Abstract
BACKGROUND: To minimize ventilator-induced lung injury, the primary clinical focus is currently expanding from measuring static indices of the individual tidal cycle (eg, plateau pressure and tidal volume) to more inclusive indicators of energy load, such as total power and its elastic components. Morbid obesity may influence these components. We characterized the relative values of elastic subcomponents of total power (ie, driving power and dynamic power) in subjects with severe hypoxemia, morbid obesity, or their combination.
METHODS: We analyzed data from subjects receiving mechanical ventilation divided into 4 groups. 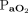 /
/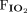 < 150 mm Hg (severe hypoxemia) indicated probable reduction of lung compliance while body mass index > 40 kg/m2 (morbid obesity) suggested a possible contribution to reduced respiratory system compliance from the chest wall. Group 1 included subjects with no expected abnormality of lung compliance or chest wall compliance; Group 2 included subjects with expected reduction of lung compliance on the basis of severe hypoxemia but with no morbid obesity; Group 3 included subjects with morbid obesity without severe hypoxemia; and Group 4 included subjects with morbid obesity and severe hypoxemia. All ventilator-induced lung injury predictors were compared among groups using mixed-effects linear models.
< 150 mm Hg (severe hypoxemia) indicated probable reduction of lung compliance while body mass index > 40 kg/m2 (morbid obesity) suggested a possible contribution to reduced respiratory system compliance from the chest wall. Group 1 included subjects with no expected abnormality of lung compliance or chest wall compliance; Group 2 included subjects with expected reduction of lung compliance on the basis of severe hypoxemia but with no morbid obesity; Group 3 included subjects with morbid obesity without severe hypoxemia; and Group 4 included subjects with morbid obesity and severe hypoxemia. All ventilator-induced lung injury predictors were compared among groups using mixed-effects linear models.
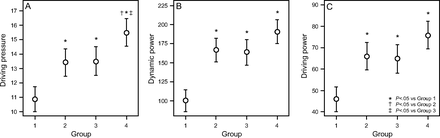
RESULTS: Groups 1–4 included 61, 52, 49, and 51 subjects, respectively. Mean body mass index averaged 28.7 kg/m2 for nonobese subjects and 52.1 kg/m2 for morbidly obese subjects. Mean driving pressure, dynamic power, and driving power of Groups 2 and 3 exceeded the corresponding values of Group 1 but fell into similar ranges when compared with each other. These values were highest in Group 4 subjects. In Group 2, mean dynamic power and driving power values were comparable to those in Group 3.
CONCLUSIONS: In mechanically ventilated subjects, stress and energy-based ventilator-induced lung injury indicators are influenced by the relative contributions of chest wall and lung to overall respiratory mechanics. Numerical guidelines for ventilator-induced lung injury risk must strongly consider adjustment for these elastic characteristics in morbid obesity.
- mechanical ventilation
- ventilator-induced lung injury
- elastic power of ventilation
- morbid obesity
- hypoxemia
- ARDS
- respiratory compliance
Introduction
Exposure to high tidal pressures is undesirable, both for previously injured lungs and for those without preexisting lung injury. Understandably, therefore, assessing the potential for ventilator-induced lung injury (VILI) from monitored pressures and volumes has centered on the characteristics of the individual tidal cycle. Prominent among these are the passive end-inspiratory and end-expiratory static airway pressures (ie, plateau pressure and PEEP) and the numerical difference between them, which is the driving pressure (ΔP).1-3 Although now incorporated into many respiratory care protocol guidelines for ventilator adjustment, such measures do not take flow or cycling frequency into account. Because both of the latter dynamic contributors to ventilating energy and power influence VILI risk,4 regulating machine work and energy-related variables is now considered a logical approach to improving lung protection.
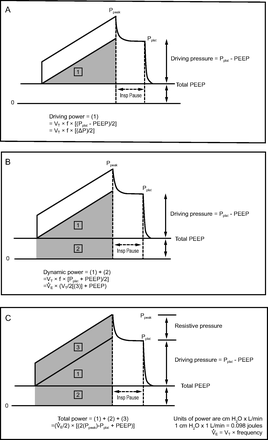
The proposed superiority of the more inclusive total inspiratory power as an indicator for VILI risk is currently debated. Total inspiratory power is defined as the total energy applied to the respiratory system over 1 min.5,6 Apart from ventilating frequency, total power includes 3 components that relate to flow resistance, tidal elastic recoil, and PEEP (Fig. 1). Of these, both the flow resistive and PEEP components of total inflation energy have been called into question as direct VILI contributors.7 Moreover, tidal elastic recoil is influenced jointly by the properties of the series-coupled lung and chest wall, which experience inflation energy and power in direct relation to their relative stiffness. Therefore, while power determined from airway pressure (Paw) may correlate well with VILI risk when tracked in any given individual, the same numerical value for total power is unlikely to influence VILI to the same extent across a spectrum of patients, including those with reduced chest wall compliance. Assuming the same lung compliance, less lung-damaging tidal energy and power would be applied in that latter group compared to patients with normal chest wall compliance.
Pressure versus time tracings under constant flow setting and inspiratory hold: (A) Driving power, (B) Dynamic Power, (C) Total power. Therefore, (1) represents the per-cycle energy that, when multiplied by frequency, comprises driving power. Similarly, areas (1) + (2) = dynamic power, and areas (1) + (2) + (3) = total power. Ppeak = peak pressure; Pplat = plateau pressure; VT = tidal volume; f = breathing frequency; 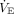 = expiratory minute volume.
= expiratory minute volume.
With the increased prevalence of morbid obesity worldwide, intensivist clinicians manage greater numbers of such ventilated patients and generally rely on Paw to determine the safety of tidal pressures and applied power.8,9 Because the lung and chest wall inflate in series, the safe range of tidal Paw values for ventilating morbidly obese patients is not known with precision. The abnormal chest walls of obese individuals may or may not restrict lung expansion, depending on body weight, position, and conformation. Naimark and colleagues10 reported that, in obese subjects, total respiratory system compliance declined in comparison to normal subjects due to decreased chest wall compliance, while lung compliance remained unchanged. Their proposed threshold of body mass index > 40 kg/m2 for morbid obesity to influence chest wall stiffness appears to be supported by more recent data.11 Yet, other elegant work, performed at higher levels of PEEP, suggests that tidal chest wall compliance may remain normal, even for very obese individuals.12 Of note, the roles of positional gas trapping and applied PEEP level may strongly influence respiratory system compliance measurements made using Paw alone.13 Understanding how morbid obesity affects bioenergetic indicators of hazard (eg, tidal energy and power) should improve our ability to ventilate these patients safely.
We hypothesized that varied influences of the recumbent morbidly obese chest wall may affect the measured values of driving power (related to ΔP) and dynamic power (related to the sum of ΔP and PEEP) in mechanically ventilated patients. Two key elements are believed to influence VILI risk: excessive strain per cycle and cycling frequency. Although not part of the tidal pressure excursion above the end-expiratory value, higher levels of PEEP do add to the baseline strain. Indeed, dynamic power, a PEEP-inclusive component of total power, has been shown in rats to be a better predictor of VILI than driving power.14 In this study, we examined a database recorded from passively ventilated patients with and without morbid obesity with the intent to characterize the ranges and distributions of components of power that relate selectively to elastic forces in these patients, and to compare specifically the relative values of bioenergetic variables in patients with morbid obesity with patients having severe hypoxemia without morbid obesity.
Quick Look
Current Knowledge
In attempts to minimize ventilator-induced lung injury, the clinical focus has been expanding from stress-based indices of tidal inflation cycles, such as plateau pressure and driving pressure, to strain-based inclusive indices, like mechanical power of ventilation. Our understanding of how morbid obesity influences these newer indicators of ventilator-induced lung injury is still developing, which limits their bedside applicability. The ranges of elastic power for intubated patients with and without morbid obesity have not yet been defined.
What This Paper Contributes to Our Knowledge
Intubated subjects characterized by morbid obesity without severe hypoxemia and those characterized by severe hypoxemia without overt chest wall abnormality required comparable elastic power to ventilate, both exceeding the currently proposed target ranges of pressure and power recorded in nonobese patients with no severe hypoxemia.
Methods
We conducted a retrospective analysis involving eligible patients supported by mechanical ventilation in our medical and surgical ICUs between July 2013 and June 2018 at Regions Hospital, St. Paul, Minnesota. The subject samples were extracted without bias or unbalance from all years in the 2015–2018 range; however to obtain our targeted number in one of the subgroups (Group 4, below), we were obligated. For each subject, we extracted 1–3 data points that were obtained within 6 h of each other and met all inclusion and exclusion criteria. Each data point was obtained after 12 h of intubation, and multiple data points were averaged. This study was approved by the HealthPartners Institutional Review Board for human research (#A19-036). Our sample was partitioned into 4 groups based on oxygen exchange and body mass index. The primary intent was to characterize the influence of morbidly obese body habitus on the elastic components of power measured by Paw that relate to VILI.  /
/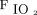 < 150 mm Hg identified candidates with severe hypoxemia; body mass index > 40 kg/m2 identified candidates with morbid obesity. We prospectively screened subjects for assignment into 1 of 4 groups differing on those characteristics: Group 1 (n = 61) included subjects with no severe hypoxemia and no morbid obesity; Group 2 (n = 52) included subjects with severe hypoxemia and no morbid obesity; Group 3 (n = 49) included subjects with morbid obesity without severe hypoxemia; and Group 4 (n = 51) included subjects with morbid obesity and severe hypoxemia.
< 150 mm Hg identified candidates with severe hypoxemia; body mass index > 40 kg/m2 identified candidates with morbid obesity. We prospectively screened subjects for assignment into 1 of 4 groups differing on those characteristics: Group 1 (n = 61) included subjects with no severe hypoxemia and no morbid obesity; Group 2 (n = 52) included subjects with severe hypoxemia and no morbid obesity; Group 3 (n = 49) included subjects with morbid obesity without severe hypoxemia; and Group 4 (n = 51) included subjects with morbid obesity and severe hypoxemia.
All subjects required mechanical ventilation for > 24 h in ICUs at Regions Hospital between July 2013 and June 2018. We excluded patients with (1) age < 18 y; (2) non-passive ventilation, as determined by the respiratory therapist and difference of ≥ 2 breaths/min between set ventilation rate and the patient’s observed breathing frequency; (3) pregnancy; (4) history of previous lung resection or deformity of the chest wall; (5) recent abdominal or chest wall surgeries; (6) primary congestive heart failure; or (7) preexisting lung parenchymal lung diseases (eg, interstitial lung disease, emphysema, cystic lung disease). A list of patients was generated automatically using electronic medical record algorithms, and the clinical research staff and investigators (Drs Syed and Selickman) reviewed the patients on the list for appropriateness of inclusion. A total of 1,463 chart records were reviewed; of these, 213 patients qualified for the study. With the exception of Group 4, the subject sample was extracted without bias or unbalance from all years in the 2015–2018 range; to obtain our targeted number of subjects for Group 4, we reviewed data extending back to 2013. Passive ventilator data generated in practice for clinical purposes were recorded by the respiratory therapist 1–3 times for each enrolled subject. When plateau pressures varied by > 20% for sequential observations, recorded data summaries used per-patient averages across the nonaberrant measurements. Body position (supine orientation and head of bed elevated in accordance with our nursing standard) was uniform for each patient from whom multiple data points were averaged.
Calculations of Bioenergetic Variables
Because subjects were exposed to a mix of flow profiles (both constant and decelerating flows), we calculated only the elastic components of total driving power of interest (ie, driving power and dynamic power). We defined driving power as the elastic energy increment in excess of PEEP needed to expand the lungs and chest wall (Fig. 1). Driving power can be estimated as 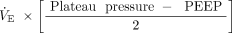 , or
, or 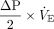 , where
, where 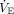 is minute ventilation (ie, tidal volume × breathing frequency); units of power are J/min (1 cm H2O×L/min = 0.098 J/min). Dynamic power includes the energy expended to overcome PEEP in addition to the driving power, that is, the inflation energy expended each minute against both the static and dynamic components of elastic recoil. This total elastic component of power, dynamic power, can be estimated as
is minute ventilation (ie, tidal volume × breathing frequency); units of power are J/min (1 cm H2O×L/min = 0.098 J/min). Dynamic power includes the energy expended to overcome PEEP in addition to the driving power, that is, the inflation energy expended each minute against both the static and dynamic components of elastic recoil. This total elastic component of power, dynamic power, can be estimated as 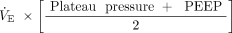 and differs from driving power only in that it includes the contribution of elastic power that results from the product of breathing frequency and the energy block related to PEEP (Fig. 1).
and differs from driving power only in that it includes the contribution of elastic power that results from the product of breathing frequency and the energy block related to PEEP (Fig. 1).
Statistical Analysis
Demographic characteristics of the participants were summarized using median (interquartile range) for continuous measures and number and frequency for categorical measures. Group sample sizes were chosen based on providing 85% power for multiplicity-adjusted detection (P < .05) of correlations of ≥ 0.5 among VILI indices within each group based on previously reported ranges of driving power.15 Correlations among VILI indices were assessed using the Spearman rank-based correlation coefficient. VILI indices were compared among groups using mixed-effects linear models and summarized by group as estimated marginal means with 95% CIs. In addition to group-wise comparisons, the relationships between VILI indices, body mass index, and 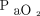 /
/ were examined using generalized additive models and visualized using contour plots. Analyses were conducted using R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria), including the nlme package (version 3.1-143) for mixed-effects models and the mgcv package (version 1.8-31) for generalized additive models.16 A 2-sided P value < .05 was regarded as statistically significant.
were examined using generalized additive models and visualized using contour plots. Analyses were conducted using R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria), including the nlme package (version 3.1-143) for mixed-effects models and the mgcv package (version 1.8-31) for generalized additive models.16 A 2-sided P value < .05 was regarded as statistically significant.
Results
The medical records of 1,463 consecutive patients were reviewed to obtain our targeted sample size of ∼ 50 eligible records for each group (Fig. 2). We included a total of 213 intubated subjects who received passive mechanical ventilation in our ICUs for > 24 h. The majority of subjects in Groups 1, 2, and 3 were consecutive subjects from 2016 through 2018 who met the inclusion criteria. For the Group 4 cohort, we reviewed data that included qualified subjects back to 2013 to obtain our required sample size. We collected ventilator data at time points in close proximity to an arterial blood gas sample taken > 12 h after intubation, provided that none of the exclusion criteria were present. Approximately 20% (21 of 100) of subjects with severe hypoxemia in Group 2 and Group 4 had ARDS. Median body mass index for Group 1 and Group 2 (both not morbidly obese) was 28.7 kg/m2 and 52.1 kg/m2 for Group 3 and Group 4 (both morbidly obese). Median 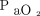 /
/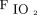 values for Group 1 and Group 3 (both not severely hypoxemic) were 335 and 229 mm Hg, respectively. Median
values for Group 1 and Group 3 (both not severely hypoxemic) were 335 and 229 mm Hg, respectively. Median  /
/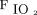 values in Group 2 and Group 4 (both severely hypoxemic) were 117 and 94 mm Hg, respectively (Table 1).
values in Group 2 and Group 4 (both severely hypoxemic) were 117 and 94 mm Hg, respectively (Table 1).
Flow chart. ABG = arterial blood gas.
Subject Characteristics and Ventilator Data
Breathing frequency and minute ventilation differed significantly among groups, but tidal volume did not. Median plateau pressure values were higher in Group 2 and Group 3 compared to Group 1. Plateau pressure was highest and respiratory system compliance was lowest in Group 4 (combined severe hypoxemia and morbid obesity), as expected. Differences of median total PEEP values for Group 2 and Group 3 were not statistically significant: 10.0 versus 9.9 cm H2O. Median total PEEP was slightly higher in Group 4 (11.3 cm H2O) and lowest in Group 1 (5.5 cm H2O).
Mean values for ΔP, driving power, and dynamic power for each group are presented in Table 2. The ΔP, driving power, and dynamic power of Group 2 and Group 3 exceeded the corresponding values of Group 1 but fell into similar ranges when compared with each other; mean ΔP, driving power, and dynamic power values in Group 2 and Group 3 did not differ significantly (P > .05). ΔP values in Group 2 and Group 3 statistically differed from Group 4 (Fig. 3). Driving power and dynamic power tended to be higher in Group 4 than in either Group 2 or Group 3, but this difference did not reach statistical significance (P > .05). The correlation coefficients of ΔP with dynamic power were 0.39, 0.37, 0.18, and 0.23 for Groups 1–4, respectively (with corresponding P values of .002, .008, .24, and .11, respectively). ΔP correlated more closely with driving power with 0.70, 0.78, 0.49, and 0.74 for Groups 1–4, respectively (with corresponding P values of < .001, .008, < .001, and < .001, respectively), as expected.
Ranges of (A) driving pressure, (B) dynamic power, and (C) driving power by group.
Driving Pressure, Dynamic Power, and Driving Power by Subject Group
We used a body mass index of 40 kg/m2 and a  /
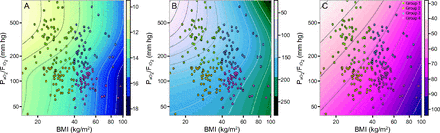
/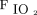 of 150 mm Hg to prospectively screen and stratify data from our patient population into 4 nominal group categories of obesity and hypoxemia. To illustrate the degree to which subject data were overlapped across categories of pressure and power, Figure 4 displays the elastic power indices plotted against those same characteristics in a continuous (noncategorical) fashion. Granular data from Group 1 are distinguished by green circles, Group 2 by yellow circles, Group 3 by purple circles, and Group 4 by pink circles. Although the group data clouds are clearly distinguishable from each other on each of the 3 pressure and power measures, some overlap across group categories did occur.
of 150 mm Hg to prospectively screen and stratify data from our patient population into 4 nominal group categories of obesity and hypoxemia. To illustrate the degree to which subject data were overlapped across categories of pressure and power, Figure 4 displays the elastic power indices plotted against those same characteristics in a continuous (noncategorical) fashion. Granular data from Group 1 are distinguished by green circles, Group 2 by yellow circles, Group 3 by purple circles, and Group 4 by pink circles. Although the group data clouds are clearly distinguishable from each other on each of the 3 pressure and power measures, some overlap across group categories did occur.
Contour plots for A: driving pressure, B: dynamic power, and C: driving power in relation to 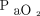 /
/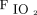 and body mass index (BMI). Contour plots illustrate how these 3 indices of pressure and power increase or decrease with
and body mass index (BMI). Contour plots illustrate how these 3 indices of pressure and power increase or decrease with 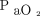 /
/ and body mass index on a continuous scale (as opposed to being segregated by group).
and body mass index on a continuous scale (as opposed to being segregated by group).
Discussion
Our key findings indicate that mechanical properties of the respiratory system associated with morbid obesity jointly influence plateau pressure, ΔP, and the static and dynamic components of tidal energy and power. We also report for the first time the ranges of the elastic components of power (ie, driving power and dynamic power) for these specific subject groups. All Paw-based indices of VILI risk were highest in Group 4 (with severe hypoxemia and morbid obesity) and lowest in Group 1 (with no severe hypoxemia and no morbid obesity). Importantly, subjects with no severe hypoxemia and morbid obesity (Group 3) exhibited numerical values of mechanical indices (ie, ΔP, dynamic power, and driving power) that overlapped the range of the subjects in Group 2 (with severe hypoxemia and no morbid obesity), but possibly with different contributions to respiratory system elastance from lung and chest wall (Fig. 3).
In overweight patients, chest wall properties are not the sole contributor to reduced respiratory system compliance, as obesity simultaneously promotes atelectasis and regional gas trapping that varies with body position and PEEP.13,17-19 Parhar et al20 reported that total power was higher in subjects with ARDS as the cause of hypoxemia than in subjects with non-ARDS causes of hypoxemia, while ΔP values were similar in both cohorts. Those data appear to indicate promise for total power (a measure that includes PEEP and frequency) to help differentiate hypoxemic patient populations into phenotypes and endotypes.21,22 A retrospective analysis relevant to our work suggested that ΔP values might be less closely associated with mortality in obese subjects.23 Notably, only 20% of our severely hypoxemic subjects (Group 2 and Group 4) were given a diagnosis of ARDS by the clinician of record. Although we did not formally apply ARDS criteria to the subjects with severe hypoxemia in our samples, this observation may be consistent with the frequent under-recognition of Berlin-defined ARDS reported in the LUNGSAFE study.24
Experimentally, the risk of VILI has been reported to increase significantly at an indistinct but critical threshold for power that varies with species and pre-exposure condition of the lungs.25-28 In humans with ARDS, the hazardous level of total power has been suggested to lie within the range of 17.0–22.0 J/min,29 but the range appropriate for morbidly obese patient populations has not been specified. While total power may be a valid indicator to follow in the same individual of whatever disease or body habitus, interpretive caution is indicated when making comparisons across individuals with different lung and chest wall characteristics. The concerning values for driving power and dynamic power, as we defined them, have not been described for any population. The relevant research is still in the phase of collecting observations to delineate the numerical boundaries for safe elastic power exposure.
To generate the excessive strain that promotes VILI, lung tissue must repeatedly be displaced from its relaxed baseline by a sufficient pressure increment.30 In morbidly obese patients, the heavy chest wall may, in theory, limit the extent of alveolar expansion associated with a given Paw increment and hence may afford some lung protection; a component of the apparently excessive power applied to these individuals must repeatedly move not only the lung but also the abnormal chest wall. In other words, the transpulmonary (lung-relevant) fraction of power is the damaging component.
In acute respiratory failure, hypoxemia is often considered a reflection of lung damage severity.30 We selected 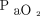 /
/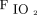 < 150 mm Hg as the cutoff value likely to be associated with lung impairment, including severe and moderately severe ARDS, as defined by the Berlin criteria.31 One problem with the use of oxygenating efficacy to define lung disease severity is that the strength of hypoxic vasoconstriction and intrapulmonary redirection of blood flow influence its applicability. Morbidly obese patients experience a heavy weight from the chest wall, which imposes a mass load that requires substantial PEEP to counterbalance and may cause both driving power and dynamic power to increase. Published work suggests that in supine, morbidly obese patients, an average of 18 cm H2O may be needed to fully recruit lung tissue collapsed solely by the weight of the chest wall.12 This value is considerably higher than the PEEP applied in our subject sample, so that some degree of atelectasis likely co-existed in our obese subjects, whether manifested by hypoxemia or not. To the extent that PEEP counterbalances the compressive effect of the chest wall, the associated Paw-based elastic power would tend to distribute to a greater extent across the lung.
< 150 mm Hg as the cutoff value likely to be associated with lung impairment, including severe and moderately severe ARDS, as defined by the Berlin criteria.31 One problem with the use of oxygenating efficacy to define lung disease severity is that the strength of hypoxic vasoconstriction and intrapulmonary redirection of blood flow influence its applicability. Morbidly obese patients experience a heavy weight from the chest wall, which imposes a mass load that requires substantial PEEP to counterbalance and may cause both driving power and dynamic power to increase. Published work suggests that in supine, morbidly obese patients, an average of 18 cm H2O may be needed to fully recruit lung tissue collapsed solely by the weight of the chest wall.12 This value is considerably higher than the PEEP applied in our subject sample, so that some degree of atelectasis likely co-existed in our obese subjects, whether manifested by hypoxemia or not. To the extent that PEEP counterbalances the compressive effect of the chest wall, the associated Paw-based elastic power would tend to distribute to a greater extent across the lung.
Clinical Implications
Our data indicate that obesity, when in the morbid range and not associated with clinically identified lung problems, influences ΔP and power indices that are based on Paw alone. In morbidly obese populations, devices like the esophageal balloon catheter should be strongly considered when Paw rises to concerning levels, so as to determine lung-relevant transpulmonary pressure. This influence of morbid obesity calls into question the magnitude of currently acceptable ΔP values in the morbidly obese, as previously shown by others.23 Because lung protection is the clinical focus, numerical values and limits for such Paw-based predictors of VILI risk are not reliable guides for safety or hazard in all circumstances. A titrated approach to setting PEEP and tidal volume, using compliance or ΔP (for the same tidal volume) as an indicator, would seem the most rational compromise when directly measured transpulmonary pressure is not available.
Total power has been linked to clinical outcomes and to experimental VILI. However, debate continues regarding whether total power is sufficiently precise or whether one of its elastic subcomponents offers a more direct measure of VILI risk.5 The driving power is another potential index being explored,32 but dynamic power might better indicate the true hazard, as PEEP adds both to tissue stretch and total power.33 There are 2 key elements to VILI risk: excessive strain per cycle and cycling frequency. Thus, although its relative contribution to ΔP might reasonably be argued, PEEP clearly adds to the baseline strain applied to most lung units. In support of its role in generating VILI, a recent experimental report indicates that total power incurred at high tidal pressures eventually produces damage, in whatever way that total power may be generated.34 The net effect of higher PEEP in those with morbid obesity might not be easily predicted, however, because it not only raises the dynamic power but tends to simultaneously recruit new lung units while redirecting more of the applied pressure and power across the lung.
Limitations
Without an estimate of pleural pressure, we could not partition the elastic power distribution across lungs and chest wall. Furthermore, the retrospective, single-center nature of our analysis imposes limits upon interpretation of our data. The rationale for our study was based on our assumption that morbid obesity contributes to ΔP and power indices to some extent by decreasing chest wall compliance. This assumption, however, is still an unsettled issue, even if our own experimental work raising abdominal pressures of pigs into the range of the very obese35 demonstrates the strong and graded impact of such elevations on ΔP,36 even as ΔP across the lung remained unchanged. We understand, however, that morbid obesity can contribute to the recorded ΔP by other means, such as position-related gas trapping or unrelieved atelectasis.13,18,19 Furthermore, even in healthy persons with such factors considered, body mass index is not the only determinant of chest wall flexibility, as it depends also on the distribution of excess body weight.37
Conclusions
Stress and energy-based VILI indicators calculated from Paw were influenced by alterations of the elastic properties of the respiratory system associated with morbid obesity. Numerical guidelines for such VILI risk indicators must consider these mechanical characteristics, with particular caution in patients with morbid obesity.
Acknowledgments
We thank John Connett PhD of the Clinical and Translational Science Institute, University of Minnesota, and Firas Elmufdi MD of Regions Hospital for their contributions to the study design. We acknowledge Paula Rupp, Katherine Grondahl, and Jenna Elizabeth of Critical Care Research Center, Regions Hospital, for their efforts in data collection.
Footnotes
- Correspondence: John J Marini MD. E-mail: marin002{at}umn.edu
Dr Hayat Syed presented a version of this paper at the 49th Annual Meeting of the Society of Critical Care Medicine, held February 16–20, 2020, in Orlando, Florida.
This research was partly supported by the National Institutes of Health’s National Center for Advancing Translational Sciences (grant UL1TR002494) and the Regions Hospital Research, Education, and Development Fund. The authors have disclosed no conflicts of interest.
- Copyright © 2021 by Daedalus Enterprises