Abstract
Recruitment maneuvers in ARDS are used to improve oxygenation and lung mechanics by applying high airway pressures to reopen collapsed or obstructed peripheral airways and alveoli. In the early 1990s, recruitment maneuvers became a central feature of a variant form of lung-protective ventilation known as open-lung ventilation. This strategy is based on the belief that repetitive opening and closing of distal airspaces induces shear injury and therefore contributes both to ventilator-induced lung injury and ARDS-associated mortality. However, the largest multi-center randomized controlled trial of open-lung ventilation in moderate to severe ARDS reported that recruitment maneuver plateau pressures of 50–60 cm H2O were associated with significantly higher mortality compared to traditional lung-protective ventilation. Despite being based on well conducted preclinical and clinical recruitment maneuver studies, the higher mortality associated with the open-lung ventilation strategy requires re-examining the assumptions and conclusions drawn from those previous studies. This narrative review examines the evidence used to design recruitment maneuver strategies. We also review the radiologic, rheologic, and histopathologic evidence regarding the nature of lung injury and the phenomena of recruitment and de-recruitment as it informs our perceptions of recruitment potential in ARDS. Major lung-protective ventilation clinical trial data and other clinical data are also examined to assess the practical necessity of recruitment maneuvers in ARDS and whether a subset of cases might benefit from pursuing recruitment maneuver therapy. Finally, a less a radical approach to recruitment maneuvers is offered that might achieve the goals of recruitment maneuvers with less risk of harm.
- acute respiratory distress syndrome
- alveolar recruitment maneuver
- intra-abdominal pressure
- plateau pressure
- positive end-expiratory pressure
- threshold opening pressure
- ventilator-induced lung injury
Introduction
ARDS is characterized by altered permeability, pulmonary edema, and decreased gas volume (ie, functional residual capacity [FRC]), which leads to low respiratory system compliance (CRS) and hypoxemia both from intrapulmonary shunting and ventilation-perfusion mismatching.1-3 Causes of decreased FRC include underinflated, unstable alveoli vulnerable to collapse and atelectatic or de-recruited alveoli.4 With the advent of low-tidal volume lung-protective ventilation (LPV), alveolar de-recruitment is exacerbated despite moderate levels of PEEP (ie, 10 ± 4 cm H2O).5
Recruitment maneuvers reverse lung collapse in ARDS by applying high airway pressures that overcome a range of threshold opening pressures (TOP). Re-opening collapsed or obstructed peripheral airways and alveoli often improves oxygenation and CRS, and may enhance alveolar fluid clearance.6-10 Historically, recruitment maneuvers consisted of inflations of 40 cm H2O sustained for ∼ 15 s (ie, the force needed to achieve vital capacity in normal subjects) to reverse intraoperative atelectasis and intrapulmonary shunting.11,12
Recruitment maneuvers as an adjunct to LPV were first described in early preclinical studies of high-frequency oscillatory ventilation for acute lung injury.13 In the early 1990s, recruitment maneuvers became a central feature of a LPV variant known as open-lung ventilation (OLV).14,15 One such technique described brief periods (eg, 10 min) of continuous mechanical ventilation at a peak airway pressure of 55 cm H2O and PEEP of 16 cm H2O.14 Over the intervening years, the mechanics, physiology, and efficacy of recruitment maneuvers were explored in numerous clinical and preclinical studies using a variety of strategies, as well as theoretical treatises.16
Some of these findings informed the largest multi-center randomized controlled trial of OLV, the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART),17 which enrolled > 1,000 subjects with moderate to severe ARDS. Despite the data-driven protocol, the ART group17 reported significantly higher mortality in the OLV treatment arm compared to the control arm using the National Institutes of Health ARDS Clinical Trials Network (ARDSNet) lower-PEEP protocol.18 Particularly vexing was that, despite significantly higher oxygenation and CRS in subjects treated with OLV, the need for rescue therapies was not different. This suggests that recruitment maneuvers were largely ineffective in stabilizing FRC. Higher mortality in the OLV arm confirmed one of the major findings of the original ARDSNet LPV trial: improved oxygenation is not necessarily a valid signifier for meaningful outcomes.18
Some have interpreted the ART results to suggest abandoning recruitment maneuvers in treating ARDS,19 whereas others20 suggest that ART-related methodological issues still cloud its interpretation and have instead advocated for a thorough post hoc analysis (which, to our knowledge, has not yet been published). Furthermore, the dramatic failure of a trial based upon numerous well-executed physiologic studies behooves re-examining the nature of recruitment and de-recruitment as well as the validity of inferences drawn from them. This narrative review re-examines the physiology and mechanics of recruitment and de-recruitment in ARDS, the results of which might suggest when and how recruitment maneuvers might be incorporated more reasonably into clinical practice.
Brief Overview of Recruitment Maneuvers in ARDS
Different approaches to recruitment maneuvers have been developed over the past 30 years (Table 1). One of the earliest and most popular strategies has been the sustained inflation maneuver using CPAP.15,21-24 Another strategy (ie, amplitude-modulated ventilation) posited that following a deep inflation the time constants for alveolar closure (of previously collapsed units) were substantially longer than the ventilatory cycle, so that recruitment achieved from a single deep inflation (or short periods of elevated PEEP) could be sustained for a period of time afterwards.25,26 This was hypothesized to allow alveolar stabilization without requiring sustained levels of higher PEEP. Strategies based on this approach include intermittent sigh breaths27,28 and intermittent or fluctuating PEEP.29-31 Another approach is a less intense variation of the recruitment maneuver technique described by Lachmann,14 in which a brief (2 min) period of ventilation at a plateau pressure (Pplat) of 45 cm H2O and PEEP of 15 cm H2O is used.32 Other, more extended iterations of this approach include the prolonged recruitment maneuver33 and the slow moderate pressure recruitment maneuver.34 Other prolonged approaches include the extended sigh maneuver35-37 and the RAMP technique.38,39 These last 4 techniques most closely resemble what is currently the most widely investigated technique (and the primary focus of this review): the staircase recruitment maneuver.40 The impact of these techniques will be discussed in varying detail throughout the course of this review in terms of what they reveal about the nature of recruitment in ARDS.
Different Recruitment Maneuver Strategies for the Treatment of Moderate to Severe ARDS
Recruitment maneuver studies have categorized ARDS subjects as either responders or nonresponders according to the presence and magnitude of improvement in oxygenation,26 resting lung volume,26 or reduction in nonaerated lung tissue by computed tomography (CT) scan, electrical impedance tomography, or ultrasonography.34,41 This implies recruitment maneuvers have a limited application under specific conditions not always discernable at the bedside. ARDS associated with either direct epithelial injury (ie, pulmonary or primary ARDS) or indirect endothelial injury (ie, extrapulmonary or secondary ARDS) both demonstrate improved oxygenation following the maneuver: those with indirect injury tended to be more responsive both in the degree of recruitment and oxygenation and of reductions in both lung resistance and elastance.21,27,42 Indirect injury typically coincides with early interstitial edema and higher chest wall elastic forces,43,44 suggesting that recruitment maneuvers are most effective when compressive and congestive atelectasis are major factors versus alveolar flooding and tissue consolidation, which are more prominent in direct injury such as pneumonia (see below).
Physics and Physiology of Recruitment: Pressure and Time
The focus of recruitment has been on alveolar re-inflation. This is a matter of conversational convenience that unintentionally results in an underappreciation of the fact that distal airway injury and inflammation is a prominent feature of ARDS and cannot be separated from alveolar injury.45 What follows is a description of the interplay between distal airway and alveolar injury as it relates to recruitment phenomena. In a later section describing the ambiguities surrounding recruitment maneuvers, a more in-depth description of associated tissue-related factors (ie, rheology and histopathology) will be provided.
Injury in distal airways (ie, airways with a diameter < 2 mm) in ARDS is characterized by bronchiolar epithelial necrosis/sloughing and the rupturing of bronchiolar-alveolar attachments that promotes distal airway instability.45,46 This in turn increases airways resistance and expiratory flow limitation.2,47 Opening collapsed small airways in ARDS is a dynamic process with a variable time course that depends upon several factors, including airway radius, the fraction of functional alveoli providing regional airway stability (ie, axial wall traction or tethering), airway fluid characteristics (ie, surface tension and viscous forces, as well as film thickness) and the presence of biologically active surfactant.48-51 As lining fluid surface tension increases, so too does the TOP to overcome it, with additional pressure required to overcome viscous forces. As airway fluid viscosity increases, both yield pressure and time required to effect airway opening also increase, which may be particularly difficult to achieve in peripheral and terminal airways.50
Regarding normal alveolar response to recruitment, an experimental microimaging study of deflated healthy lungs undergoing stepwise inflation from 0 to 35 cm H2O observed an unusual U-shaped pattern, whereby mean alveolar size first increased, stabilized (at 25 cm H2O), and then decreased. The number of inflated alveoli decreased and then markedly increased again at pressures of 25–35 cm H2O, causing a doubling of lung volume size.52 In other words, at higher pressures, alveoli paradoxically become both smaller and more plentiful. It was hypothesized that stretching the alveolar wall increases the diameter of the pores of Kohn. This in turn thins the alveolar lining fluid normally covering the pores, thus facilitating pressure transmission between adjacent mother-daughter alveoli and resulting in the latter’s recruitment.
These experimental conditions, however, diverge from those encountered in ARDS such that similar behavior (if it in fact occurs during a recruitment maneuver) might require a prolonged time period. In early ARDS altered permeability pulmonary edema fluid contains protein concentrations similar to plasma.53,54 Protein and fibrin-rich alveolar edema, along with oxygen radicals, inactivates surfactant, resulting in higher TOP for both distal airways and alveoli.55
Depending upon the severity of pulmonary capillary leakage, when alveolar flooding involves the alveolar ducts, liquid bridge formation rises exponentially, particularly when FRC decreases and elastic recoil forces increase.56 Bronchiolar epithelial damage and inflammation also are present and associated with ARDS severity,45,57 thereby increasing the likelihood of inflammatory exudate obstructing both the airway lumen and the pores of Kohn. Because fluid viscosity increases with increasing protein concentrations,58 enhanced viscosity of airway and alveolar lining fluid (along with other cellular debris accumulating in the peripheral airspaces) may prolong the time necessary to achieve maximum recruitment for any targeted Pplat during a recruitment maneuver.
The time necessary to reopen collapsed or obstructed small airways also depends upon the extent of menisci formation or plugs in sequentially collapsed or obstructed airways, which may be amplified either by the presence of mucus in pneumonia-associated ARDS or in patients with substantial smoking histories.48,50,59 In other words, the level of recruitment reported at a specific Pplat cited in recruitment maneuver studies that were sustained for 1–2 min is not definitive proof of maximum efficacy at that level of applied pressure (see below).
Temporal Aspects of Lung Recruitment
Two temporal aspects influence the effectiveness of recruitment: (1) the duration of any particular recruitment maneuver itself, and (2) the clinician-set inspiratory time chosen during the maneuver. Some of what is discussed below reflects this ambiguity as to precisely what occurs when we observe recruitment. Some of this (but by no means all) has been clarified by the advent of lung parenchymal microimaging in animal models, as discussed above. The following 2 sections provide a historical narrative on the development of our understanding as well as the persistent ambiguity surrounding recruitment from the 1960s to the 1990s.
Creep: Fast Versus Slow Pulmonary Compartments
The term “creep” was coined in the 1960s to describe the progressive increase in volume over time when the lungs are subjected to “constant” pressure inflations.60,61 More broadly referred to as hysteresis or stress adaptation, creep expresses how tissue, once deformed, resists returning to its former shape. This is attributed to adaptive surface tension forces in the lungs and intrinsic viscoelastic properties of both lung and chest wall tissue (eg, the presence of elastic fibers in smooth muscle, skeletal muscle, ligaments, and tendons) as well as abdominal organs.61
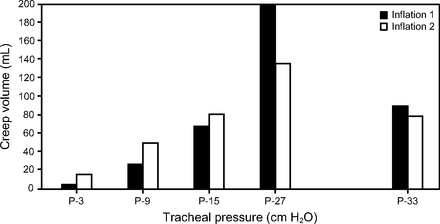
Under normal physiology, a 2-phase process consisting of fast and slow compartments was described in animals.60 During a 10-s inflation hold, an initial rapid (2 s) phase is followed by a slow (8 s) phase of continued tissue stretching; the latter was attributed primarily to alveolar recruitment and reduced alveolar surface tension, and to a lesser degree alterations in tissue viscoelastic properties.62 Stress adaptation was directly associated with increasing driving pressures and reaching maximum creep at 33 cm H2O. Increasing sustained inflation intensity (ie, 120 s at 33 and 39 cm H2O) effected additional stress adaptation (Fig. 1).60
Association between increasing driving pressures and alveolar stress adaptation in an animal model with normal chest mechanics. Data from Reference 60.
Stress adaptation has been observed in anesthetized normal subjects undergoing step inflations (similar to constructing a pressure-volume curve), stabilizing at 5–7 s at different volumes.61 Two thirds of stress adaptation was attributed to the lungs, with the chest wall exhibiting a smaller, slower time course. This was ascribed to tissue properties in both structures rather than alveolar recruitment and gas redistribution.61
Evidence Supporting Creep Phenomenon in ARDS
In ARDS, slow volume changes following a 10 cm H2O PEEP increase were first reported by Katz et al,4 whereby 67% of volume change occurred during the first breath and 90% by the fifth breath. The remaining increase occurred over 40 min and was attributed either to stress adaptation or alveolar recruitment. Similar to other findings,61 62% of the changes were attributed to the lungs and 38% to the chest wall.4
Slowly distensible pulmonary compartments in severe ARDS have been reported by others.63 Using a 5 cm H2O PEEP increment, only 37% of subjects exhibited a slow recruitment compartment, whereas 79% displayed a slow de-recruitment compartment, consistent with other studies reporting delayed de-recruitment following step-decreases in PEEP.26 The mean inflation time constant (τ) of the slow compartment was 9.4 ± 7.3 s. As 95% equilibration occurs at 3 τ and 99% at 5 τ,64 recruitment (or stress adaptation) of slow pulmonary compartments would reach 95–99% volume equilibration at mean times of 28–47 s with an upper 95% confidence limit of 43–72 s. As a reference, in normal subjects under general anesthesia, atelectasis reversal occurs at τ of 2.6 s (95–99% reversal at 8–13 s).12
In contrast, when oxygenation is the variable of interest, the temporal impact on recruitment is exaggerated. Several studies examined the time required to establish steady state oxygenation in ARDS following a PEEP increase or after initiating sigh breaths.27,65,66 Setting PEEP above the lower inflection point (PEEP 14 ± 3 cm H2O), 90% of maximal improvement occurred at 20 ± 19 min.65 However, in another study, a 10 cm H2O PEEP increase produced no apparent oxygenation plateau (ie, 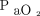 progressively increased by 5–60 min).66 When augmenting LPV with intermittent sigh breaths (Pplat 45 cm H2O; PEEP 14 cm H2O) maximum improvements occurred at 30 min for both
progressively increased by 5–60 min).66 When augmenting LPV with intermittent sigh breaths (Pplat 45 cm H2O; PEEP 14 cm H2O) maximum improvements occurred at 30 min for both  and end-expiratory lung volume.27
and end-expiratory lung volume.27
An intriguing aspect of recruitment are transient (pulmonary) states observed in ARDS when the ventilatory pattern was altered.67 Prolonged effects of recruitment were noted after various manipulations, including a single PEEP step, a PEEP wave maneuver, and an undulating PEEP pattern. One hour following a PEEP increase from 13 to 21 cm H2O, FRC rose 150% greater than that predicted by CRS of the fast pulmonary compartment (ie, baby lung).68 These results were similar to those reported by Katz et al.4
In the PEEP wave study, a brief repetitive cycle of incremental ascending and descending PEEP with a maximum PEEP change of 10 cm H2O was repeated 5 times over several hours. When PEEP was returned to the initial settings,  stabilized at 10 mm Hg above the previous baseline. The phenomenon occurred with each successive PEEP wave so that, at the end of the experimental run,
stabilized at 10 mm Hg above the previous baseline. The phenomenon occurred with each successive PEEP wave so that, at the end of the experimental run, 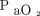 was 80 mm Hg higher than at the initial baseline.67
was 80 mm Hg higher than at the initial baseline.67
The undulating PEEP study assessed the upper limit of time constant distributions using a PEEP cycle above and below a baseline PEEP of 14 cm H2O. PEEP was titrated in increments of 7 cm H2O up to 29 cm H2O and down to 0 cm H2O over 9 h. FRC measured 1 h following any PEEP change did not indicate a steady state in terms of recruitment or de-recruitment. The overall impression was that “the length of individual time constants in ARDS may exist in the region of hours.”67
Slow, progressive lung recruitment frequently observed during prone position therapy supports the existence of slow pulmonary compartments in ARDS.69 Initial improvement in oxygenation typically occurred within 30–60 min, yet it is not uncommon for improvements to become apparent only after 6 h, with continuing improvements sometimes observed over 20–36 h.69 Prolonged recruitment maneuver (ie, 6–14 h) reversing profound refractory hypoxemia has been reported anecdotally in ARDS complicated by abdominal compartment syndrome70 and in pronounced obesity when combined with prone position.69
These findings underscore the considerable difference in the time frames chosen to evaluate oxygenation response following a recruitment maneuver. Several recruitment maneuver studies that will be discussed in the next section used 2-min equilibration periods between all or some of the PEEP steps,17,40,71-73 which is consistent with classic physiologic studies.60,67,74 While the 2-min limit allows for stress adaptation, it also limits exposure to severe respiratory acidemia40 and potential cardiovascular instability from alterations in right and left ventricular function.75-78 Most importantly, these necessary time constraints imposed by very high pressure recruitment maneuver techniques limits our ability to fully understand the actual recruitment potential in ARDS.
Selecting Inspiratory Time During Recruitment Maneuver
The other temporal aspect is whether the inspiratory time per breath impacts the overall effectiveness of a recruitment maneuver. This is likely dependent upon whether the clinician-set inspiratory time is appropriate for the inspiratory time constant of individual patients. In general, ARDS subjects have an inspiratory τ of 0.17–0.41 s,2 which (depending upon syndrome severity) would result in 95% and 99% estimated equilibration between airway and alveolar pressures at ∼ 0.5–1.2 s and 0.9–2.1 s, respectively. However, these estimates are based on assumptions of mono-exponential functions of constant elastances and resistances throughout inspiration, and therefore neglect the impact of mechanical inhomogeneity in ARDS.79 Moreover, they ignore the impact of continued gas mixing and redistribution (ie, pendelluft motion) and increased diffusion time on both oxygenation and dead-space ventilation by which recruitment maneuver efficacy often is assessed.
The range of inspiratory time reported in recruitment maneuver studies have varied: 1.5 s (single PEEP step),63 2.5 ± 1.1 s (for sigh breaths), 27 2–3 s17,71,72,80 (for staircase recruitment maneuver studies or unspecified),40 whereas others used an end-inspiratory pause of 5–7 s.34,81 In a lung lavage model of acute lung injury, in vivo microscopic studies of subpleural alveoli during a recruitment maneuver at 40 cm H2O found that, over a period of 40 s, ∼ 85% of recruitment occurred by 2 s.82 These data support clinical use of inspiratory times of 2–3 s to maximize per-breath recruitment potential during an recruitment maneuver.
Mechanics of Recruitment and De-Recruitment
The majority of physiologic and clinical studies investigating recruitment in ARDS began in earnest in the first decade of this century and has produced the majority of our current knowledge base and clinical evidence. Because of this, the narrative in this section mostly derives from select studies we believe constitute the most important findings regarding recruitment maneuver and PEEP titration informing current practice.
In 2000, a variant of the original recruitment maneuver used in OLV,14 was introduced by Medoff et al,83 who applied pressure control ventilation at a Pplat of 60 cm H2O and PEEP of 40 cm H2O for 2 min. In a subsequent study comparing pressure control ventilation recruitment maneuvers to sustained inflation recruitment maneuvers (CPAP of 45 cm H2O), a 2-min trial pressure control ventilation recruitment maneuver (using a lower Pplat of 45 cm H2O and inspiratory time of 2.5 s) produced substantially greater oxygenation improvement (80% vs 19%).75
Since the case report by Medoff et al,83 the pressure control ventilation recruitment maneuver has become a popular approach and was the basis of the strategy used in the ART trial.17 A generalized description of this approach is as follows: an initial Pplat of 40 cm H2O is slowly increased in increments of 5 cm H2O to levels of 50 or 60 cm H2O. The recruitment maneuver is done using 2–3 min stepwise escalation/de-escalation of super-PEEP (ie, 20–45 cm H2O) with a fixed driving pressure (ie, the difference between Pplat and PEEP) of 15 cm H2O (Fig. 2).40,84 This is based on observations that TOP (Pplat being the clinical correlate) progressively increases from nondependent to dependent lung between 20–60 cm H2O.40,85 A variation of this technique was used in the ART trial but with stabilization periods of only 1 or 2 min between steps (Fig. 3).17
Representation of a “staircase” recruitment maneuver inflation steps. P drive = driving pressure (plateau pressure – PEEP); Paw = airway pressure; RM = recruitment maneuver. Data from Reference 84.
Representation of a “staircase” recruitment maneuver inflation steps as used initially during the ART trial. P drive = driving pressure (plateau pressure – PEEP); Paw = airway pressure; RM = recruitment maneuver. Data from Reference 17.
When absorption atelectasis is pervasive, Pplat up to 70 cm H2O is required,85 and pressures up to 80 cm H2O have been used in ARDS associated with blunt chest trauma86 or abdominal compartment syndrome.70 To place these extraordinary pressures into perspective, the first few post-natal breaths, which expand gasless, partially liquid-filled lungs (ie, 100 times more viscous than air), require TOP of 40 cm H2O and peak transpulmonary pressures of 60–100 cm H2O to achieve full inflation.87,88 The unique circumstances in the moments following birth, in which completely deflated, non-injured lungs are initially expanded, are markedly different from that of heterogenous lung and distal airway injury present in ARDS (in addition to pathological alterations in chest wall mechanics). Nonetheless, the physics illustrates the circumstantial necessity that sometimes requires applying extraordinarily high transpulmonary pressures to displace liquid and re-expand the lungs under extreme conditions.
Distribution of TOP and Recruitment
Small physiologic studies suggest varying degrees of recruitment occur throughout the lung. An early CT study reported that potentially recruitable lung in moderate-to-severe ARDS averaged 21 ± 10% and required a Pplat of 45 cm H2O (whereas ∼ 25% remained collapsed).80 Also, there may exist nodal points whereby full recruitment transitions down the lungs from mid-to-dorsal regions when Pplat of 30, 35, and 45 cm H2O are reached (with the least amount of nonaerated tissue observed at 45 cm H2O).40,85 Subsequent studies have reaffirmed that TOP varies down the ventral-dorsal axis in ARDS. Upper zones had a negligible TOP of 0–4 cm H2O, whereas middle zones had a TOP of 4–7 cm H2O and dorsal lung recruitment commenced at ∼ 20 cm H2O.81
Similar to initial post-natal breaths, achieving a TOP in ARDS is not synonymous with full recruitment. Early pressure-volume curve studies of ARDS interpreted the lower inflection point as the TOP needed to recruit collapsed peripheral airways and alveoli, but it was misconstrued as the anchoring point for setting best PEEP.89 It later became apparent that recruitment merely commenced in the upper lung zones at the lower inflection point and continued throughout the inspiratory pressure-volume limb.90,91 Likewise, despite TOPs of 4–7 cm H2O (middle) and ∼ 20 cm H2O (dorsal), maximum recruitment in these regions occurs at 20–30 cm H2O and 45 cm H2O, respectively.81,85
Other CT imaging studies reported that nonaerated lung tissue progressively decreased from 55% (at a baseline ventilation with 10 cm H2O of PEEP) to 23% at a Pplat of 40 cm H2O and to 10% at a Pplat of 50 cm H2O.71 Improved recruitment was observed even when Pplat increased from a baseline of 28–32 cm H2O to 36–41 cm H2O at essentially the same PEEP level.5 Most importantly (in light of the ART study results), extending a recruitment maneuver to a Pplat of 60 cm H2O only reduced nonaerated tissue by 5%. Full recruitment has been reported at Pplat of 40–51 cm H2O,72 whereas others have reported increasing percentages of subjects achieving full recruitment as Pplat increased: 46% at 40 cm H2O, ∼ 60% at 45 cm H2O, and ∼ 70% at 50 cm H2O).40 In the seminal study on the time course of FRC improvement with PEEP in subjects with acute respiratory failure, the majority of whom likely would have met the current definition of ARDS, a Pplat of 40 cm H2O and PEEP of 18 cm H2O were needed to return FRC to normal.4 Thus, in the context of refractory hypoxemia even at moderate to high levels of PEEP, a recruitment maneuver Pplat of at least 40 cm H2O probably should be targeted.
Furthermore, there is speculation that the recruitable lung represents a penumbra of inflamed tissue surrounding a core nidus of compartmentalized injury, constituting a mixture of collapsed or partially flooded air spaces.21,92 The remaining ∼ 25% of nonaerated lung tissue, despite recruiting pressures of 45 cm H2O,80 likely signifies consolidated tissue, at least in those with normal body habitus (see below).
Interpretive Limitations of Mechanistic Studies of Recruitment
Interpreting these recruitment maneuver studies raises several issues. First, confounding factors influence the potential effectiveness of recruitment maneuvers in ARDS. These may include: (1) the inherently heterogenous nature and unique spatial patterns of acute lung injury among individual patients; (2) apparent differences in the response to recruitment maneuver related either to initiating pathways (direct vs indirect, interstitial vs alveolar edema) or the severity of injury (eg, the degree of inflammation and magnitude of edema formation); (3) timing of recruitment maneuver relative to syndrome onset; (4) the ventilatory strategy used prior to initiating recruitment maneuver; (5) alterations in chest wall mechanics; and (6) hemodynamic status (eg, various vasoactive drugs that might affect cardiac output and pulmonary blood flow distribution).24,93-98 Moreover, mechanistic recruitment maneuver studies require highly complex, clinically impractical methodologies that limit the number of subjects who can be studied and thus limits the generalizability of results to individual patients.
Second, variables chosen to signify full recruitment differed between studies, which introduces interpretative ambiguity. Borges et al40 used 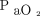 +
+  > 400 mm Hg. Adjusting for the range of mean
> 400 mm Hg. Adjusting for the range of mean  across recruitment maneuver steps (70–95 mm Hg) yields a corresponding
across recruitment maneuver steps (70–95 mm Hg) yields a corresponding  of ∼ 300–330 mm Hg. Povoa et al72 used a
of ∼ 300–330 mm Hg. Povoa et al72 used a 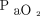 of 250 mm Hg on an
of 250 mm Hg on an  of 1, and de Matos et al71 reported the Pplat at which nonaerated alveoli was minimal. Studies by both Crotti et al85 and Caironi et al92 merely reported the degree of recruitment observed at a fixed Pplat of 45 cm H2O as a surrogate measure of total lung capacity.
of 1, and de Matos et al71 reported the Pplat at which nonaerated alveoli was minimal. Studies by both Crotti et al85 and Caironi et al92 merely reported the degree of recruitment observed at a fixed Pplat of 45 cm H2O as a surrogate measure of total lung capacity.
Third, over the years, recruitment maneuver studies have utilized different measurement techniques that have influenced both the results and their interpretation.32 Recruitment inferred from chest mechanics (eg, change in end-expiratory lung volume measured during construction of pressure-volume curves or after step changes in PEEP) reflects increased aeration of partially and fully inflated alveoli, as well as recruitment of previously collapsed or noncommunicating alveoli. In this review, we have focused on CT-based studies. Although ambiguities exist with this technique, it nonetheless provides a high degree of differentiation between non-, poorly and well-inflated alveoli (see below). As would be anticipated, recruitment inferred from chest mechanics analysis estimate much greater recruitment than those based on CT analysis.32
Taken together, these potential confounding variables (ie. relatively small numbers of study subjects, variations in both technique and primary endpoints) limit the generalizability of recruitment maneuver study results to individual patients, let alone navigating the contentious discourse regarding their interpretation.
Sponge Theory and Superimposed Hydrostatic Pressures
Setting aside common clinically induced causes (eg, circuit disconnection, endotracheal suctioning), lung de-recruitment in ARDS is thought to be caused largely by super-imposed hydrostatic pressure of overlying edematous lung tissue and mediastinal structures, as well as increased weight of the chest wall (eg, thoracic anasarca, ascites).94 This is based upon the sponge theory posited to explain rapid redistribution of lung densities on CT scans from dorsal to ventral regions during placement in prone position.76 Two facts support the notion that this represents a shift in gravitational forces applied to the lungs. First, overall lung density was unchanged, suggesting lung tissue mass (ie, edema, blood, cellular content or debris) had remained stable.99 Second, although pulmonary edema clearance in ARDS is severely impaired (6%/h),100 edema fluid is removed through the lymphatic system and does not freely redistribute through lung tissue.99
PEEP and De-Recruitment
A recurring and relatively uniform finding in many of the early recruitment maneuver studies was that, when ventilation was resumed at the previous PEEP level, oxygenation improvements dissipated rapidly over time despite relatively high baseline PEEP (∼ 12–15 cm H2O).22,23,27,101,102 In contrast, oxygenation improvements could be sustained following recruitment maneuver when higher post-recruitment maneuver PEEP levels were maintained (eg, ∼ 6–7 cm H2O above baseline).21 Acute lung injury models also reported that oxygenation after a recruitment maneuver was PEEP-dependent, with the highest sustained improvement occurring at PEEP of 16 cm H2O (vs 12 or 8 cm H2O).77 That the sustained improvement was independent of recruitment maneuver methodology suggests recruitment and de-recruitment occur through different mechanisms.
When pleural pressure exceeds alveolar pressure at end-expiration, de-recruitment occurs over time irrespective of previous volume history.73 In ARDS, de-recruitment is a continuous process that becomes prominent at PEEP < 15 cm H2O.85 De-recruitment appears to cease in the upper and hilar lung zones at PEEP of 10 cm H2O, whereas it continues in dorsal regions, reaching a maximum collapse rate at 5 cm H2O.85 De-recruitment modeling suggests the speed of collapse also increases as PEEP decreases.59 Similarly, a decremental PEEP study noted that pleural pressure exceeded alveolar pressure once PEEP decreased below ∼ 9 ± 5 cm H2O, whereas in some subjects de-recruitment occurred at PEEP < 20 cm H2O.73
These findings suggest 3 potential PEEP targets that might reduce de-recruitment during the acute phase of ARDS: (1) minimum PEEP of 10–12 cm H2O, (2) a general target of 16 cm H2O, and (3) ≥ 20 cm H2O in very severe cases, particularly those with reduced chest wall compliance. This is similar to the better PEEP strategy proposed by Gattinoni and colleagues.103
Superimposed Pressure, De-Recruitment, and ARDS Severity
The largest and perhaps most comprehensive CT study reported the maximum range of ventral-dorsal superimposed hydrostatic pressure was 6–18 cm H2O.94 Interestingly, the mean hydrostatic pressure was similar between Berlin classifications of mild, moderate, and severe ARDS (12 ± 3, 12 ± 2, 13 ± 1 cm H2O, respectively, P = .053). Factoring in PEEP required to counter both superimposed hydrostatic pressure and chest wall elastance yielded PEEP estimates of 16 ± 8, 16 ± 5, and 18 ± 5 cm H2O, respectively (P = .48).
A particularly interesting finding was that PEEP requirements did not differ between those characterized as having low or high recruitment potential and based on the observation that maximum superimposed hydrostatic pressure between the 2 groups differed by only 1–2 cm H2O. Thus, neither superimposed hydrostatic pressure nor chest wall elastance correlated appreciably with recruitment potential.
This implies that superimposed hydrostatic pressure enters the calculus of setting PEEP to preserve lung stability following recruitment rather than causing recruitment. These observations led the authors to dissuade clinicians from reflexively treating low recruitability (ie, lobar ARDS) with PEEP levels of ∼ 15 cm H2O simply to prevent shear injury in “a few grams of lung tissue,” given the greater risk of hemodynamic compromise and regional overdistention in middle and ventral lung zones.94
Elevated Intra-Abdominal Pressure in ARDS
The ventral-dorsal pleural pressure gradient in the supine position determines resting alveolar size and largely reflects gravitational forces imposed by the abdomen, which is a more dense, fluid-like compartment with a volume twice that of an air-filled thorax.104,105 Normal intra-abdominal pressure (IAP) is ∼ 5–7 mm Hg (7–10 cm H2O), whereas intra-abdominal hypertension is defined as IAP > 12 mm Hg (16 cm H2O) with 20–60% pressure transmission to the thorax.106 Therefore, severe hypoxemia coinciding with intra-abdominal hypertension is a compelling indication for OLV.
Thoracoabdominal Mechanics, De-Recruitment, and Intra-Abdominal Hypertension
Elevated IAP displaces the diaphragm cephalad into the thorax and stiffens the abdominal portion of the chest wall, such that pleural pressure becomes more positive. This is particularly acute in the dorsal-caudal regions in the supine position, causing reduced lung and chest wall compliance, increased tissue and airways resistance, and compressive atelectasis.107-109 Under these conditions, alveolar de-recruitment from tissue compression (vs alveolar consolidation) is more likely the primary cause of refractory hypoxemia, hence a recruitment maneuver is more likely to be effective.
Abdominal compartment syndrome (IAP > 25 mm Hg; > 34 cm H2O)110 is associated with substantial nonaerated and poorly aerated lung tissue (23% and 18%, respectively).111 At these extraordinary pressures, respiratory system inertance, normally considered negligible, may become significant and therefore would increase TOP.112 Inertance refers to the acceleration of gas molecules as well as displacement of resting lung and chest wall tissues, including the abdominal contents.109
During quiet breathing with normal body habitus, inertance accounts for < 5% of driving pressure.113 In morbid obesity, inertance is ∼ 4-fold higher, and up to 68% can be accounted for by chest wall tissue.109 Although its relevance to ARDS is unknown, it is notable that, in morbidly obese subjects, the driving pressure required to overcome inertance alone during maximal ventilation maneuvers reaches 40 cm H2O.109 In a case of ARDS and abdominal compartment syndrome, a similar driving pressure (Pplat 80 cm H2O and PEEP 45–50 cm H2O) was required to increase 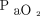 from 23 to 350 mm Hg when surgical decompression could not be attempted.70
from 23 to 350 mm Hg when surgical decompression could not be attempted.70
Intra-Abdominal Hypertension and ARDS
Intra-abdominal hypertension is common in severe ARDS108 and is particularly prevalent in extrapulmonary cases.43 It occurs in pulmonary ARDS complicated by morbid obesity (ie, mass loading), where IAP is ∼ 12–19 cm H2O,114 as well as other conditions such as ascites from abdominal sepsis, pancreatitis, or hepatic failure.115,116 In acute lung injury models, IAP of 20 cm H2O greatly exacerbated pulmonary edema formation and increased intrapulmonary shunting.117,118 Mean IAP of ∼ 22 cm H2O43,116,119 and end-expiratory esophageal pressures of ∼ 20 cm H2O have been reported in cases of severe ARDS.120
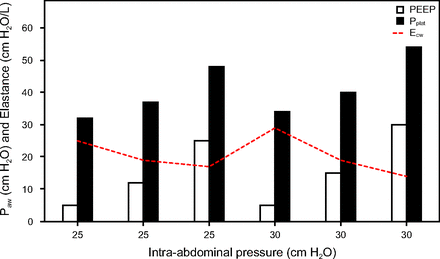
IAP is particularly relevant in treating refractory hypoxemia. A preclinical study reported that, at IAP of 24–35 cm H2O, high PEEP (ie, 15 cm H2O) was equally ineffective as low to moderate PEEP (ie, 5–12 cm H2O) in improving FRC and 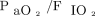 .121 This led to a follow-up study of IAP-matching PEEP in acute lung injury with intra-abdominal hypertension (ie, 16–25 cm H2O). Higher levels of Pplat and PEEP used in the pressure control ventilation recruitment maneuver strategies described above were needed to improve FRC and oxygenation (Fig. 4).117
.121 This led to a follow-up study of IAP-matching PEEP in acute lung injury with intra-abdominal hypertension (ie, 16–25 cm H2O). Higher levels of Pplat and PEEP used in the pressure control ventilation recruitment maneuver strategies described above were needed to improve FRC and oxygenation (Fig. 4).117
Relationship between PEEP, plateau pressure (Pplat) and chest wall elastance (Ecw) at increasing levels of intra-abdominal pressure in an animal model of acute lung injury. Data from Reference 118.
Adding half of the measured IAP to the recruitment maneuver pressure targets has been suggested.106 For example, a recruitment maneuver of 45 cm H2O92 applied to IAP representing conditions of intra-abdominal hypertension (16 cm H2O), average IAP in reported in ARDS (22 cm H2O) or severe abdominal compartment syndrome (≥ 50 cm H2O)110,122 would require adjusting Pplat upward to 53, 56, and 70 cm H2O, respectively.
Attempting a recruitment maneuver in a patient with intra-abdominal hypertension requires assessing overall risk/benefit ratio. Elevated pleural and intra-abdominal pressures impede hemodynamic function and lymphatic drainage and therefore carries the risk of worsening both pulmonary edema and intra-abdominal hypertension as well as risking hemodynamic collapse.123 In the context of abdominal compartment syndrome, it should probably be considered only when surgical decompression carries an even greater risk.
Impact of PEEP on Volume Distribution in ARDS
Finally, regardless of Pplat or PEEP, gas distribution in ARDS steadily decreases down the ventral-dorsal axis with an upper-to-lower lung volume distribution ratio of 2.2:1 at ambient end-expiratory pressure. At PEEP of 20 cm H2O ventral-dorsal gas distribution was essentially equivalent (1.1:1).81 For the dorsal regions (ie, those having the greatest impact on gas exchange) this volume redistribution translated into increased end-expiratory lung volumes from ∼ 10% to 25% and increased end-inspiratory lung volumes from 15% to 35%. These findings were supported by an electrical impedance tomography study of OLV wherein ventral/dorsal tidal volume ratio decreased from 2.01 ± 0.36 to 1.19 ± 0.10 (P < .01).124
Ambiguous and Perplexing Nature of Recruitment Phenomena
In ARDS, improvements in radiologic imaging, gas exchange, and lung mechanics during and following recruitment maneuver represent complex, histopathologic responses of injured lungs and chest wall forces to applied pressure, and thus are open to interpretation. This section describes some of the vagaries that limit our interpretation of the efficacy of recruitment maneuver.
Radiologic Factors
CT scans are the accepted standard for evaluating topographic distribution of aerated and nonaerated lung tissue in ARDS, inferred by the lungs ability to attenuate x-rays.125 The radiologic definition of consolidation is markedly increased lung attenuation obscuring pulmonary vessels caused by atelectasis or alveolar filling, whereas in pathology the term specifically refers to the latter.126 Attenuation is measured by the Hounsfield linear density scale that assigns a numeric value (Hounsfield units [HU]) differentiating between bone (+1,000 HU), water (0 HU) and air (–1,000 HU).127 Values between these 3 points are used to convey various states of pulmonary tissue, with values between −100 HU and +100 HU considered to represent collapsed tissue (Table 2).12,81,94,128
Interpretation of Lung Function Based on Computed Tomography Imaging
From these interpretations, pulmonary gas-tissue ratios are calculated and used to infer the response to recruitment maneuver and PEEP. Yet, the designation of lung “tissue” also includes extravascular fluid and blood.127 Thus, CT imaging represents “the quantity of air being introduced into a diseased lung,” hence the statement, “one pixel is not an alveolus.”115 Lung CT imaging interpretation relies upon an unprovable assumption of homogenous alveolar filling in condensed lung tissue, whereas in reality it likely includes already aerated alveoli.56,115 In addition, estimating the reduction in nonaerated tissue is dependent upon the number of CT sections sampled (compared to whole lung scans). For example, a single juxta-diaphragmatic section may result in either over- or underestimation of recruitment, whereas adding samples of apical and hilar regions tends to overestimate recruitment.129
In spite of the strong association found between radiologic assessment of alveolar recruitment and oxygenation,40,130 a complex interaction of other factors contributes to improved oxygenation (eg, increased ventilation-perfusion matching,131 decreased cardiac output with redistribution of pulmonary perfusion,132 reduced edema formation,133 and its redistribution to the perivascular spaces56,134). Skeptics claim radiologic evidence supporting lung recruitment are “inferences about alveolar micromechanics from measurements made on a scale several orders of magnitude greater than that of the structures of interest.”135 The volume element of a CT image (ie, the voxel) is ∼ 2−2.6 mm3,32,127 whereas a single alveolus is ∼ 0.12 mm3.136 Thus, a single voxel may represent a tissue section consisting of ∼ 15 discrete alveoli.
The importance of this limitation becomes apparent in lung microimaging of gas dynamics within alveolar clusters. Animal models of acute lung injury observed pronounced pendelluft motion between adjacent alveoli (some slowly inflating during expiration, some deflating during inspiration), as well as paradoxically simultaneous recruitment and de-recruitment, while still others either synchronously inflate and deflate or appear stunned (ie, remaining motionless at a constant volume).137,138 This localized inter-alveolar asynchrony and instability results from mechanical interdependency between neighboring alveoli and increases with the severity of injury.138 Although CT imaging studies provide invaluable information on the nature of recruitment and de-recruitment, they are clinically impractical for routine use; in addition, there remains assumptive ambiguity and therefore a risk of over-interpretation.
Rheologic Factors
During expiration, distal airway de-recruitment occurs as increasing surface tension causes liquid bridges to reform, drawing airway and alveolar walls together.56,139 An in vivo study of acute lung injury confirmed the presence of liquid menisci forming dense bridges across small peripheral airways.138 Therefore, the perception of alveolar recruitment in acutely injured lungs may be explained as the breaking of foam bridges and displacement of pulmonary edema fluid, resulting in increased alveolar ventilation.56,135,140 Thus, other factors determine the force required to re-open the lungs: surface-tension forces (accounting for 50–60% of lung elastance), the presence of biologically active surfactant (in both alveoli and distal airways), viscosity and thickness of airway edema, and overcoming strain energy in collapsed small airways (see below).48-50,56,139,141
Even sponge model proponents acknowledge that compression atelectasis likely represents a mixture of alveolar and small airway collapse.81 What remains undisputed is that specific and reproducible ranges of airway pressures transmitted to the lung parenchyma are required to improve regional aeration and gas exchange in ARDS, and that the recruitment of collapsed or obstructed airways and alveoli invariably involves epithelial cell deformation and therefore likely causes shear injury139,140 and exacerbates baseline airway epithelial injury associated with ARDS.45 Greater injury is thought to occur with reopening collapsed versus obstructed airways.140
Histopathologic Factors
Ambiguity surrounding the effectiveness and appropriateness of recruitment maneuvers partly depends upon whether atelectasis (ie, degassed alveoli), intra-alveolar edema (ie, flooded alveolar units and peripheral airways), or interstitial edema is the predominant lesion causing refractory hypoxemia, as well as the intensity of edema.98 Historically the most prominent autopsy findings in early ARDS included some combination of interstitial and alveolar edema or hemorrhage and hyaline membranes,142-152 along with a substantial subset reporting atelectasis.144,145,147,149,153 In what eventually would be called ARDS, the term congestive atelectasis was used to describe “diffuse non-obstructive collapse of pulmonary alveoli and intense interstitial edema and pulmonary capillary congestion,”147 leading to excessive surface tension forces causing collapse.148 More recently, this has been redefined as inflammatory (ie, congestive) atelectasis versus compression atelectasis.154
These characteristics defined diffuse alveolar damage, the histopathologic hallmark of ARDS.142 During the first week of ARDS confirmed with diffuse alveolar damage, intra-alveolar edema tends to be highest (90% of cases) but remains prevalent during subsequent weeks (74% of cases).155 Only ∼ 50% of ARDS cases now present with diffuse alveolar damage,156 its decrease coinciding with the emergence of LPV.157 ARDS without diffuse alveolar damage has been associated primarily associated with pneumonia. This is characterized less by intense interstitial edema and alveolar neutrophil infiltration localized in the terminal bronchioles.156,158
A study that matched PEEP responsiveness to lung biopsy and autopsy samples reported that subjects exhibiting minimal oxygenation response had complete alveolar filling with purulent or hemorrhagic material. Those exhibiting the greatest oxygenation response had less intense alveolar edema and were distinguished by hyaline membrane formation, interstitial edema, and atelectasis.159
Direct Versus Indirect Injury and Injury Severity
Both direct and indirect forms of ARDS include alveolar collapse,42 yet direct (ie, alveolar epithelial) injury has been characterized more by intense collapse and alveolar edema but minimal interstitial edema, whereas indirect (ie, capillary endothelial) injury is associated with more intense interstitial edema than alveolar edema.160 Similar findings were reported in other studies.126 Moreover, direct injury has been associated with higher pulmonary microvascular permeability that, over a period of days, coincides with higher levels of extravascular lung water.161 Some evidence suggests that recruitment maneuver (at least when using the sustained inflation technique) might be ineffective in the presence of high extravascular lung water (∼ 16 mL/kg).98
Inconsistencies between histologic findings in ARDS likely have many sources due to the limited number of samples, the heterogeneous nature of ARDS and associated lesions, and its timing relative to syndrome onset. Irrespective of these, such inconsistencies suggest that simplistic conceptual models guiding recruitment maneuver have limited utility because the varied histopathologic changes in ARDS coexist across a spectrum and that lesions evolve over time.159 Moreover, direct injury from pneumonia disrupting alveolar membrane integrity (ie, loss of bacterial compartmentalization) can induce indirect, secondary injury to noninfected lung regions through systemic cytokine release.162 In fact, a substantial number of subjects with ARDS with either aspiration or pneumonia as primary etiology also have sepsis as a secondary source of lung injury (20% and 40%, respectively).163
Furthermore, secondary analysis of several recruitment maneuver CT studies concluded that recruitment is likely determined more by the severity of injury and corresponding edema formation than injury mechanism per se.93 As lung injury severity increases, so too does the degree of pulmonary capillary permeability and the magnitude of extravascular lung water.164 In general, regardless of injury mechanisms, greater recruitment potential is present in ARDS characterized by diffuse versus predominant dorsal opacities.101,165-167 Unfortunately, this is not a distinction that can be made by clinicians when chest radiographs are the only practical tool available when contemplating whether to pursue treating refractory hypoxemia with recruitment maneuver.
In a secondary analysis of recruitment maneuver studies evaluated with CT, estimates of recruitability were actually higher in direct injury.93 Several factors were cited that provide important insights into the interpretation of recruitment maneuver studies. First, the timing of recruitment maneuver relative to ARDS onset influences recruitability. Over time, edema fluid is slowly reabsorbed while concurrently fibrotic and tissue repair mechanisms evolve. Second, the duration and fidelity to LPV prior to initiating a recruitment maneuver will influence recruitment potential regardless of injury mechanism. Third, the higher correlation between direct injury and ARDS severity may reflect the degree of bacterial diffusion throughout the lung parenchyma.93 This in turn suggests relatively greater consolidation in direct injury (with a corresponding brisk reactive edema formation) possibly producing greater edema than that caused by distant organ injury. However, the investigators stressed that extrapulmonary injury or infection can cause equivalent severity. Thus, when only a small number of subjects are studied (ie, selection bias), the results may suggest equivalence, relatively greater, or relatively lesser lung recruitability between direct versus indirect injury.
Pplat and PEEP During LPV
Oxygenation goals in LPV tend to align with the least-PEEP philosophy, whereby the objective is using the lowest PEEP that provides a reasonable  (≥ 70 mm Hg) at a relatively non-toxic
(≥ 70 mm Hg) at a relatively non-toxic  (≤ 0.60).168 Only when clearly toxic levels of
(≤ 0.60).168 Only when clearly toxic levels of  (≥ 0.70)169 are necessary are higher PEEP levels generally used (> 10 cm H2O).18
(≥ 0.70)169 are necessary are higher PEEP levels generally used (> 10 cm H2O).18
In traditional LPV, tidal volume is titrated to achieve a Pplat ≤ 30 cm H2O.18 Slightly more stringent LPV variants have focused on minimizing the risk of right-ventricular dysfunction and cor pulmonale (Pplat ≤ 26 cm H2O)170,171 or the risk of tidal overdistention (Pplat ≤ 27 cm H2O).172 Given the heterogeneity of ARDS and large variability in oxygenation dysfunction, it is important to have some perspective as to how often traditional LPV goals fall short of securing adequate oxygenation at relatively non-toxic levels of  .
.
The mechanistic studies reviewed above suggest that a Pplat of 30 cm H2O effects almost complete recruitment in the mid-lung (CT regions 4–7) and simultaneously the largest incremental changes in the dorsal lung (regions 8–10). In addition, de-recruitment becomes apparent at PEEP < 15 cm H2O and is particularly prominent in the dorsal regions only at PEEP < 10 cm H2O.85,92 Therefore, assuming normal body habitus, a PEEP of 10–15 cm H2O and Pplat of 26–30 cm H2O appears sufficient to ensure adequate oxygenation at relatively non-toxic levels of 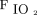 in the majority of ARDS cases.
in the majority of ARDS cases.
Data from 3 major LPV trials173-175 involving > 2,300 subjects with early ARDS compared 2 PEEP strategies support this interpretation. These studies found that: (1) moderate PEEP of 8–10 cm H2O produced a mean Pplat of 21–25 cm H2O and was generally sufficient to achieve an adequate to normal 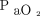 on relatively non-toxic levels of
on relatively non-toxic levels of  ; (2) higher PEEP (ie, ∼ 15 cm H2O) with mean Pplat < 30 cm H2O further improved
; (2) higher PEEP (ie, ∼ 15 cm H2O) with mean Pplat < 30 cm H2O further improved 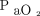 at a decidedly less toxic levels of
at a decidedly less toxic levels of  ; and (3) by the third study day, oxygenation had either stabilized or improved regardless of PEEP strategy. These findings strongly suggest that a recruitment maneuver is unnecessary to manage the majority of ARDS cases and needlessly increases the risk/benefit ratio (Table 3).
; and (3) by the third study day, oxygenation had either stabilized or improved regardless of PEEP strategy. These findings strongly suggest that a recruitment maneuver is unnecessary to manage the majority of ARDS cases and needlessly increases the risk/benefit ratio (Table 3).
Oxygenation and Pplat Differences in 3 Trials of Lower vs Higher PEEP During Lung-Protective Ventilation
Mean data, however, cannot elucidate whether Pplat generated by PEEP levels used during traditional LPV would: (1) likely reach suggested nodal points of TOP associated with full recruitment of dorsal regions; (2) estimate the percentage of subjects requiring toxic levels of  ; and (3) gauge how many subjects would be reasonable candidates for recruitment maneuver therapy. We examined these issues by querying databases used in our prior studies.163,176 Our results are discussed in detail in online supplementary materials (see the supplementary materials at http://www.rcjournal.com); however, the 2 main findings are: (1) tidal volume titration effectively limited Pplat to desired levels despite high PEEP levels; and (2) this limited the likelihood for substantial dorsal lung recruitment in severe refractory hypoxemia, as even at PEEP > 16 cm H2O only 5% of subjects reached a sufficiently high recruitment threshold of 45 cm H2O. Thus, there is a subset of severe ARDS cases in which traditional LPV is insufficient and a recruitment maneuver would appear to be a reasonable option to reverse refractory hypoxemia.
; and (3) gauge how many subjects would be reasonable candidates for recruitment maneuver therapy. We examined these issues by querying databases used in our prior studies.163,176 Our results are discussed in detail in online supplementary materials (see the supplementary materials at http://www.rcjournal.com); however, the 2 main findings are: (1) tidal volume titration effectively limited Pplat to desired levels despite high PEEP levels; and (2) this limited the likelihood for substantial dorsal lung recruitment in severe refractory hypoxemia, as even at PEEP > 16 cm H2O only 5% of subjects reached a sufficiently high recruitment threshold of 45 cm H2O. Thus, there is a subset of severe ARDS cases in which traditional LPV is insufficient and a recruitment maneuver would appear to be a reasonable option to reverse refractory hypoxemia.
Optimizing Oxygenation and Minimizing Risk of Atelectrauma
FRC represents the alveolar volume and is the primary determinant of 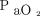 .177 Therefore, increased
.177 Therefore, increased 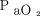 in response to increased PEEP or recruitment maneuver is a bedside convenience to infer changes in FRC and, by extension, shear injury risk. Unfortunately, the logic linking these 3 phenomena is precarious.
in response to increased PEEP or recruitment maneuver is a bedside convenience to infer changes in FRC and, by extension, shear injury risk. Unfortunately, the logic linking these 3 phenomena is precarious.
Depending upon Pplat, a substantial portion of early FRC increase (ie, the fast pulmonary compartment) represents expansion of normally inflated or underinflated alveoli and not recruitment.4 In addition, arbitrary  thresholds used to signify full recruitment (ie, 250–330 mm Hg)40,72 are literally false. Full recruitment implies normal pulmonary oxygen diffusion function (eg,
thresholds used to signify full recruitment (ie, 250–330 mm Hg)40,72 are literally false. Full recruitment implies normal pulmonary oxygen diffusion function (eg,  ≥ 450 mm Hg). This does not occur in ARDS because of varying degrees of tissue consolidation and slow resolution of pulmonary edema. Thus, the
≥ 450 mm Hg). This does not occur in ARDS because of varying degrees of tissue consolidation and slow resolution of pulmonary edema. Thus, the  thresholds of 250–330 mm Hg used to evaluate recruitment maneuver effectiveness suggest a tacit acknowledgment that the term full recruitment is meant figuratively.
thresholds of 250–330 mm Hg used to evaluate recruitment maneuver effectiveness suggest a tacit acknowledgment that the term full recruitment is meant figuratively.
Beyond these vagaries lies the crux of the debate: Does OLV materially reduce the risk of repetitive shear injury compared to traditional LPV? This is unlikely for the majority of ARDS cases. First, a Pplat of ≥ 40 cm H2O is needed to reopen distal airways and alveoli deep within the dorsal lung; these units remain closed and protected from shear injury when Pplat is limited to ≤ 30 cm H2O. Second, in ARDS substantial portions of lung tissue appear to reach full recruitment at Pplat ≤ 30 cm H2O, and its stability appears to be maintained when PEEP is set at 10–15 cm H2O. In addition, evidence from several preclinical studies suggests that atelectatic areas are relatively protected from shear injury by intra-alveolar edema, with most damage caused by excessive stress developed in the peripheral airways.178 In these studies, tidal overdistention was a more important contributor to pro-inflammatory cytokine expression than shear injury. Third, microimaging of subpleural alveoli in acute lung injury models revealed that, despite stable levels of driving pressure and PEEP, there exist patterns of recruitment and de-recruitment between interdependent alveoli, even at high PEEP levels, that appear to fluctuate minute by minute.138 Thus, the notion of eliminating de-recruitment and atelectrauma in ARDS appears illusory.
Implications of Slow Pulmonary Compartments
Integrating the temporal issues involved in recruitment, with evidence that most recruitment occurs at ≤ 50 cm H2O, and the increased mortality risk reported in the ART study,17 it behooves us to reflect upon the need for a recruitment maneuver and how it might be approached going forward. Compelling evidence of slow pulmonary compartments in ARDS is at odds with the current recruitment maneuver strategy and raises questions of whether brief recruitment periods reflect the actual effectiveness of a specific Pplat. By extension, this influences the decision to use higher pressures with increasing risk of injury and hemodynamic compromise. Moreover, limited intensity recruitment maneuver studies such as the extended sigh, the prolonged, and the slow moderate recruitment maneuvers cited above all observed substantial recruitment at pressures ≤ 40 cm H2O over a period of several minutes.33-35,37,38 To date, no study has investigated whether an extended trial of super-PEEP limited to 25–30 cm H2O and driving pressures of 15 cm H2O might provide sufficiently stable oxygenation over a period of several hours.
Also, the relative importance of using an inspiratory time of 2–3 s during a recruitment maneuver, while supported by preclinical data, has not been evaluated clinically. This strategy substantially limits recruitment maneuvers because it restricts minute ventilation in more severe manifestations of ARDS that are associated with highly elevated physiologic dead space179 and places additional strain on right-ventricular function.78,170 In preclinical studies, an inspiratory time of 1.4 s is generally sufficient for recruiting the fast pulmonary compartment.180 In light of studies describing transient [pulmonary] states, as well as those on prone positioning, it is worth considering whether more clinically appropriate inspiratory times used during LPV, if not optimal, might be sufficient to effect sufficient recruitment over time to reach oxygenation goals.
Hemodynamic Consequences of OLV
Although it is not the focus of this review, the unexpectedly higher mortality in the OLV arm of the ART study, and its association with a higher incidence of hemodynamic impairment, requires a brief review of cardiothoracic inter-relationships in ARDS and the potential impact of OLV strategies. The pulmonary vasculature functions as a low-resistance, high-capacitance system reflected in the thin-walled right ventricle, which readily shows signs of dysfunction and eventually fails under sustained work demands imposed by high pulmonary vascular resistance in ARDS.170
Acute pulmonary hypertension commonly develops in ARDS due to hypoxemia, hypercapnia, acidosis, and pulmonary vascular obstruction from interstitial edema, and disseminated arterial and microvascular embolization.181-184 Under mechanical ventilation, conditions of high end-inspiratory volume (eg, high PEEP, driving pressure, or a combination of both) markedly increase pulmonary vascular resistance negatively impacts right-ventricular function.185-187 Right-ventricular function is further compromised due to the simultaneous reduction in venous return and ventricular preload. Acute cor pulmonale develops when the right ventricle becomes ischemic from sustained excessive workloads; this occurs in 22–25% of patients with ARDS, with the incidence increasing to 50% in patients with severe ARDS.188 Thus higher PEEP strategies and the potential for recruitment maneuver overuse in response to incidents of desaturation risks the development of either short-term transient hemodynamic instability, which is a common finding in recruitment maneuver studies,189 or, more importantly, the potential for longer-term problems of right-ventricular dysfunction and the development of cor pulmonale, which increases mortality risk in patients with ARDS.190
Potential Risk of Ventilator-Induced Lung Injury
A brief comment also seems appropriate regarding the potential risk of pressure control ventilation recruitment maneuver strategies for ventilator-induced lung injury. Although driving pressure is controlled at a seemingly safe level during stepwise increases of super-PEEP (ie, 15 cm H2O), the overall magnitude of step-changes in airway pressure increase abruptly from 5 or 10 cm H2O to 20 cm H2O as PEEP increases from 25 to 45 cm H2O40,72 or by continuous incremental changes of 10 cm H2O.17 Regardless, these manipulations culminate in extraordinarily high end-inspiratory pressures of 60 cm H2O. More concerning is that, during the subsequent PEEP decrement trials in some studies, once the optimal PEEP level is determined, “patients underwent another recruitment maneuver using the same recruiting pressures used in the last step of the maximum recruitment maneuver.”40 This procedure was incorporated into the ART trial.17 As others have noted, regardless of the perceived safety of limiting driving pressure to 15 cm H2O, there is an upper limit of lung stress that can be tolerated without resulting in severe lung injury.140 As described in this review, there appears to exist highly circumspect situations in which this might be appropriate (eg, morbid obesity, abdominal compartment syndrome), although clinicians should always be cognizant of this danger.
Implications of the ART Study
Finally, the discouraging results of the ART trial underscore the primary clinical problem with recruitment maneuver therapy in ARDS, namely the necessary reliance upon chest radiographs and inferences drawn from mechanistic studies that limits clinicians to mere speculation about the likelihood of therapeutic success. As a result, the vexing problem for clinicians is discerning whether an apparent nonresponder reflects inadequate TOP, insufficient time allotted for recruitment, or simply poor recruitment potential. From what little we are able to discern from the ART trial, this appears to be what occurred: the majority of ARDS cases were from direct injury exhibiting only mild responses to recruitment maneuver, with a correspondingly higher incidence of lung overdistention (surmised from the incidence of barotrauma and need for vasopressor therapy) that was significantly associated with mortality risk in the OLV study arm.17
As mentioned above, the accepted standard for assessing lung recruitment is CT imaging, which provides superior information to identify both the patients who are most likely to benefit from a recruitment maneuver (eg, diffuse injury pattern) and the limits of Pplat and PEEP based on real-time imaging. Unfortunately, this is clinically impractical, and we therefore remain in the same predicament as the ART study investigators. However, the utilization of bedside ultrasonography in the assessment of lung recruitment is a promising tool and should be incorporated into clinical management when assessing the effectiveness of a recruitment maneuver.
Summary
In the context of severe ARDS, consideration of a recruitment maneuver should be reserved for a minority of cases with persistent or recurring bouts of hypoxemia that occur despite PEEP levels of 15–20 cm H2O and require prolonged exposure (ie, days) to  ≥ 0.70 to stabilize oxygenation, particularly patients with either intra-abdominal hypertension or severe obesity. Under these circumstances, the risk of exacerbating lung injury from oxidative stress from prolonged exposure to toxic levels of
≥ 0.70 to stabilize oxygenation, particularly patients with either intra-abdominal hypertension or severe obesity. Under these circumstances, the risk of exacerbating lung injury from oxidative stress from prolonged exposure to toxic levels of  enters prominently into the calculus.169 When elevated IAP is not a prominent factor, a prolonged trial of super-PEEP and low driving pressures that generate a Pplat of 40–45 cm H2O (perhaps in concert with prone position) may be a more prudent approach to stabilize oxygenation. Finally, a recruitment maneuver in those with direct injury and a higher likelihood of pronounced tissue consolidation is probably of limited benefit and has been associated with greater mortality risk.
enters prominently into the calculus.169 When elevated IAP is not a prominent factor, a prolonged trial of super-PEEP and low driving pressures that generate a Pplat of 40–45 cm H2O (perhaps in concert with prone position) may be a more prudent approach to stabilize oxygenation. Finally, a recruitment maneuver in those with direct injury and a higher likelihood of pronounced tissue consolidation is probably of limited benefit and has been associated with greater mortality risk.
Footnotes
- Correspondence: Richard H Kallet MSc RRT FAARC. E-mail: richkallet{at}gmail.com
Supplementary material related to this paper is available at http://www.rcjournal.com.
Mr Burns presented a version of this paper at AARC Congress 2018, held December 4–7, 2018, in Las Vegas, Nevada.
Mr Kallet has disclosed a relationship with Nihon Kohden. The remaining authors have disclosed no conflicts of interest.
- Copyright © 2021 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.
- 8.
- 9.
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.
- 96.
- 97.
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.
- 144.↵
- 145.↵
- 146.
- 147.↵
- 148.↵
- 149.↵
- 150.
- 151.
- 152.↵
- 153.↵
- 154.↵
- 155.↵
- 156.↵
- 157.↵
- 158.↵
- 159.↵
- 160.↵
- 161.↵
- 162.↵
- 163.↵
- 164.↵
- 165.↵
- 166.
- 167.↵
- 168.↵
- 169.↵
- 170.↵
- 171.↵
- 172.↵
- 173.↵
- 174.
- 175.↵
- 176.↵
- 177.↵
- 178.↵
- 179.↵
- 180.↵
- 181.↵
- 182.
- 183.
- 184.↵
- 185.↵
- 186.
- 187.↵
- 188.↵
- 189.↵
- 190.↵