 h, HCO3– and HFJV rate significantly decreased, whereas pH, base excess/deficit, set PEEP, and
decreased (64 mm Hg vs 51 mm Hg, P = .001), and HCO3– increased (26 vs 29 mEq/L, P = .007). A linear effects model revealed that, in subjects who remained on HFJV for 72 h, HCO3– and HFJV rate significantly decreased, whereas pH, base excess/deficit, set PEEP, and 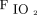 increased significantly. Subgroup analysis of only those 12 subjects remaining on HFJV for > 72 h revealed results similar to those for the complete cohort (Table 2).
increased significantly. Subgroup analysis of only those 12 subjects remaining on HFJV for > 72 h revealed results similar to those for the complete cohort (Table 2).
Change in Oxygenation and Ventilation Variables Over Time With HFJV
Eighteen (51%) subjects were ultimately transitioned from HFJV to conventional mechanical ventilation without ECMO or another high-frequency mode after a median (IQR) time on HFJV of 5.4 (0.8–11.1) d. Three subjects were transitioned to conventional ventilation within 4–6 h, while 5 were transitioned within 24 h, 2 within 48 h, and 1 within 72 h, with the remaining 9 subjects being transitioned after > 72 h of HFJV. Of the 17 (49%) subjects who were unable to be transitioned back to conventional mechanical ventilation, 9 (53%) received ECMO, 5 (29%) received high-frequency oscillatory ventilation, 1 (6%) received high-frequency percussive ventilation, and 2 (12%) died while on HFJV. One subject transitioned successfully from HFJV but expired later. Median (IQR) HFJV settings prior to transition to conventional ventilation were PIP 39 (32–50) cm H2O, 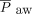 14 (11–17) cm H2O, and
14 (11–17) cm H2O, and 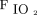 0.50 (0.35–0.56). (Table 3). Conventional mechanical ventilation settings at transition from HFJV were median (IQR) set breathing frequency of 30 (26–33) breaths/min, set inspiratory pressure of 20 (16–23) cm H2O, set PEEPof 8 (6–10) cm H2O, and
0.50 (0.35–0.56). (Table 3). Conventional mechanical ventilation settings at transition from HFJV were median (IQR) set breathing frequency of 30 (26–33) breaths/min, set inspiratory pressure of 20 (16–23) cm H2O, set PEEPof 8 (6–10) cm H2O, and 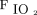 of 0.45 (0.39–0.60).
of 0.45 (0.39–0.60).
Post-HFJV Oxygenation and Ventilation Variables
Nonsurvivors had higher PIM2 scores (6.3% vs 1.6%, P = .01), were more likely to be immunocompromised (44% vs 8%, P = .01), were less likely to have a documented infection (44% vs 85%, P = .02), and had lower airway resistance (111 cm H2O/L/s vs 66 cm H2O/L/s, P = .02). There were no differences for age, weight, initial OI, initial 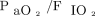 , ventilatory index, pH,
, ventilatory index, pH, 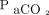 ,
,  , HCO3–, base excess/deficit, OI at 4–6 h post-HFJV initiation, and
, HCO3–, base excess/deficit, OI at 4–6 h post-HFJV initiation, and 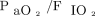 at 4–6 h post-HFJV initiation (Table 4). There were no differences for time on mechanical ventilation prior to HFJV (P = .78), total time on HFJV (P = .12), reason for mechanical ventilation (P = .56), history of congenital heart disease (P = .43), prematurity (P = .25), congenital syndromes (P = .14), or prior surgery (P = .75). There was no difference in the need for ECMO (P = .78), or inhaled nitric oxide (P = .21). There were no differences in conventional ventilator settings, observed
at 4–6 h post-HFJV initiation (Table 4). There were no differences for time on mechanical ventilation prior to HFJV (P = .78), total time on HFJV (P = .12), reason for mechanical ventilation (P = .56), history of congenital heart disease (P = .43), prematurity (P = .25), congenital syndromes (P = .14), or prior surgery (P = .75). There was no difference in the need for ECMO (P = .78), or inhaled nitric oxide (P = .21). There were no differences in conventional ventilator settings, observed 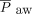 , lung compliance, tidal volume, volume of exhaled carbon dioxide, or initial HFJV settings (see the supplementary materials at http://www.rcjournal.com).
, lung compliance, tidal volume, volume of exhaled carbon dioxide, or initial HFJV settings (see the supplementary materials at http://www.rcjournal.com).
Comparison of Survivors vs. Non-Survivors
Discussion
In this study, we describe our experience with HFJV in infants with acute respiratory failure from multiple etiologies. To our knowledge, this is the largest and most varied HFJV cohort in non-neonatal pediatric subjects. Short-term success was seen in 71% of subjects, with 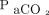 and pH improved at 4–6 h post-HFJV initiation and remaining stable for those remaining on HFJV for > 72 h. The improvement in gas exchange variables was likely overestimated because the 29% of subjects who failed HFJV would not have had improved gas exchange, or potentially would have had worsening gas exchange. While the PIP was much higher than during conventional ventilation, there is significant attenuation of PIP during HFJV throughout the respiratory system.6 However, a total of 43% of subjects ultimately required transition to other high-frequency modalities or ECMO. There was no effect on OI or
and pH improved at 4–6 h post-HFJV initiation and remaining stable for those remaining on HFJV for > 72 h. The improvement in gas exchange variables was likely overestimated because the 29% of subjects who failed HFJV would not have had improved gas exchange, or potentially would have had worsening gas exchange. While the PIP was much higher than during conventional ventilation, there is significant attenuation of PIP during HFJV throughout the respiratory system.6 However, a total of 43% of subjects ultimately required transition to other high-frequency modalities or ECMO. There was no effect on OI or  observed at 4–6 h or after 72 h.
observed at 4–6 h or after 72 h.
Survivors had a high rate of viral infection, predominantly respiratory syncytial virus, underscoring that respiratory failure as the result of respiratory syncytial virus has a better prognosis than other etiologies.17 As expected, nonsurvivors had a statistically significant higher illness severity as indicated by immunocompromised state and higher PIM2 scores, and they trended toward a lower pH and lower base deficit, although these were not statistically significant. There were also no differences in OI, ventilation index, 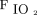 , and set breathing frequency between survivors and nonsurvivors.
, and set breathing frequency between survivors and nonsurvivors.
There is a paucity of data evaluating HFJV outside of the neonatal population, and most prior studies have focused on single-disease states such as viral bronchiolitis. Valentine et al11 described the use of HFJV in a series of 11 infants and children (1.7–14.2 kg, age 2 weeks to 39 months) with respiratory syncytial virus. They observed increased pH and decreased 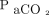 , and a 91% (10 of 11) survival to discharge.11 The majority (9 of 11) of their subjects were born prematurely, compared to only 26% in our cohort. Their median ventilation index was 55 and the OI was 14, higher than what was observed in our study. The median pH,
, and a 91% (10 of 11) survival to discharge.11 The majority (9 of 11) of their subjects were born prematurely, compared to only 26% in our cohort. Their median ventilation index was 55 and the OI was 14, higher than what was observed in our study. The median pH,  , or PIM2 scores were not reported in that study. The differences in outcomes between that study and ours are likely attributed to the etiology of acute respiratory failure; our study included subjects with undifferentiated acute respiratory failure from multiple etiologies, whereas theirs included only subjects with respiratory syncytial virus, who are expected to have a high survival rate.17,18
, or PIM2 scores were not reported in that study. The differences in outcomes between that study and ours are likely attributed to the etiology of acute respiratory failure; our study included subjects with undifferentiated acute respiratory failure from multiple etiologies, whereas theirs included only subjects with respiratory syncytial virus, who are expected to have a high survival rate.17,18
The change in gas exchange observed in our study is similar to those reported by Zhang et al,12 who described a series of 25 infants (mean weight of 2.8 kg) with congenital diaphragmatic hernia managed with HFJV. In that study, HFJV was associated with minimal complications and resulted in a significant increase in pH and decrease in  over an unreported timeframe, with a mortality rate of 64%.12 Infants with congenital diaphragmatic hernia are also managed with HFJV in our center; however, they were not included in this study because they are managed within our neonatal ICU by a separate clinical team.19 Smith et al10 evaluated HFJV use in 29 pediatric subjects with pediatric ARDS complicated by air leak syndrome and found a survival rate of 64%. In that study, survivors spent significantly less time on conventional ventilation prior to HFJV than nonsurvivors (3.7 d vs 9.6 d), suggesting that early application of HFJV might be beneficial, although this study was published in 1993 and subjects were receiving a PIP of 49 cm H2O prior to HFJV. Our clinical practice embraces this early HFJV strategy, as evidenced by our median time on conventional ventilation prior to HFJV of 0.6 days.
over an unreported timeframe, with a mortality rate of 64%.12 Infants with congenital diaphragmatic hernia are also managed with HFJV in our center; however, they were not included in this study because they are managed within our neonatal ICU by a separate clinical team.19 Smith et al10 evaluated HFJV use in 29 pediatric subjects with pediatric ARDS complicated by air leak syndrome and found a survival rate of 64%. In that study, survivors spent significantly less time on conventional ventilation prior to HFJV than nonsurvivors (3.7 d vs 9.6 d), suggesting that early application of HFJV might be beneficial, although this study was published in 1993 and subjects were receiving a PIP of 49 cm H2O prior to HFJV. Our clinical practice embraces this early HFJV strategy, as evidenced by our median time on conventional ventilation prior to HFJV of 0.6 days.
The clinically important improvement in ventilation observed in our study suggests that HFJV can be of value in patients with increased airway resistance, such as bronchiolitis, with significant respiratory acidosis that is refractory to conventional ventilation. HFJV can significantly decrease 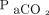 and should be considered for patients in whom conventional ventilation requires an elevated plateau pressure, PIP, or driving pressure for adequate ventilation. In our study, there may have been room to increase the set breathing frequency because the median frequency was 30 breaths/min; however, there was no way to assess whether air-trapping and auto-PEEP were present prior to HFJV due to our methodology. In the future, it is possible that
and should be considered for patients in whom conventional ventilation requires an elevated plateau pressure, PIP, or driving pressure for adequate ventilation. In our study, there may have been room to increase the set breathing frequency because the median frequency was 30 breaths/min; however, there was no way to assess whether air-trapping and auto-PEEP were present prior to HFJV due to our methodology. In the future, it is possible that 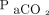 could be managed by increasing the breathing frequency, increasing inspiratory time, or adjusting PEEP to increase lung recruitment prior to transitioning to HFJV. Despite PEEP being increased following HFJV initiation, there were no statistically significant differences in PEEP or
could be managed by increasing the breathing frequency, increasing inspiratory time, or adjusting PEEP to increase lung recruitment prior to transitioning to HFJV. Despite PEEP being increased following HFJV initiation, there were no statistically significant differences in PEEP or 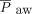 . There was no change in oxygenation at 4–6 h, indicating that HFJV may not have a short-term impact on oxygenation; while
. There was no change in oxygenation at 4–6 h, indicating that HFJV may not have a short-term impact on oxygenation; while 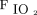 decreased over time, there were no differences for OI or
decreased over time, there were no differences for OI or 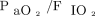 . The effect of HFJV on oxygenation may be related to minimal lung recruitment despite the higher PIP. Lung recruitment during HFJV can be achieved by increasing the PEEP or breathing frequency on the conventional ventilator. PEEP set on the conventional ventilator is the primary driver of
. The effect of HFJV on oxygenation may be related to minimal lung recruitment despite the higher PIP. Lung recruitment during HFJV can be achieved by increasing the PEEP or breathing frequency on the conventional ventilator. PEEP set on the conventional ventilator is the primary driver of 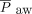 during HFJV, although higher HFJV rates are associated with increased air-trapping, a strategy that may be counterproductive in patients with increased airway resistance, such as bronchiolitis. For patients with acute lung injury, our protocol calls for increasing the
during HFJV, although higher HFJV rates are associated with increased air-trapping, a strategy that may be counterproductive in patients with increased airway resistance, such as bronchiolitis. For patients with acute lung injury, our protocol calls for increasing the 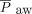 by 5–6 cm H2O at initiation; however, there was no significant difference in
by 5–6 cm H2O at initiation; however, there was no significant difference in 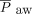 after HFJV initiation.
after HFJV initiation.
While a randomized controlled trial of HFJV would be ideal, we feel this is likely unfeasible because HFJV is not widely used in pediatric ICUs. Future case series should focus on the feasibility of HFJV in larger patients and patient populations in which HFJV has not been extensively studied. These studies should attempt to incorporate the use of advanced imaging techniques such as electric impedance tomography to evaluate the effect of HFJV on lung volumes.
This study has several limitations, including a small sample size. As a retrospective study, we were limited to data that were available in the medical record. This was a single-center study at an academic medical center with extensive experience using HFJV in its various neonatal and pediatric critical care units, and thus the results may not be generalizable to other centers. The lack of a control group limits the generalizability of the study, and we were unable to evaluate a group that was not treated with HFJV. We did not record adverse events such as barotrauma because it was not possible to attribute adverse events to HFJV, high pre-HFJV ventilator settings, or manual ventilation with high pressure due to our methodology. The PIM2 score may not be predictive of mortality risk in a cohort of children predominantly admitted for viral respiratory failure.20 There was potential selection bias by the clinical team when initiating HFJV. The plateau pressure was not documented, which prevented us from determining driving pressure. We were unable to perform multivariable analyses or subgroup analyses to further delineate various associations due to our limited sample size. Only 3 subjects weighed > 10 kg (with 2 being 10.1 and 10.4 kg), so we were unable to evaluate whether there was an upper weight limit to HFJV.
Conclusions
In subjects who remained on HFJV for 4–6 h after initiation, HFJV was associated with improved ventilation but no significant change in oxygenation. HFJV was moderately successful with many subjects requiring other high-frequency modes or ECMO.
Footnotes
- Correspondence: Andrew G Miller MSc RRT RRT-ACCS RRT-NPS FAARC, Duke University Medical Center, 2301 Erwin Rd, Durham, NC 27710. E-mail: andrew.g.miller{at}duke.edu
See the Related Editorial on Page 349
Supplementary material related to this paper is available at http://www.rcjournal.com.
Mr Miller has disclosed a relationship with Ventec Life Systems. Dr Cheifetz has disclosed relationships with Philips, Medtronic, Tim Peters and Co., and the National Heart, Lung, and Blood Institute. Dr Rotta has disclosed relationships with Vapotherm, Breas, and Elsevier. The remaining authors have disclosed no conflicts of interest.
- Copyright © 2021 by Daedalus Enterprises








