Abstract
BACKGROUND: The efficacy of noninvasive oxygenation strategies (NIOS) in treating COVID-19 disease is unknown. We conducted a prospective observational study to assess the rate of NIOS failure in subjects treated in the ICU for hypoxemic respiratory failure due to COVID-19.
METHODS: Patients receiving first-line treatment NIOS for hypoxemic respiratory failure due to COVID-19 in the ICU of a university hospital were included in this study; laboratory data were collected upon arrival, and 28-d outcome was recorded. After propensity score matching based on Simplified Acute Physiology (SAPS) II score, age,  and
and  at arrival, the NIOS failure rate in subjects with COVID-19 was compared to a previously published cohort who received NIOS during hypoxemic respiratory failure due to other causes.
at arrival, the NIOS failure rate in subjects with COVID-19 was compared to a previously published cohort who received NIOS during hypoxemic respiratory failure due to other causes.
RESULTS: A total of 85 subjects received first-line treatment with NIOS. The most frequently used methods were helmet noninvasive ventilation and high-flow nasal cannula; of these, 52 subjects (61%) required endotracheal intubation. Independent factors associated with NIOS failure were SAPS II score (P = .009) and serum lactate dehydrogenase at enrollment (P = .02); the combination of SAPS II score ≥ 33 with serum lactate dehydrogenase ≥ 405 units/L at ICU admission had 91% specificity in predicting the need for endotracheal intubation. In the propensity-matched cohorts (54 pairs), subjects with COVID-19 showed higher risk of NIOS failure than those with other causes of hypoxemic respiratory failure (59% vs 35%, P = .02), with an adjusted hazard ratio of 2 (95% CI 1.1–3.6, P = .01).
CONCLUSIONS: As compared to hypoxemic respiratory failure due to other etiologies, subjects with COVID-19 who were treated with NIOS in the ICU were burdened by a 2-fold higher risk of failure. Subjects with a SAPS II score ≥ 33 and serum lactate dehydrogenase ≥ 405 units/L represent the population with the greatest risk.
Introduction
Although international guidelines have been unable to provide recommendations on the use of noninvasive oxygenation strategies (NIOS) for the early treatment of acute hypoxemic respiratory failure,1–3 NIOS have been extensively used during the COVID-19 pandemic, both for resource limitations and for clinicians' confidence in this tool.4
Caution is required with the use of NIOS, including high-flow nasal cannula, CPAP, and noninvasive ventilation (NIV), to treat hypoxemic respiratory failure.5 Maintaining spontaneous breathing in hypoxemic respiratory failure may show some benefits,6–12 but it carries the inherent risk of self-inflicted lung injury,12–17 delayed intubation, and increased mortality.18,19 These considerations prompt careful selection of patients and analysis of risk factors to limit the rate of treatment failure and delayed intubation.20,21
Variable NIOS failure rates have been reported during other viral pandemics,22–26 but few data elucidate the use of NIOS in acute hypoxemic respiratory failure due to COVID-19 in the ICU.27,28 We conducted a prospective, observational study to describe the outcomes of critically ill subjects with hypoxemia due to COVID-19 who received first-line NIOS, to identify possible early predictors of treatment failure, and to compare the rate of endotracheal intubation of subjects with COVID-19 with that of historical matched-control subjects affected by other causes of acute hypoxemic respiratory failure.
Quick Look
Current Knowledge
Noninvasive oxygenation strategies are widely used to treat acute hypoxemic respiratory failure due to COVID-19 and other etiologies. While potentially beneficial, these strategies carry the risk of delayed intubation and patient self-induced lung injury, and caution is required.
What This Paper Contributes To Our Knowledge
Subjects with COVID-19 who received noninvasive oxygenation strategies in the ICU due to acute hypoxemic respiratory failure had a 2-fold greater risk for endotracheal intubation than that of subjects experiencing hypoxemic respiratory failure of other origins. In COVID-19 disease, a NIOS-induced improvement in oxygenation was not able to anticipate treatment success; however, higher SAPS II score and serum lactate dehydrogenase were independently associated with treatment failure.
Methods
Study Design
This prospective, observational study was conducted at Fondazione Policlinico Universitario A. Gemelli IRCCS in Italy. Approval was granted by the local institutional review board, and informed consent was obtained according to committee recommendations.
All consecutive adult patients admitted to the ICU between March 12 and April 20 due to hypoxemic respiratory failure with confirmed microbiological diagnosis of COVID-19 (defined as positive real-time polymerase chain reaction for viral RNA performed on an upper or lower respiratory tract specimen) who received noninvasive oxygenation (ie, NIV or high-flow nasal cannula) as first-line treatment for hypoxemic respiratory failure were included in the analysis. Hypoxemic respiratory failure was defined as acute-onset symptoms and  < 300 mm Hg during oxygen therapy via an air-entrainment mask and bilateral pulmonary infiltrates on chest radiography or computed tomography scan. Severe COPD,29 chronic respiratory diseases requiring oxygen therapy prior to hospital admission, and do-not-intubate orders were the main exclusion criteria.
< 300 mm Hg during oxygen therapy via an air-entrainment mask and bilateral pulmonary infiltrates on chest radiography or computed tomography scan. Severe COPD,29 chronic respiratory diseases requiring oxygen therapy prior to hospital admission, and do-not-intubate orders were the main exclusion criteria.
Subjects received NIOS according to the choice of attending physicians: the devices available in our ICU were helmet NIV, face mask NIV, and high-flow nasal cannula. The interface for NIOS, the duration of treatments, as well as every other clinical decision were made by the clinician in charge.
The following demographic, clinical, and laboratory data were collected upon admission: age, gender, Simplified Acute Physiologic Score (SAPS) II, underlying diseases, D-dimer, blood urea nitrogen, total bilirubin, ferritin, C-reactive protein, lactate dehydrogenase (LDH), and lymphocyte count. Arterial blood gas analysis was performed upon arrival in the ICU, 1 h and 6 h after start of NIOS, and then on a 24-h basis up to ICU discharge. Duration of NIOS treatment, treatment failure (ie, the need for endotracheal intubation) within 28 d, and 28-d clinical outcome were prospectively collected.
Enrolled subjects with COVID-19 were compared to a control group of subjects with hypoxemic respiratory failure and bilateral infiltrates from other etiologies, who had received NIOS in a previous observational study conducted in the same clinical context with a similar approach.30 One-to-one propensity score matching between subjects with COVID-19 and the historical control subjects was performed. Three investigators (DLG, SMM and MA) selected a priori the variables for matching: 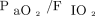 and
and 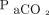 at enrollment, SAPS II score, and age. These represent widely acknowledged factors associated with the need for endotracheal intubation in subjects undergoing NIV for hypoxemic respiratory failure.18–20,30 Subjects with similar propensity scores in the 2 groups were matched (1:1 match without replacement), using a caliper of 0.15 standard deviation of the logit of the propensity score. For matching, subjects with COVID-19 who received high-flow nasal cannula alone were excluded. This choice was driven by the absence of subjects treated with high-flow nasal cannula in the historical cohort.
at enrollment, SAPS II score, and age. These represent widely acknowledged factors associated with the need for endotracheal intubation in subjects undergoing NIV for hypoxemic respiratory failure.18–20,30 Subjects with similar propensity scores in the 2 groups were matched (1:1 match without replacement), using a caliper of 0.15 standard deviation of the logit of the propensity score. For matching, subjects with COVID-19 who received high-flow nasal cannula alone were excluded. This choice was driven by the absence of subjects treated with high-flow nasal cannula in the historical cohort.
End Points
The primary aim of this study was to describe the rate of endotracheal intubation of hypoxemic patients receiving NIOS in the ICU due to COVID-19. Secondary aims were to identify early factors associated with the need for endotracheal intubation and to report any differences in the outcomes with the historical cohort.
Finally, we separated subjects with COVID-19 who were intubated after NIOS into 2 groups according to the median time from treatment start and failure, and compared the 28-d clinical outcomes in these 2 cohorts. In both the COVID-19 cohort and the historical cohort, the decision to intubate and any other clinical decisions were made by the clinician in charge, independent of study procedures.
Statistical Analysis
At the time of the study design, systematic data on NIOS in subjects with COVID-19 were lacking. Hence, to provide a timely report, we decided to evaluate for enrollment patients who were treated in our ICU up to April 20, 2020.
Data were reported as median (interquartile range [IQR]) or proportions, as appropriate. Student t test, Wilcoxon rank-sum test, Kruskal-Wallis test, chi-square test, and Fisher test were used for univariate comparisons, as appropriate. Additional analyses were performed to separate survival analyses regarding the time to treatment failure and time to death up to day 28 using the log-rank test; Kaplan-Meier curves are displayed for most significant results. Cox regression was used to investigate the relationship between potential covariates and outcomes (ie, NIOS failure, 28-d mortality). All variables with P ≤ .05 in the univariate analysis were included in the multivariate analyses. For all tests, a 2-sided P ≤ .05 was considered significant. Statistical analysis was performed with SPSS 26.0 (IBM, Armonk, New York) and MATLAB (MathWorks, Natick, Massachusetts). Manuscript figures were prepared with GraphPad Prism (La Jolla, California).
Results
Subject Characteristics
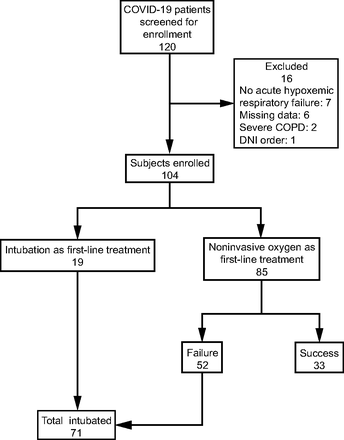
Up to April 20, 2020, 120 patients with COVID-19 were admitted to the dedicated ICU of our institution. Eighty-five subjects received first-line treatment with NIOS (Fig. 1) due to acute hypoxemic respiratory failure. Clinical characteristics of the subjects are displayed in Table 1. The most-used interface was the helmet NIV, followed by high-flow nasal cannula and face mask NIV (Table 1). In subjects treated with NIV, median PEEP was 10 cm H2O (IQR 7–12), and pressure support was 12 cm H2O (IQR 10–14). During high-flow nasal cannula, flows of 50–60 L/min were initially applied in all treated subjects. NIOS was used for a median time of 66 h (IQR 21–109); the group that avoided endotracheal intubation received NIOS for longer time (median time, 91 h (IQR 71–153) (Fig. 2, Table 1).
Flow chart. DNI = do not resuscitate order.
Subject management after enrollment. A: Subjects with noninvasive oxygenation success, and B: subjects who failed noninvasive oxygenation (ie, underwent endotracheal intubation). Each bar represents a single subject's treatment received over time. NIV = noninvasive ventilation; HFNC = high-flow nasal cannula.
Characteristics and Clinical Outcomes of Subjects Receiving NIOS
Treatment Failure
Fifty-two (61%) subjects required endotracheal intubation. All subjects were intubated due to the lack of improvement in oxygenation and dyspnea, while no subject developed hypercapnia during the NIOS. Upon arrival,  was slightly lower in subjects who subsequently needed intubation (Table 1). After 1 h of NIOS, however,
was slightly lower in subjects who subsequently needed intubation (Table 1). After 1 h of NIOS, however, 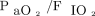 increased both in subjects who were afterwards intubated and in those who were not (Fig. 3).
increased both in subjects who were afterwards intubated and in those who were not (Fig. 3).  was not different between cohorts and did not change after 1 h of treatment.
was not different between cohorts and did not change after 1 h of treatment.
Subjects treated with noninvasive oxygenation strategies had improved  regardless of the subsequent need for intubation. The change in
regardless of the subsequent need for intubation. The change in 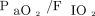 induced by noninvasive oxygenation strategies was not significantly different between subjects who avoided (A) or who needed (B) endotracheal intubation within 28 d of treatment start.
induced by noninvasive oxygenation strategies was not significantly different between subjects who avoided (A) or who needed (B) endotracheal intubation within 28 d of treatment start.
In the univariate analysis, subjects who required endotracheal intubation were older (unadjusted odds ratio for age 1.04 [95% CI 1.01–1.08]), had higher SAPS II score (odds ratio 1.10 [95% CI 1.04–1.16]), were more often affected by hypertension (odds ratio 3.3 [95% CI 1.3–8.4]), had higher blood urea nitrogen (odds ratio 1.073 [95% CI 1.024–1.123]), and serum LDH (odds ratio 1.005 [95% CI 1.001–1.009]).
In the Cox regression analysis, the independent factors associated with NIOS failure were SAPS II score (adjusted hazard ratio per unit increase 1.039 [95% CI 1.018–1.061], P < .001) and serum LDH at arrival (adjusted hazard ratio per unit increase 1.002 [95% CI 1.000–1.004], P = .01).
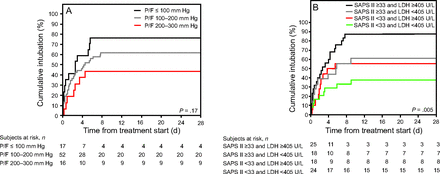
We dichotomized subjects according to the median SAPS II score and LDH levels (median values of 33 and 405 units/L, respectively). The rate of endotracheal intubation was higher in subjects with SAPS II scores ≥ 33 and LDH ≥ 405 units/L (88% [95% CI 74–99]; sensitivity and specificity for endotracheal intubation were 43% and 91%, respectively, positive predictive value 88%, and negative predictive value 50%) and lower in subjects with both SAPS II score < 33 and LDH < 405 units/L (32% [95% CI 11–53], sensitivity and specificity for avoidance of endotracheal intubation 45% and 85%, respectively, positive predictive value 68%, and negative predictive value 71%) (Fig. 4).
Kaplan-Meier curves of the cumulative incidence of intubation according to severity of hypoxemia at treatment start (A) and according to SAPS II score and serum lactate dehydrogenase (LDH) at enrollment (B), which were the factors independently associated with treatment failure. P/F =  , SAPS II = Simplified Acute Physiology Score II.
, SAPS II = Simplified Acute Physiology Score II.
We stratified subjects according to the median duration of treatment before endotracheal intubation and categorized subjects as “early failure” versus “late failure” (ie, ≤ 42 and > 42 h, respectively). These groups did not show significant differences in baseline characteristics, and had similar 28-d mortality (42% vs 38%, P > .99) (see the supplementary materials at http://www.rcjournal.com). The nonsignificant difference in mortality between these 2 cohorts was confirmed after adjustment for age, SAPS II score, and 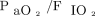 at enrollment, with a hazard ratio for the late/early failure group of 1.3 (95% CI 0.5–3.3) (P = .54).
at enrollment, with a hazard ratio for the late/early failure group of 1.3 (95% CI 0.5–3.3) (P = .54).
Hypoxemic Respiratory Failure and COVID-19
Fifty-four subjects with COVID-19 were matched with 54 subjects with hypoxemic respiratory failure from other causes. Demographics of these matched cohorts are detailed in Table 2. Subjects with COVID-19 were mainly treated with helmet NIV, whereas subjects with hypoxemic respiratory failure from other causes mainly underwent face mask NIV with lower PEEP and higher pressure support. The intubation rate was higher in subjects with COVID-19 (59% vs 35%, P = .02). This difference remained significant after adjustment for age, SAPS II score, and 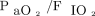 at study inclusion, with a hazard ratio for subjects with COVID-19 of 2.0 (95% CI 1.1–3.6) (Fig. 5).
at study inclusion, with a hazard ratio for subjects with COVID-19 of 2.0 (95% CI 1.1–3.6) (Fig. 5).
After propensity score matching with a historical cohort, subjects with COVID-19 who were treated with noninvasive oxygenation strategies had higher incidence of intubation than subjects affected by acute hypoxemic respiratory failure due to other causes. This difference remained significant after adjustment for age, SAPS II, and 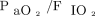 at study inclusion, with a hazard ratio of 2.0 (95% CI 1.1–3.6) for subjects with COVID-19. SAPS II = Simplified Acute Physiology Score II.
at study inclusion, with a hazard ratio of 2.0 (95% CI 1.1–3.6) for subjects with COVID-19. SAPS II = Simplified Acute Physiology Score II.
Characteristics and Clinical Outcomes of Subjects Receiving NIOS With or Without COVID-19
Discussion
The results of this study on the use of NIOS to treat subjects with COVID-19 can be summarized as follows. First, the use of NIOS as a first-line treatment of COVID-19 disease was common in our ICU; 61% of the subjects receiving NIOS required invasive mechanical ventilation within 28 d from treatment start. Second, NIOS-induced changes in 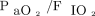 after 1 h of treatment did not predict treatment success. Third, SAPS II score and serum LDH were the factors capable of anticipating the need of endotracheal intubation. Finally, compared with a propensity-matched historical cohort of subjects with acute hypoxemic respiratory failure from other causes, subjects with COVID-19 showed higher risk of NIOS failure.
after 1 h of treatment did not predict treatment success. Third, SAPS II score and serum LDH were the factors capable of anticipating the need of endotracheal intubation. Finally, compared with a propensity-matched historical cohort of subjects with acute hypoxemic respiratory failure from other causes, subjects with COVID-19 showed higher risk of NIOS failure.
NIOS is commonly used for the treatment of acute hypoxemic respiratory failure, but its safety and efficacy are debated.6 The COVID-19 pandemic is imposing stress on critical care resources, and the use of NIOS is common in the most heterogeneous clinical settings.3 However, whether and to what extent patients with COVID-19 may benefit from NIOS is unknown. In some preliminary reports, high-flow nasal cannula has been used to treat patients with COVID-19; while it seemed effective in subjects with mild hypoxemia (eg,  > 200 mm Hg), it had a high failure rate in subjects with more severe hypoxemia.31,32 To our knowledge, this is the first study specifically designed to assess the rate and predictors of NIOS failure in subjects with COVID-19 treated in the ICU setting, and to determine whether any difference exists with acute hypoxemic respiratory failure from other causes.
> 200 mm Hg), it had a high failure rate in subjects with more severe hypoxemia.31,32 To our knowledge, this is the first study specifically designed to assess the rate and predictors of NIOS failure in subjects with COVID-19 treated in the ICU setting, and to determine whether any difference exists with acute hypoxemic respiratory failure from other causes.
NIOS Failure Rate
We report a high rate of NIOS failure (61%); in acute hypoxemic respiratory failure from other causes, NIOS failure might detrimentally affect clinical outcomes by delaying intubation and allowing self-inflicted lung injury during treatment.13,33,34 While this has not been yet demonstrated in patients with COVID-19, the high failure rate observed in our cohort prompts the detection of easy-to-use predictors that may anticipate the need for endotracheal intubation in COVID-19 disease. In hypoxemic respiratory failure from other causes, the lack of improvement in 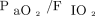 after 1 h of NIOS is associated with NIOS failure.20,30,35 Conversely, subjects with COVID-19 had improved
after 1 h of NIOS is associated with NIOS failure.20,30,35 Conversely, subjects with COVID-19 had improved  values regardless of the subsequent need for intubation. This may represent a disease-specific feature and is consistent with previous reports showing a remarkable PEEP-induced improvement in oxygenation during the early stages of COVID-19 respiratory failure.36,37 In our results, 97% of subjects receiving invasive mechanical ventilation had improved oxygenation with higher PEEP, regardless of recruitability.38 These results warrant careful monitoring of subjects with COVID-19 who are treated with NIOS, given the inability to predict the outcome from the initial oxygenation response.
values regardless of the subsequent need for intubation. This may represent a disease-specific feature and is consistent with previous reports showing a remarkable PEEP-induced improvement in oxygenation during the early stages of COVID-19 respiratory failure.36,37 In our results, 97% of subjects receiving invasive mechanical ventilation had improved oxygenation with higher PEEP, regardless of recruitability.38 These results warrant careful monitoring of subjects with COVID-19 who are treated with NIOS, given the inability to predict the outcome from the initial oxygenation response.
SAPS II score and serum LDH were the independent factors associated to NIOS failure in subjects with COVID-19. These parameters can be assessed easily in most clinical scenarios. The SAPS II score incorporates age, the degree of oxygenation impairment, organ dysfunction, and the most relevant comorbidities; this result seems to align with data from subjects with acute hypoxemic respiratory failure of other etiologies, in whom a SAPS II score > 34 represents an independent risk factor for treatment failure.20,30 Serum LDH is a cytoplasmic enzyme harbored in all organ systems. Its appearance in serum reflects cell damage. Although highly sensitive, serum LDH is poorly specific of lung injury.39 However, in the early stage of the disease, patients with COVID-19 usually develop isolated lung injury, which could increase the specificity of this enzyme in stratifying the severity of lung injury in the early stage of COVID-19 disease.
In our cohort, subjects with high SAPS II scores and high serum LDH values showed a likelihood of intubation close to 90%, whereas subjects with low LDH and low SAPS II scores were successfully treated with NIOS in > 70% of cases. To avoid delaying intubation, first-line NIOS treatment should be reserved solely for patients with a high likelihood of treatment success; from the perspective of our results, it appears wise to suggest that first-line treatment with NIOS should be avoided in patients with high SAPS II scores and serum LDH values.
Clinical Outcome
Subjects treated with NIOS had a 28% mortality at 28 d, which is consistent with the available data about COVID-19 mortality in ICU.4 Mortality of subjects who needed endotracheal intubation was 42%, while it was 6% in the group for whom NIOS was successful.
After stratifying subjects who required endotracheal intubation according to duration of NIOS treatment, we noted similar mortality in subjects who were intubated earlier or later, ie, the 2 groups were similar for comorbidities and hypoxemia severity. Subjects enrolled in our study were treated with different NIOS methods, but mostly with high-PEEP helmet NIV. High PEEP during spontaneous breathing reduces inspiratory effort and enhances lung homogeneity, limiting the risk of self-inflicted lung injury.40–42 The choice of the helmet interface may have contributed to limit the risk of lung injury progression during the treatment, possibly mitigating any detrimental effect of delayed intubation on clinical outcome. However, due to the relatively small sample size, it appears wise not to draw conclusions on the safety of prolonged NIOS treatments that, to our knowledge, should be avoided.43
Impact of COVID-19
After 1:1 matching on disease severity and age, we compared the NIOS failure rate for subjects with COVID-19 and acute hypoxemic respiratory failure with that for subjects with acute hypoxemic respiratory failure from other causes. The intubation rate was significantly higher in subjects with COVID-19 (59% vs 35%). Intubation is a clinical decision; while in the control cohort predefined criteria of intubation were available,30 this was not the case for subjects with COVID-19 and might constitute a limitation of the comparison. However, while this was not defined by an institutional protocol, in our institution a NIOS trial of ≥ 1 h was allowed; if a subject did not improve significantly according to the treating physician (eg, persistent dyspnea, tachypnea, activation of accessory respiratory muscles, inability to protect the airways, copious tracheal secretions, hemodynamic instability), they were promptly intubated to avoid self-inflicted lung injury.
It is possible that nonventilatory features (eg, the microvascular involvement of the disease and the unavailability of etiologic treatments) play an important role in determining NIOS treatment outcome in COVID-19 disease. This comparison strengthens the general message of this study, which addresses caution in the use of NIOS in COVID-19 disease.
In our study, subjects with COVID-19 were treated mostly with helmet NIV, higher levels of PEEP, and lower pressure support, whereas the control subjects were treated mostly with face mask NIV. We intentionally excluded subjects with COVID-19 who received high-flow nasal cannula alone from the comparison because this treatment was not available when the historical cohort was studied. The difference in the interfaces and settings used reflects the evolution of NIOS respiratory support in acute hypoxemic respiratory failure13; this does not alter, and could even strengthen, the significance of this investigation, as helmets may reduce the need for endotracheal intubation compared to face mask NIV, and high PEEP during spontaneous breathing exerts lung-protective effects.6,40,44 Hence, patients with COVID-19 should have been protected from treatment failure by high PEEP and lower pressure support, but this was not the case.
Limitations
This study has several limitations. First, our single-center report involved subjects treated in the ICU, and our results may not be generalizable to clinical contexts beyond the critical care setting (ie, emergency department, medical units). Second, we did not use standardized criteria for intubation; however, these results reflect the effect of NIOS use in a real-life scenario in a center with expertise in NIOS. Third, at the time of the study no evidence of the potentially beneficial effects of steroids on acute hypoxemic respiratory failure due to COVID-19 were available, and our subjects were treated without dexamethasone. While this might have affected the overall mortality and intubation rates, this does not alter in a clinically important manner the overall message of the study. Finally, while the propensity-matched comparison with the historical cohort of subjects affected by respiratory failure of other causes has several strengths, we cannot exclude that other uncontrolled factors affected treatment outcome.
Conclusions
Hypoxemic subjects with COVID-19 who received NIOS in the ICU were burdened by high risk of needing endotracheal intubation. This risk appeared to be 2-fold greater than that of subjects experiencing acute hypoxemic respiratory failure of other origins. Differently from what was previously reported for other causes of acute hypoxemic respiratory failure, NIOS-induced improvement in oxygenation did not predict treatment success in COVID-19 disease, whereas higher SAPS II scores and serum LDH values were independently associated with treatment failure. To avoid any delay in endotracheal intubation, treatment of hypoxemic respiratory failure due to COVID-19 with NIOS requires extreme caution and careful clinical monitoring, especially in patients with severe hypoxemia (SAPS II score ≥ 33) with serum LDH ≥ 405 units/L.
Footnotes
- Correspondence: Domenico Luca Grieco MD, Department of Anesthesiology and Intensive Care Medicine, Catholic University of The Sacred Heart, Fondazione Policlinico Universitario A. Gemelli IRCCS, L. go F. Vito, 00168 Rome, Italy. E-mail: dlgrieco{at}outlook.it
Dr Grieco is supported in part by research grants by ESICM and SIAARTI, and has disclosed relationships with Maquet, Getinge, Air Liquide, and GE Healthcare. Dr Maggiore is the principal investigator of the RINO trial (Clinicaltrials.gov, NCT02107183), which was supported by Fisher & Paykel Healthcare. Dr Antonelli has disclosed relationships with Maquet, Toray, and GE Healthcare. All other authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com
See the Related Editorial on Page 878
- Copyright © 2021 by Daedalus Enterprises