Abstract
Providing supplemental oxygen to hospitalized adults is a frequent practice and can be administered via a variety of devices. Oxygen therapy has evolved over the years, and clinicians should follow evidence-based practices to provide maximum benefit and avoid harm. This systematic review and subsequent clinical practice guidelines were developed to answer questions about oxygenation targets, monitoring, early initiation of high-flow oxygen (HFO), benefits of HFO compared to conventional oxygen therapy, and humidification of supplemental oxygen. Using a modification of the RAND/UCLA Appropriateness Method, 7 recommendations were developed to guide the delivery of supplemental oxygen to hospitalized adults: (1) aim for  range of 94–98% for most hospitalized patients (88–92% for those with COPD), (2) the same
range of 94–98% for most hospitalized patients (88–92% for those with COPD), (2) the same  range of 94–98% for critically ill patients, (3) promote early initiation of HFO, (4) consider HFO to avoid escalation to noninvasive ventilation, (5) consider HFO immediately postextubation to avoid re-intubation, (6) either HFO or conventional oxygen therapy may be used with patients who are immunocompromised, and (7) consider humidification for supplemental oxygen when flows > 4 L/min are used.
range of 94–98% for critically ill patients, (3) promote early initiation of HFO, (4) consider HFO to avoid escalation to noninvasive ventilation, (5) consider HFO immediately postextubation to avoid re-intubation, (6) either HFO or conventional oxygen therapy may be used with patients who are immunocompromised, and (7) consider humidification for supplemental oxygen when flows > 4 L/min are used.
Introduction
Adult patients admitted to critical care services for acute respiratory failure often require supplemental oxygen.1 Generally thought to be harmless, there is an increasing interest in the potentially harmful effects of oxygen delivery and excessive  1,2 Various oxygen delivery devices are used, and selection is often based on comfort and the level of supplemental oxygen needed. When escalation in care is required, high-flow nasal cannula (HFNC) can be used to potentially avoid noninvasive or invasive mechanical ventilation.3-8
1,2 Various oxygen delivery devices are used, and selection is often based on comfort and the level of supplemental oxygen needed. When escalation in care is required, high-flow nasal cannula (HFNC) can be used to potentially avoid noninvasive or invasive mechanical ventilation.3-8
Available evidence shows varying outcomes for supplemental oxygen therapy in adult acute and intensive care. In a systematic review of 25 randomized control trials (RCTs), Chu et al1 reported increased mortality with liberal use of oxygen in ICU subjects. Their review suggested unfavorable outcomes as a result of 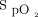 > 94–96%.
> 94–96%.
Our literature review focused on monitoring and methods of oxygen delivery. The clinical practice guidelines were developed from our review to address 6 questions regarding oxygen therapy in postoperative and critical care:
In adult patients requiring supplemental oxygen, does a specific oxygenation target improve hospital length of stay (LOS), ICU LOS, mortality, and cognitive function?
In critically ill adult patients requiring supplemental oxygen, does a specific oxygenation target improve hospital LOS, ICU LOS, mortality, and cognitive function?
In adult patients receiving postoperative supplemental oxygen, does continuous monitoring prevent adverse events compared to intermittent or no monitoring?
In adult patients requiring supplemental oxygen, does early initiation of HFNC decrease hospital LOS, decrease ICU LOS, decrease escalation of care to invasive or noninvasive ventilation (NIV), and improve morbidity versus late initiation of high-flow oxygen?
In adult patients requiring supplemental oxygen, does HFNC decrease hospital LOS, decrease ICU LOS, decrease escalation of care to invasive ventilation or NIV, and decrease morbidity versus standard oxygen delivery?
In adult patients requiring supplemental oxygen, does heated or nonheated humidification of oxygen improve patient outcomes, improve patient comfort, and reduce adverse events versus no humidification?
Committee Composition
A committee was selected by the American Association for Respiratory Care (AARC) leadership based on their known experience related to the topic, their interest in participating in the project, and their commitment to the process details. The committee first met face to face, where they were introduced to the process of developing clinical practice guidelines. At that time, the committee selected a chair and wrote a first draft of patient, intervention, comparison, and outcome (PICO) questions. Subsequent meetings occurred as needed by conference call. Frequent e-mail communication occurred among committee members and AARC staff. The committee members received no remuneration for their participation in the process, though the AARC covered their face-to-face meeting expenses.
Search Strategy
A literature search was conducted using the PubMed, CINAHL via EBSCOhost, and Scopus.com databases for studies on oxygen therapy care in hospitalized adult patients. The search strategies used a combination of relevant controlled vocabulary (ie, Medical Subject Headings and CINAHL Headings) and key word variations related to oxygen therapy, oxygenation techniques, and outcomes. The searches were limited to English language studies about human populations. The searches were also designed to filter out citations indexed as commentaries, editorials, interviews, news, or reviews. No date restrictions were applied to the searches. Refer to the online supplemental material for available at http://rc.rcjournal.com the complete search strategy executed in each database on January 21, 2021. Duplicate citations were identified and removed using the EndNote X8 (Clarivate, Philadelphia, Pennsylvania) citation management software.
Study Selection
At least two reviewers assessed the eligibility in the Covidence (Melbourne, Australia) systematic review software. If there was disagreement regarding eligibility, a third reviewer would be used to resolve the dispute. Inclusion criteria used to assess eligibility were (1) oxygen therapy, (2) adult population, and (3) clinical outcomes. The exclusion criteria used were (1) not oxygen therapy, (2) pediatric population, (3) wrong route of oxygen administration, (4) no clinical outcomes relevant to oxygen therapy, (5) wrong setting, (6) not empirical research (eg, theory, opinion, or review articles), and (7) published prior to 1987.
Development of Recommendations
It is recognized that a process is necessary to combine the best available evidence with committee members’ collective experience. To achieve this, a modification of the RAND/UCLA Appropriateness Method9 was used. The literature was collapsed into evidence tables according to PICO question (Table 1). Individual panel members were assigned the task of writing a systematic review of the topic, drafting one or more recommendations, and suggesting the level of evidence supporting the recommendation:
Convincing scientific evidence based on randomized controlled trials of sufficient rigor;
Weaker scientific evidence based on lower levels of evidence such as cohort studies, retrospective studies, case-control studies, and cross-sectional studies;
Based on the collective experience of the committee.
Summary of Evidence for each PICO Question Included in the Systematic Review
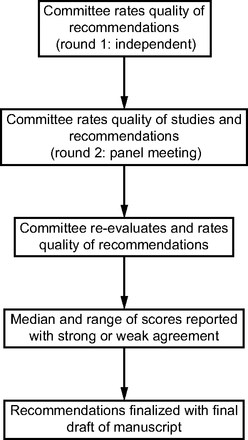
Committee members reviewed the first draft of evidence tables, systematic reviews, recommendations, and evidence levels. Each committee member rated each recommendation using a Likert scale of 1 to 9, with 1 meaning expected harms greatly outweigh the expected benefits and 9 meaning expected benefits greatly outweigh the expected harms. The ratings were returned to the committee chair. The first ratings were done with no interaction among committee members. A conference call was convened, during which time the individual committee ratings were discussed. Particular attention was given to any outlier scores and the justification. Recommendations and evidence levels were revised with input from the committee members. After discussing each PICO question, committee members re-rated each recommendation. The final median and range of committee members’ scores are reported (Table 2). Strong agreement required that all committee members rank the recommendation 7 or higher; weak agreement meant that one or more committee members ranked the recommendation below 7, but the median vote was at least 7. For recommendations with weak agreement, the percentage of committee members who rated 7 or above was calculated and reported after each weak recommendation. Figure 1 illustrates the process flow the panel used to rate the appropriateness and quality of the literature selected through the search process.
Summary of Recommendations for Each PICO Question
Process used by the committee to appraise the literature.
Drafts of the report were distributed among committee members in several iterations. When all committee members were satisfied, the document was submitted for publication. The report was subjected to peer review before final publication.
Assessment and Recommendations
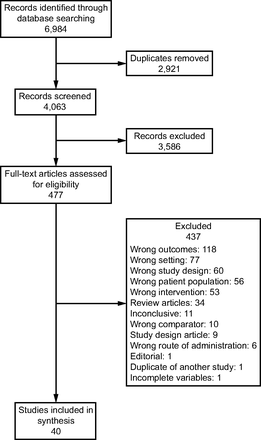
The search strategies retrieved a total of 6,984 articles. After the removal of duplicates, 4,063 articles remained for screening, of which 3,586 were excluded at the title and abstract level. Of the remaining 477 articles, 437 were excluded following full-text review against the inclusion and exclusion criteria, leaving 40 articles included for synthesis (Fig. 2).
Flow chart.
Specific Oxygenation Targets in Acutely Ill Adults
There is increased attention given to determine safe levels of oxygen therapy in ICUs, emergency departments (ED), and specific diseases. Several studies use 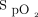 , versus
, versus 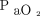 , to assess oxygenation as
, to assess oxygenation as  is readily available, noninvasive, cost-effective, and easily measured.10,11
is readily available, noninvasive, cost-effective, and easily measured.10,11
A large single-center RCT randomized 434 subjects to a conservative oxygen protocol versus a conventional control group.11 The conservative group was assigned to receive oxygen therapy to maintain 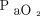 between 70–100 mm Hg or
between 70–100 mm Hg or 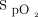 between 94–98%. The conventional group allowed
between 94–98%. The conventional group allowed  up to 150 mm Hg or PO2 between 97–100%. The median
up to 150 mm Hg or PO2 between 97–100%. The median 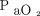 values during the ICU LOS were significantly higher (P < .001) in the conventional group (median
values during the ICU LOS were significantly higher (P < .001) in the conventional group (median  102 mm Hg [interquartile range [IQR] 88–116]) vs the conservative group (median
102 mm Hg [interquartile range [IQR] 88–116]) vs the conservative group (median 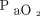 87 mm Hg [IQR 79–97]). Mortality rates were lower in the conservative group. The study also reported fewer episodes of shock, liver failure, and bacteremia in the conservative group.
87 mm Hg [IQR 79–97]). Mortality rates were lower in the conservative group. The study also reported fewer episodes of shock, liver failure, and bacteremia in the conservative group.
A 2009 retrospective observational study by van den Boom and colleagues10 analyzed and compared the 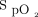 of 26,723 records of ICU patients from the eICU Collaborative Research Database and 8,546 records of patients from the Medical Information Mart for Intensive Care III database to hospital mortality rate. The results demonstrated that the optimal range of
of 26,723 records of ICU patients from the eICU Collaborative Research Database and 8,546 records of patients from the Medical Information Mart for Intensive Care III database to hospital mortality rate. The results demonstrated that the optimal range of 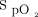 associated with decreasing mortality was 94–98%. Conversely, it was also noted that an
associated with decreasing mortality was 94–98%. Conversely, it was also noted that an 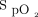 < 94% was associated with increased mortality. The authors’ results are a reminder of the importance of oxygen therapy in preventing hypoxemia and limiting its usage to prevent hyperoxia.
< 94% was associated with increased mortality. The authors’ results are a reminder of the importance of oxygen therapy in preventing hypoxemia and limiting its usage to prevent hyperoxia.
Raksakietisak et al12 studied 2 oxygen therapy devices to prevent hypoxemia. Hypoxemia was defined as an  < 94% and the threshold in which to initiate oxygen therapy. This was an RCT comparing nasal cannula to a simple face mask in 500 low-risk post-anesthesia subjects in the post-anesthesia care unit. The first group received 4 L/min of oxygen via nasal cannula, whereas the second group received 5 L/min through an oxygen mask. Both methods resulted in a comparable
< 94% and the threshold in which to initiate oxygen therapy. This was an RCT comparing nasal cannula to a simple face mask in 500 low-risk post-anesthesia subjects in the post-anesthesia care unit. The first group received 4 L/min of oxygen via nasal cannula, whereas the second group received 5 L/min through an oxygen mask. Both methods resulted in a comparable 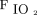 (0.35). There was no significant difference in
(0.35). There was no significant difference in 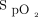 between the 2 devices. This study concluded that both nasal cannula and a simple face masks can prevent hypoxemia.
between the 2 devices. This study concluded that both nasal cannula and a simple face masks can prevent hypoxemia.
Other studies focused on oxygen therapy for specific diseases. High 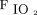 delivered to patients with COPD with hypercapnia in respiratory failure can lead to worsening gas exchange, increased morbidity, and mortality.13,14 Studies in this population have recommended administering 2 L/min of oxygen or 0.28
delivered to patients with COPD with hypercapnia in respiratory failure can lead to worsening gas exchange, increased morbidity, and mortality.13,14 Studies in this population have recommended administering 2 L/min of oxygen or 0.28 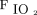 to minimize these effects.13-15 Joosten et al13 performed a retrospective review of subjects admitted to the ED with a COPD exacerbation. Subjects with
to minimize these effects.13-15 Joosten et al13 performed a retrospective review of subjects admitted to the ED with a COPD exacerbation. Subjects with  > 45 mm Hg were considered CO2 retainers. Results demonstrated an increase in LOS, use of NIV, and a higher admission rate to an ICU for persons with COPD who were CO2 retainers and received supplemental O2 > 4 L/min. This study emphasizes the importance of managing the amount of oxygen for patients with COPD. Two other studies focused on in-hospital mortality with subjects receiving supplemental oxygen and admitted with COPD exacerbation. Cameron et al16 found an increase in adverse outcomes in this population with
> 45 mm Hg were considered CO2 retainers. Results demonstrated an increase in LOS, use of NIV, and a higher admission rate to an ICU for persons with COPD who were CO2 retainers and received supplemental O2 > 4 L/min. This study emphasizes the importance of managing the amount of oxygen for patients with COPD. Two other studies focused on in-hospital mortality with subjects receiving supplemental oxygen and admitted with COPD exacerbation. Cameron et al16 found an increase in adverse outcomes in this population with 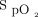 < 88% or > 96%. Echevarria and colleagues17 also studied in-hospital mortality with subjects receiving supplemental oxygen and admitted with COPD exacerbation, hypercapnia, and normocapnia. In-hospital mortality was lowest in both groups when targeting oxygen saturation of 88–92%.
< 88% or > 96%. Echevarria and colleagues17 also studied in-hospital mortality with subjects receiving supplemental oxygen and admitted with COPD exacerbation, hypercapnia, and normocapnia. In-hospital mortality was lowest in both groups when targeting oxygen saturation of 88–92%.
Hoffman et al18 conducted an RCT to compare the routine use of oxygen in subjects with an acute myocardial infarction without hypoxemia. A sample of 6,629 subjects > 30-y old and with an SpO2 > 90% received either supplemental oxygen or ambient air. The authors found no impact on mortality with the routine administration of oxygen at 1-y in subjects with suspected myocardial infarction with no sign of hypoxemia.
Smit and colleagues19 evaluated the use of oxygen therapy following coronary artery bypass graft (CABG) surgery in an RCT of 50 subjects to either a moderate hyperoxia state or a near-physiologic oxygen state. The results demonstrated no decrease in myocardial damage to the CABG with a near-physiological oxygen strategy. There were no increases in lactate levels or hypoxic events.
The available literature and experiences of the committee support using 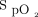 to monitor oxygenation to prevent hypoxemia and hyperoxia to decrease mortality. The literature also suggests that there is no benefit to hyperoxia and rather supports the importance of maintaining normoxia among patients with myocardial infarction and CABG. Therefore, the committee supports an optimal
to monitor oxygenation to prevent hypoxemia and hyperoxia to decrease mortality. The literature also suggests that there is no benefit to hyperoxia and rather supports the importance of maintaining normoxia among patients with myocardial infarction and CABG. Therefore, the committee supports an optimal 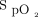 range of 94–98% for most patients requiring supplemental oxygen and a range of 88–92% for patients with COPD who require supplemental oxygen. Finally, it is important to manage the oxygen given to an exacerbation of COPD that is a CO2 retainer (Table 3) (Evidence level C; all committee members responded 7).
range of 94–98% for most patients requiring supplemental oxygen and a range of 88–92% for patients with COPD who require supplemental oxygen. Finally, it is important to manage the oxygen given to an exacerbation of COPD that is a CO2 retainer (Table 3) (Evidence level C; all committee members responded 7).
Recommended  Range by Population
Range by Population
Specific Oxygenation Targets in Critically Ill Adults
The harmful effects of hyperoxemia have been debated for decades.20,21 Protocols designed to limit hyperoxemia have become more common in intensive care.22 Although the contributive effects of conservative oxygenation goals are not fully understood, several studies aimed to identify positive outcomes.1,11,22,23 It has been hypothesized that maintaining 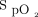 within specific parameters negatively impacts patients with ventilatory failure.24 Whereas several studies have used oxygen targets to titrate
within specific parameters negatively impacts patients with ventilatory failure.24 Whereas several studies have used oxygen targets to titrate 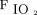 in mechanically ventilated patients, there is a dearth of data available in patients requiring supplemental oxygen.
in mechanically ventilated patients, there is a dearth of data available in patients requiring supplemental oxygen.
In a crossover RCT, Pilcher and colleagues24 reported that high concentrations of oxygen positively correlated with increased 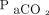 in morbidly obese subjects. Subjects with a body mass index > 40 kg/m2 were placed on 8 L/min face masks (n = 12) and compared with those placed on low-flow oxygen (n = 12). Subjects exposed to higher oxygen concentration were more likely to have higher transpulmonary
in morbidly obese subjects. Subjects with a body mass index > 40 kg/m2 were placed on 8 L/min face masks (n = 12) and compared with those placed on low-flow oxygen (n = 12). Subjects exposed to higher oxygen concentration were more likely to have higher transpulmonary 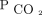 (
(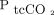 ) than those on low-flow oxygen (outcome difference 3.2 [1.3–5.2], Ρ = .002). The authors’ main finding was that high-concentration oxygen therapy increased
) than those on low-flow oxygen (outcome difference 3.2 [1.3–5.2], Ρ = .002). The authors’ main finding was that high-concentration oxygen therapy increased 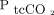 in morbidly obese in-patients to a significantly greater degree than titrating oxygen to achieve a target
in morbidly obese in-patients to a significantly greater degree than titrating oxygen to achieve a target  of 88–92%.
of 88–92%.
Sepehrvand et al20 studied oxygen titration to maintain either high (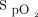 ≥ 96%) or low (
≥ 96%) or low (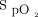 90–92%) oxygen saturation range in subjects admitted for acute heart failure. Hospital LOS was significantly higher in the low
90–92%) oxygen saturation range in subjects admitted for acute heart failure. Hospital LOS was significantly higher in the low 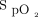 group (9.5 d vs 4.7 d, P = .01). However, when the population was adjusted for age, sex, residence times, and prior medical history (including cardiac devices), no difference was found (P = .07). Overall, no differences were found between groups when oxygen was adjusted to meet targets in the first 72 h after admission. In a retrospective analysis of the association between admission
group (9.5 d vs 4.7 d, P = .01). However, when the population was adjusted for age, sex, residence times, and prior medical history (including cardiac devices), no difference was found (P = .07). Overall, no differences were found between groups when oxygen was adjusted to meet targets in the first 72 h after admission. In a retrospective analysis of the association between admission 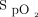 and all-cause in-hospital mortality, Yu and colleagues25 found that the optimal range for
and all-cause in-hospital mortality, Yu and colleagues25 found that the optimal range for 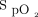 for subjects with acute myocardial infarction was 94–96%.
for subjects with acute myocardial infarction was 94–96%.
Guidance from the ARDSNet studies includes a recommended safe oxygenation range of 88–95% (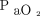 55–80 mm Hg) for patients with severe acute hypoxemia. In a 2021 study, Schjørring et al26 found no difference in mortality when targeting
55–80 mm Hg) for patients with severe acute hypoxemia. In a 2021 study, Schjørring et al26 found no difference in mortality when targeting  60 mm Hg versus 90 mm Hg (
60 mm Hg versus 90 mm Hg ( 90–97%). Although the authors did not address
90–97%). Although the authors did not address  < 60 mm Hg or > 90 mm Hg, it is reasonable to consider an oxygenation range of 90–97% as safe.
< 60 mm Hg or > 90 mm Hg, it is reasonable to consider an oxygenation range of 90–97% as safe.
Despite no difference found in mortality for patients with low or high 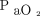 , patients with severe ARDS may require high levels of
, patients with severe ARDS may require high levels of 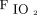 to maintain acceptable oxygenation. In preclinical animal data, high levels of
to maintain acceptable oxygenation. In preclinical animal data, high levels of  delivered for prolonged periods of time have consistently shown to contribute to oxidative injury.27 Furthermore, studies in both adults and pediatric patients receiving invasive mechanical ventilation have shown a reduction in functional residual capacity that occurs due to de-nitrogenation atelectasis.28 However, these effects may be mitigated if a high PEEP strategy can be utilized safely.28 Many randomized trials of mechanical ventilation for ARDS have utilized an oxygenation target saturation of 88–93% to facilitate the use of the lowest possible
delivered for prolonged periods of time have consistently shown to contribute to oxidative injury.27 Furthermore, studies in both adults and pediatric patients receiving invasive mechanical ventilation have shown a reduction in functional residual capacity that occurs due to de-nitrogenation atelectasis.28 However, these effects may be mitigated if a high PEEP strategy can be utilized safely.28 Many randomized trials of mechanical ventilation for ARDS have utilized an oxygenation target saturation of 88–93% to facilitate the use of the lowest possible 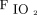 .29-31
.29-31
The available evidence is weak to suggest that titration of oxygen saturation improves outcomes such as mortality and hospital LOS in critically ill adults. It is unknown whether the scant amount of available literature can be generalized to wider populations. Despite these findings, the committee recommends an 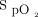 range of 94–98% for critically ill patients. However, based on previous ARDS studies and the experience of the committee, an oxygen saturation target of 88–93% should be used when critically ill patients require
range of 94–98% for critically ill patients. However, based on previous ARDS studies and the experience of the committee, an oxygen saturation target of 88–93% should be used when critically ill patients require 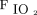 of 0.70 or higher to maintain oxygen, particularly when they are not undergoing invasive ventilation with a high PEEP strategy (Evidence level C; all committee members responded 7).
of 0.70 or higher to maintain oxygen, particularly when they are not undergoing invasive ventilation with a high PEEP strategy (Evidence level C; all committee members responded 7).
Postoperative Continuous Monitoring
Monitoring the postoperative hospitalized patient with noninvasive monitoring, such as a pulse oximeter or capnography, has been suggested to detect respiratory depression and prevent adverse events, including death.32-34 Continuous monitoring via pulse oximetry and/or capnography may provide earlier detection of these abnormalities but may also contribute to the cacophony of alarms that lead to alarm fatigue.35
In response to concerns about pulse oximetry allowing detection of deterioration early enough for intervention in patients receiving supplemental oxygen, Taenzer et al36 studied the rate of desaturation in subjects receiving supplemental oxygen versus those not receiving supplemental oxygen. They reported that the speed of desaturation was not different between the groups, concluding that pulse oximetry-based surveillance can be used in the patient receiving supplemental oxygen to detect deterioration.
A 2017 systematic review by Lam et al37 focused on the effectiveness of continuous pulse oximetry versus routine care and the effectiveness of continuous capnography with or without pulse oximetry in the postoperative population, though not all studies included in the review included supplemental oxygen delivery. Routine care was defined as vital signs obtained every 4–6 h. They identified 4 studies comparing continuous pulse oximetry to routine care and found that the odds of recognizing desaturation were significantly higher with continuous pulse oximetry versus routine care. In addition, they identified 5 studies evaluating the use of capnography with or without pulse oximetry. Those studies revealed that the odds of recognizing postoperative respiratory distress were significantly higher using capnography than with the use of pulse oximetry.
Kisner et al38 studied the incidence of cardiac arrhythmias identified postoperatively via remote pulse oximetry monitoring versus no monitoring in subjects that required CABG or cardiac valve replacements. They found that subjects who were monitored had a lower incidence of atrial fibrillation than those who were not monitored, although this did not reach statistical significance.
Though there is evidence to support the use of monitoring via pulse oximetry and capnography to prevent postoperative respiratory distress, there is a paucity of evidence comparing continuous monitoring to intermittent or no monitoring in patients receiving supplemental oxygen, including impact on mortality. At this time, no recommendation can be made.
Early Initiation of High-Flow Nasal Cannula
Early initiation of HFNC is not clearly defined in the literature. For this review, the committee included studies that explored the impact of time to initiation of HFNC and those studying HFNC applied postextubation to prevent or reverse postextubation respiratory failure. Even with these qualifiers, there is a paucity of literature exploring the timing of initiating HFNC or comparing early versus late initiation.
Gaunt et al39 in a retrospective study identified the timing of initiation of HFNC and the occurrence of adverse events, ICU LOS, and post-ICU LOS. They found that the number of days to the initiation of HFNC was associated with an increased post-ICU LOS (P = .003) and the number of days between admission to the ICU and initiation of HFNC was associated with an increased ICU LOS (P < .001). The timing of the initiation of HFNC was not significantly related to escalation of care (P = .06).
In a prospective evaluation, Lamb et al40 studied extubation directly to HFNC or HFNC after extubation only if oxygen requirements escalated to 4 L/min via standard nasal oxygen. Both groups had a control group from a retrospective analysis in the pre-study period. In the group extubated directly to HFNC, neither hospital LOS nor ICU LOS differed significantly between the study and control groups (P = .27 and P = .79, respectively), nor did the study group differ in need for re-intubation (P = .99). However, in the other group, they identified that, when used early, hospital and ICU LOS were reduced (P = .007 and P = .03, respectively).
The limited available evidence and experience of the committee support early initiation of HFNC versus late initiation of HFNC based on the clinical condition of the patient (Evidence level C; median appropriateness score 8, range 7–9).
High-Flow Nasal Cannula Versus Standard Oxygen
Several studies compared HFNC to conventional oxygen therapy. Many of these studies can be placed into the following categories of patient areas: ED, postoperative care, ICU, and postextubation. Additionally, several studies assessed HFNC in subjects with immunosuppression. Each of these areas, and studies of immunosuppression, will be presented separately for the effects of HFNC compared to conventional oxygen therapy on ICU and hospital LOS, escalation of care (to NIV and intubation), and morbidity.
Studies included in this analysis that compared conventional oxygen therapy to early initiation of HFNC in the ED found no difference in hospital or ED LOS.41-44 Bell et al41 found a significant reduction in breathing frequency (6.7% vs 38.5%, P = .005) and escalation of care (4.2% vs 19.0%, P = .02) and improvements in dyspnea. A reduction in breathing frequency was also found in other studies conducted in the ED.42,43 Jones et al44 assessed the impact of HFNC treatment in the ED, including mortality, and found no difference between subjects treated with conventional oxygen therapy.
Several studies assessed the impact of HFNC after surgery when supplemental oxygen was required.45-49 Hospital and ICU LOS were not significantly different in most of the included studies. However, Zochios et al45 found significantly lower hospital LOS (P = .01) and significantly fewer ICU readmissions (P = .03) in subjects treated with HFNC prophylactically after cardiac surgery compared to conventional oxygen therapy. Vourc’h et al46 found less need for escalation to NIV in subjects treated with HFNC after cardiac surgery (P = .007). Yu et al47 also reported less escalation to NIV (P = .01) and fewer reintubations (P = .031) using HFNC compared to conventional oxygen therapy in subjects treated with HFNC after thoracic surgery. Improved oxygenation and less dyspnea likely led to less perceived respiratory distress and, therefore, less escalation of care. In postoperative studies that assessed mortality as an outcome, no difference was found between subjects treated with HFNC compared to conventional oxygen therapy.
Few randomized trials compared HFNC to conventional oxygen therapy in subjects meeting criteria of respiratory failure admitted to the ICU (separate from the ED discussed above or subjects with immunosuppression discussed later).50,51 None of the studies found a significant difference in hospital or ICU LOS. Parke et al50 reported higher success of therapy with HFNC compared to face mask (P = .006), with significantly fewer desaturation episodes (P = .009). However, no difference was found in the rate of intubation. Frat et al51 assessed rate of intubation as the primary outcome and found no difference overall between subjects treated with HFNC compared to conventional oxygen therapy. However, in a post hoc analysis, they found lower intubation rates in subjects with  /
/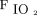 ≤ 200 mm Hg. This 2015 research was the only study reporting a lower 90-d mortality for subjects treated with HFNC compared to conventional oxygen therapy and NIV. This finding of lower mortality was likely influenced by the reduction in intubation found in subjects with more severe hypoxemia.
≤ 200 mm Hg. This 2015 research was the only study reporting a lower 90-d mortality for subjects treated with HFNC compared to conventional oxygen therapy and NIV. This finding of lower mortality was likely influenced by the reduction in intubation found in subjects with more severe hypoxemia.
Assessment of the use of HFNC compared to conventional oxygen therapy immediately after extubation was the objective of several RCTs.52-59 Hospital and ICU LOS were not different. Hernández et al52 found lower re-intubation within 72 h of extubation using HFNC compared to conventional oxygen therapy in subjects at low risk of extubation failure (P = .004). Hou et al53 found less escalation to NIV (P = .02) and lower re-intubation (P = .036) using HFNC compared to an air-entrainment mask. However, neither Gaspari et al54 nor Matsuda et al55 found a difference in escalation of care or ICU LOS when they compared HFNC with a heated humidified face mask, suggesting perhaps that humidification plays an important role. No difference was found in mortality using HFNC or conventional oxygen therapy.53,56
A total of 4 studies were included in our analysis that compared HFNC with conventional oxygen therapy in a specific patient population of immunosuppression.60-63 Two of the studies were post hoc analysis of previous RCTs60,61 and 2 were prospective RCTs.62,63 No difference was found using HFNC compared to conventional oxygen therapy for hospital or ICU LOS, escalation of care, or mortality. Frat et al61 found lower 90-d mortality using HFNC compared to NIV in their analysis (P = .02) but no difference compared to conventional oxygen therapy (P = .65).
Based on the available evidence and the experience of the committee, HFNC may avoid escalation to NIV and the need for intubation in patients with significant hypoxemia, likely due to its effects on oxygenation and dyspnea compared to conventional oxygen therapy. However, HFNC does not reduce LOS compared to conventional oxygen therapy. Further evidence is required to confirm a mortality benefit using HFNC compared to conventional oxygen therapy (Evidence level B; median appropriateness score 8, range 7–9).
Compared to conventional oxygen therapy, HFNC reduces re-intubation when used immediately postextubation (Evidence level B; all committee members responded 8).
There are no benefits in LOS, escalation of care, or morbidity of HFNC compared to conventional oxygen therapy in immunocompromised patients (Evidence level B; all committee members responded 8).
Humidification of Oxygen
There is a lack of high-level evidence to support an impact of humidity on patient outcomes or adverse events. Most evidence centers on patient comfort. In a prospective crossover study, Chanques et al64 compared the comfort level associated with high-flow face mask with bubble humidification versus high-flow face mask with heated humidification (HH). Subjects indicated that, when HH was used, they had less discomfort. As compared to bubble humidification, the oxygen delivery system using HH scored more favorably on the dryness scale and was preferred by subjects.
The only study (N = 30) that measured nasal airway caliber (cross-sectional area by acoustic rhinometry) failed to document a difference between HFNC and conventional oxygen therapy. A blinded evaluation by an otorhinolaryngologist demonstrated significantly greater nasal dryness in the standard oxygen group. Subjects’ assessment of nasal dryness was judged better with HFNC. Dryness of the mouth and throat, dysphagia, and throat pain was not significantly different between the HFNC and conventional oxygen therapy. Subjects noted a significant overall subjective preference of HFNC over conventional oxygen despite relative noise induced by the device.65
Vourc’h et al46 randomized adult subjects with severe hypoxemia after cardiac surgery to either HFNC at 45 L/min or non-rebreathing mask at 15 L/min. They studied self-reported subject satisfaction, mucus dryness, and nasal bleeding. The HFNC cohort experienced more satisfaction (P < .001), less mucus dryness (P = .003), and fewer instances of nasal bleeding (P = .36).
The heated nature of the humidification used with HFNC allows the patient to tolerate higher flows. However, the temperature of the device is variable. Mauri et al66 studied the effect of temperature on patient comfort with HFNC. Using 2 flows (30 L/min and 60 L/min) at 2 temperatures (31°C and 37°C), they studied patient comfort. Their study revealed a higher comfort rate at a lower temperature, regardless of flow.
Even given the above evidence, studies have not been able to determine that nonhumidified supplemental oxygen is inferior to humidified supplemental oxygen. Poiroux et al67 studied humidified and nonhumidified oxygen delivered via nasal cannula at various flows. They reported that nonhumidified oxygen at flows > 4 L/min may be associated with higher levels of discomfort but, overall, oxygen therapy-related discomfort was low. They also assessed the effects of oxygen humidification and outcomes such as the incidence of intubation, NIV, ICU LOS, and mortality. They found no significant difference between the outcomes of the humidified and nonhumidified cohorts. They also found no significant difference in the incidence of ear, nose, or throat infection or in the need for bronchoscopy between the 2 groups.
The available evidence and the experience of the committee suggest that humidification may be considered for oxygen flows > 4 L/min to improve patient comfort (Evidence level C; median appropriateness score 8, range 7–9).
Summary
Providing supplemental oxygen to patients in a critical care environment is essential to the management of hypoxemia. Drug delivery of any kind requires thoughtful and evidence-based recommendations regarding how the appropriate dose should be given, and if alternative delivery methods exist, the benefits associated with them should be determined. In this review, we evaluated the available evidence regarding 6 specific PICO questions related to oxygenation targets (dosing), continuous monitoring in the postoperative setting, the increasingly common delivery method of HFNC, and humidification of supplemental oxygen. For the PICO outcomes, the committee agreed to focus on the clinically-relevant patient outcomes of LOS (hospital and ICU) and improved morbidity.
The available evidence was evaluated for the appropriate clinical targets of oxygen saturation for both acutely ill and critically ill adult patients. For acutely ill adult patients, van den Boom et al10 found an optimal 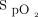 target of 94–98%. However, the available data do not uniformly support a specific oxygen saturation target. The use of pulse oximetry should be used to maintain normoxemia. Additionally, there was no benefit found for hyperoxemia in subjects with myocardial infarction and post CABG.18,19 High-quality evidence was reported in a 2018 systematic review and meta-analysis. Chu et al1 found that liberal oxygen targets led to higher 30-d mortality across 25 RCTs including 16,000 subjects. Median
target of 94–98%. However, the available data do not uniformly support a specific oxygen saturation target. The use of pulse oximetry should be used to maintain normoxemia. Additionally, there was no benefit found for hyperoxemia in subjects with myocardial infarction and post CABG.18,19 High-quality evidence was reported in a 2018 systematic review and meta-analysis. Chu et al1 found that liberal oxygen targets led to higher 30-d mortality across 25 RCTs including 16,000 subjects. Median 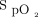 in these trials was 96% among subjects in the liberal oxygen group. The relative risk of in-hospital mortality increased as oxygen targets were liberalized, although there were no differences found in other morbidities. Patients with COPD exacerbation and CO2 retention require a more individualized approach for target oxygenation. Patients with ARDS that require
in these trials was 96% among subjects in the liberal oxygen group. The relative risk of in-hospital mortality increased as oxygen targets were liberalized, although there were no differences found in other morbidities. Patients with COPD exacerbation and CO2 retention require a more individualized approach for target oxygenation. Patients with ARDS that require 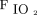 of 0.70 or more are at a higher risk of de-nitrogenation atelectasis. Therefore, based on our collective clinical experience, and despite the low-level evidence in the current literature review, the committee recommends
of 0.70 or more are at a higher risk of de-nitrogenation atelectasis. Therefore, based on our collective clinical experience, and despite the low-level evidence in the current literature review, the committee recommends  94–98% for acutely and critically ill adults, 88–92% for critically ill adults with CO2 retention and/or COPD, and 88–93% for critically ill patients requiring
94–98% for acutely and critically ill adults, 88–92% for critically ill adults with CO2 retention and/or COPD, and 88–93% for critically ill patients requiring  of 0.70 or higher who are not invasively ventilated with a high PEEP strategy.
of 0.70 or higher who are not invasively ventilated with a high PEEP strategy.
Postoperative complications are a clinically relevant concern, and patient monitoring plays a significant role. Due to the limited data comparing continuous monitoring with intermittent monitoring, the committee was unable to provide a recommendation for continuous oxygen saturation monitoring. There is growing evidence supporting the use of capnography, but this practice was not evaluated as part of the PICO question. For these reasons, the committee has no recommendations related to capnography.
Early versus late initiation of HFNC was defined as studies that compared HFNC initiation early rather than later (as escalation of therapy) in their clinical course. The committee found only 2 studies meeting these criteria, and results suggest that earlier application of HFNC may reduce ICU and hospital LOS. However, the evidence available is low quality due to a lack of randomized controlled trials.
There have been several studies published comparing HFNC to conventional oxygen therapy for the treatment of respiratory failure. The available evidence suggests treatment of hypoxemic respiratory failure with HFNC may avoid escalation to NIV and reduce the need for intubation in patients particularly with 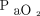 /
/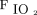 ≤ 200 mm Hg and immediately postextubation when compared to conventional oxygen therapy. Our findings are consistent with a published systematic review and meta-analysis8 and subsequent clinical practice guidelines by Rochwerg et al.68 These clinical practice guidelines gave a strong recommendation for HFNC over conventional oxygen therapy for hypoxemic respiratory failure, and a conditional recommendation for use immediately postextubation, and postoperatively in cardiac and/or thoracic surgery patients. Further data are required to demonstrate mortality benefits or confirm benefits in ICU or hospital LOS with HFNC compared to conventional oxygen therapy in any patient population.
≤ 200 mm Hg and immediately postextubation when compared to conventional oxygen therapy. Our findings are consistent with a published systematic review and meta-analysis8 and subsequent clinical practice guidelines by Rochwerg et al.68 These clinical practice guidelines gave a strong recommendation for HFNC over conventional oxygen therapy for hypoxemic respiratory failure, and a conditional recommendation for use immediately postextubation, and postoperatively in cardiac and/or thoracic surgery patients. Further data are required to demonstrate mortality benefits or confirm benefits in ICU or hospital LOS with HFNC compared to conventional oxygen therapy in any patient population.
Though the evidence does not demonstrate a clinical benefit to adding humidification to oxygen therapy, studies have demonstrated that additional humidification does improve patient comfort. Though some sources cite conflicting information about the superiority of HFNC over conventional oxygen therapy with patient comfort and nasal dryness,69 this systematic review identified that adding humidification to delivered supplemental oxygen may not improve patient outcomes but could improve tolerability of the device.46,64-66 Considering the relative level of discomfort experienced by the patient during their hospitalization, adding humidification to reduce discomfort associated with supplemental oxygen flows > 4 L/min is a small concession and easily accomplished.
For several of the research questions in this systematic review, supporting literature focused on the identified outcomes was sparse. The most studied area of interest during the development of this guideline was focused on HFNC. Based on the volume of research published related to HFNC, the committee acknowledges that the quality of evidence related to HFNC is likely to strengthen recommendations for or against its use in certain clinical contexts over the coming years. We are confident that these recommendations for HFNC are consistent with recently published systematic reviews and meta-analyses and clinical practice guidelines and can positively impact patient outcomes. However, there is also a need for more rigorously designed studies to guide clinical decision-making in other areas of oxygen delivery in the acute care setting.
Acknowledgements
The authors would like to thank Ms Shawna Murray for her contribution to the development of the research questions and literature review.
Footnotes
- Correspondence: Thomas Piraino RRT FCSRT FAARC, St. Michael's Hospital, 36 Queen St E, Toronto, ON M5B 1W8, Toronto, Canada. E-mail: thomaspiraino{at}gmail.com
Mr Piraino discloses relationships with Dräger, Fisher & Paykel, and Philips. Ms Madden discloses relationships with ICON and Dräger. Dr Lamberti discloses relationships with Boehringer Ingelheim, Janssen, Sanofi/Regeneron, Philips, and Genentech. The remaining authors declare no conflicts of interest.
At the time of this work, Dr Strickland was affiliated with the American Association for Respiratory Care.
Supplementary material related to this paper is available at http://rc.rcjournal.com.
- Copyright © 2022 by Daedalus Enterprises