Abstract
During the coronavirus disease 2019 (COVID-19) pandemic, noninvasive respiratory support has played a central role in managing patients affected by moderate-to-severe acute hypoxemic respiratory failure, despite inadequate scientific evidence to support its usage. High-flow nasal cannula (HFNC) treatment has gained popularity because of its effectiveness in delivering a high fraction of humidified oxygen, which improves ventilatory efficiency and the respiratory pattern, as well as its reported high tolerability, ease of use, and application outside of ICUs. Nevertheless, the risk of infection transmission to health-care workers has raised some concerns about its use in the first wave of the pandemic outbreak, with controversial recommendations provided by different scientific societies. This narrative review provides an overview of the recent evidence on the physiologic rationale, risks, and benefits of using HFNC instead of conventional oxygen therapy and other types of noninvasive respiratory support devices, such as continuous positive airway pressure and noninvasive ventilation in patients affected by COVID-19 pneumonia with associated acute hypoxemic respiratory failure. It also summarizes the available evidence with regard to the clinical use of HFNC during the current pandemic and its reported outcomes, and highlights the risks of bioaerosol dispersion associated with HFNC use.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has impacted the health-care system and resulted in an unprecedented number of patients who are critically ill with moderate-to-severe acute hypoxemic respiratory failure, which requires a high 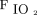 , noninvasive respiratory support or a rapid escalation to endotracheal intubation (ETI), and invasive mechanical ventilation. Although the best option for noninvasive respiratory support systems in the management of acute hypoxemic respiratory failure is still a matter of debate, high flows nasal cannula (HFNC) has emerged as an effective and well-tolerated respiratory support technique in various clinical scenarios.1,2 Furthermore, by providing high-flows of oxygen-enriched gas with low-level PEEP, HFNC has been suggested as an alternative to conventional oxygen therapy or noninvasive ventilation (NIV) in a well-selected group of patients with acute hypoxemic respiratory failure.1 Similarly, HFNC could be a valuable and feasible treatment option for patients with COVID-19 pneumonia, with remarkable clinical advantages. Its easy setup allows for rapid training, even for non-expert personnel with heterogeneous backgrounds.3 Thus, its implementation in a non-ICU setting 4 might be crucial for countries and health-care systems with shrinking critical care and invasive ventilation resources.5,6
, noninvasive respiratory support or a rapid escalation to endotracheal intubation (ETI), and invasive mechanical ventilation. Although the best option for noninvasive respiratory support systems in the management of acute hypoxemic respiratory failure is still a matter of debate, high flows nasal cannula (HFNC) has emerged as an effective and well-tolerated respiratory support technique in various clinical scenarios.1,2 Furthermore, by providing high-flows of oxygen-enriched gas with low-level PEEP, HFNC has been suggested as an alternative to conventional oxygen therapy or noninvasive ventilation (NIV) in a well-selected group of patients with acute hypoxemic respiratory failure.1 Similarly, HFNC could be a valuable and feasible treatment option for patients with COVID-19 pneumonia, with remarkable clinical advantages. Its easy setup allows for rapid training, even for non-expert personnel with heterogeneous backgrounds.3 Thus, its implementation in a non-ICU setting 4 might be crucial for countries and health-care systems with shrinking critical care and invasive ventilation resources.5,6
Despite the theoretical physiologic rationale and HFNC’s potential clinical usefulness, the use of HFNC was limited and variable during the first wave of the pandemic.4,7 In addition, the application of high gas flows initially raised doubts and controversies about the safety of the device in terms of aerosolization of droplets and infection transmission.6 Here, we summarized the role of HFNC in patients affected by COVID-19-associated acute hypoxemic respiratory failure, the rationale for its use, the advantages of HFNC over standard oxygen and other types of noninvasive respiratory support devices (CPAP and NIV), the evidence for its aerosol generation and clinical applications, and recommendations for its use during the pandemic.
Methods
MEDLINE and PubMed were searched to identify observational studies, randomized clinical trials (RCT), meta-analyses, and clinical practice guidelines by using the search terms: “(high-flow nasal cannula or HFNC) and (COVID-19 or coronavirus).” To identify ongoing clinical trials, we sought trials registered to study HFNC treatment for COVID-19 and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at https://clinicaltrials.gov. The last update of the search was performed in January 2021. A single reviewer (CC) screened all potential references for inclusion.
Rationale and Physiologic Effects
The main physiologic effects of HFNC are summarized in Table 1. These findings were obtained from the bench and from the clinical studies performed in healthy volunteers8-12 and heterogeneous groups of subjects affected by acute respiratory failure.13-20 When considering the rationale mentioned above, HFNC might have a potential role in the clinical management of acute hypoxemic respiratory failure associated with COVID-19 pneumonia.
Physiological Effects of High-Flow Nasal Cannula
HFNC and COVID-19 Pneumonia
HFNC might have potential clinical benefits for patients when provided early in the course of acute hypoxemic respiratory failure.9,21,22 Evidence that emerged during the COVID-19 pandemic showed that the application of HFNC was feasible to treat patients with acute hypoxemic respiratory failure due to COVID-19 in non-ICU settings or for patients with a poorer prognosis who have been denied admission to the ICU.4,23,24 Many centers have reported their experiences in observational studies, as shown in Table 2. To date, there is a lack of robust data from RCTs on the timely use of HFNC in COVID-19-associated acute hypoxemic respiratory failure. Nevertheless, a few RCTs are ongoing and registered in trial registry databases, as shown in Table 3.
Published Clinical Studies on HFNC Use and COVID-19-Associated Acute Hypoxemic Respiratory Failure
Characteristics of RCTs Found in the Trial Registries, Studying the Efficacy and Safety of HFNC in Subjects With COVID-19-Associated Acute Hypoxemic Respiratory Failure
Advantages and Disadvantages Compared With Standard Oxygen
HFNC decreased the need for intubation or escalation of treatment compared with standard oxygen in patients without COVID-19 who were critically ill and with acute hypoxemic respiratory failure, and recent clinical practice guidelines strongly recommended its use1; similarly, HFNC performs better than standard oxygen in COVID-19 settings.6,25 This beneficial effect is probably due to its ability to more adequately match patients’ respiratory flow demands, reduce inspiratory effort, and decrease the risk of patients’ self-inflicted lung injury,26 which may have important implications for the management of the heterogeneous pulmonary manifestations of patients with COVID-19.27 Furthermore, the heat and humidification delivered by HFNC help to maintain hydration and mobilize secretions, which positively affect the mucus hypersecretion of patients with COVID-19,28-31 even if the delivered heated air may initially bother the patient.32
Advantages and Disadvantages Compared With NIV and CPAP
Current guidelines do not recommend the routine use of NIV in patients with de novo acute hypoxemic respiratory failure without previous chronic respiratory and cardiac disease,33 which suggests that an NIV trial might be attempted only by an experienced clinical team in a “protective” environment, such as an ICU. Moreover, a lung-protective strategy required to avoid the triggering and/or worsening of ventilator-induced lung injury is challenging to achieve in most patients who receive NIV for de novo acute hypoxemic respiratory failure.34 The patient’s high inspiratory effort contributes to generating a large tidal volume despite the relatively low inspiratory pressure during NIV. Thus, lung injury can be caused by the ventilator, the patient’s own breathing, or both, and can be predicted by a high tidal volume (>9.5 mL/kg)35 and high inspiratory effort as assessed with an esophageal pressure swing.36 A recent study showed better outcomes and decreased risk of death when using helmet NIV than mask NIV in patients with ARDS37 due to the ability of the helmet to keep higher PEEP levels without worsening leaks. Nevertheless, a physiologic study showed that, although the helmet improved oxygenation compared with HFNC, the patients with low inspiratory effort on HFNC increased their transpulmonary pressure if shifted onto helmet NIV.38 Indeed, NIV intolerance is common in patients with acute respiratory failure, and it is a determinant of NIV failure,39 which is associated with a worse prognosis and increased mortality.33
In this regard, HFNC is generally better tolerated than NIV.40 Therefore, the treatment can be maintained for several hours with less risk of skin breakdown,41 which avoids alveolar de-recruitment and oxygen reduction, which typically occur during NIV interruption. Moreover, HFNC can reduce inspiratory effort42 similar to NIV, without the downsizes and concerns of air leakage, patient-ventilator synchrony, and the need of sedation,43 which offers a good balance between adequate oxygenation and comfort.44 However, unlike NIV, HFNC cannot deliver high PEEP levels. In addition, it is crucial to consider amid a pandemic that HFNC is easier to set up and implement than NIV.3 Therefore, HFNC success is less dependent on the expertise of the team, as required for NIV,3 to avoid asynchronies, leaks, and intolerance,45 even if adequate staff training is essential to ensure proper use of the technique and patient safety. Furthermore, the soft and easy-to-fit HFNC interface creates less burden on the patient’s face, and it is more “patient friendly,” which allows the patient to be unimpeded while speaking, coughing, or eating meals.46 Indeed, in critical-care resource-limited settings, HFNC could represent an alternative strategy to NIV to support patients who are hypoxemic with comfort care only,23,47,48 as usually happens in a non-pandemic time.49
HFNC Initiation: Setting Considerations
The amount of flow that should be used for patients with COVID-19 is still a matter of debate due to the lack of RCTs, and the published evidence shows significant variability in the settings used (Table 2). However, evidence from observational studies suggests that higher flows, between 50 and 60 L/min, are mainly used, similar to the scientific evidence on non-COVID-19 acute hypoxemic respiratory failure,21,50-55 according to the described flow-dependent physiologic effects of HFNC, and data from systematic reviews, which reported a flow of >45 L/min in most of the included studies.56-59 Moreover, a recent bench study showed that HFNC set at a higher flow and larger cannula size might generate higher positive pressure.60
Clinical Application
HFNC has been reported as a valuable therapeutic resource during the pandemic to ration ICU resources (both beds and ventilators),4,61,62 and its use has been widely and heterogeneously reported in the literature.3-5,23,61-70 Thus, in resource-constrained health systems, HFNC was feasible for successfully treating severe COVID-19 acute hypoxemic respiratory failure in almost half of those who received it, with no need for invasive mechanical ventilation, in a non-ICU environment when using an affordable pulse oximetry-based monitoring.61 In a retrospective observational study, Wang et al3 reported the use of HFNC as primary respiratory support in 17 subjects hospitalized with COVID-19 pneumonia (63%), with NIV and invasive ventilation in only 9 (33%) and 1 subject (4%), respectively. Interestingly, among the 17 subjects treated with HFNC, 7 (41%) with lower  /
/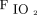 switched to NIV due to HFNC failure.3 However, only 29% of the subjects received intubation after NIV escalation.3 Similarly, an early report from the Italian experience during the first wave of the pandemic reported the use of HFNC in 28 subjects admitted to a respiratory intermediate care unit with a success rate of 67.8% and a lower initial
switched to NIV due to HFNC failure.3 However, only 29% of the subjects received intubation after NIV escalation.3 Similarly, an early report from the Italian experience during the first wave of the pandemic reported the use of HFNC in 28 subjects admitted to a respiratory intermediate care unit with a success rate of 67.8% and a lower initial  /
/ associated with treatment failure.63
associated with treatment failure.63
A recent multi-center retrospective study demonstrated the feasibility of noninvasive respiratory support application to treat COVID-19-related acute hypoxemic respiratory failure outside the ICU and showed that HFNC was used in 163 of 671 subjects (24.3%), of whom, 71% avoided intubation.23 Although limited by the study design, interestingly, no significant difference in the primary outcomes was shown compared with CPAP and NIV (intubation rate of 29% for HFNC vs 25% for CPAP vs 28% for NIV, and an unadjusted mortality rate of 16% for HFNC vs 30% for CPAP vs 30% for NIV).23 However, HFNC was applied in subjects who were less ill (mean ± SD 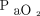 /
/ , 166 ± 65 mm Hg) compared with NIV (mean ± SD
, 166 ± 65 mm Hg) compared with NIV (mean ± SD 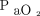 /
/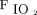 , 138 ± 66 mm Hg) and CPAP (mean ± SD
, 138 ± 66 mm Hg) and CPAP (mean ± SD 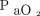 /
/ , 151 ± 90 mm Hg), which reflected the clinicians’ attitudes to start the latter two in subjects in whom a relatively high level of external PEEP might be indicated.23 Another retrospective study performed in the ICU compared the outcome of subjects treated with HFNC with a case-matched group treated with conventional oxygen therapy in subjects with COVID-19.64 The median
, 151 ± 90 mm Hg), which reflected the clinicians’ attitudes to start the latter two in subjects in whom a relatively high level of external PEEP might be indicated.23 Another retrospective study performed in the ICU compared the outcome of subjects treated with HFNC with a case-matched group treated with conventional oxygen therapy in subjects with COVID-19.64 The median 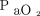 /
/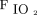 did not differ between the 2 groups median (IQR) (126 [86–189] mm Hg in the subjects on HFNC vs 130 [97–195] mm Hg in the subjects on standard oxygen therapy).64 However, the proportion of the subjects who required invasive mechanical ventilation on day 28 was significantly lower in the HFNC group (55% vs 72%; P < .001), although no difference was found in the mortality rate.64
did not differ between the 2 groups median (IQR) (126 [86–189] mm Hg in the subjects on HFNC vs 130 [97–195] mm Hg in the subjects on standard oxygen therapy).64 However, the proportion of the subjects who required invasive mechanical ventilation on day 28 was significantly lower in the HFNC group (55% vs 72%; P < .001), although no difference was found in the mortality rate.64
In a retrospective analysis of subjects with COVID-19 and with moderate-to-severe acute hypoxemic respiratory failure, Patel et al65 found that application of HFNC may substantially reduce the need for invasive mechanical ventilation and escalation of NIV, with no apparent effect on mortality. In particular, 23.3% of the subjects were initially treated with HFNC, of whom 64.4% remained on HFNC, which showed a significant improvement in oxygenation and reduction in the incidence of hospital-acquired pneumonia compared with those who progressed to intubation or NIV.65 A single-center RCT on 22 subjects with severe COVID-19 showed that early treatment with HFNC improved oxygenation and breathing frequency, even infectious indices, and reduced ICU length of stay compared with conventional oxygen therapy.66 In addition, the early use of HFNC is an effective respiratory strategy according to a recent retrospective multi-center study,67 which showed that patients with HFNC failure had a poor prognosis, with a hospital mortality rate of 65%.
A recent propensity-matched cohort study assessed the role of HFNC in 122 subjects who were critically ill with COVID-19-associated acute hypoxemic respiratory failure and who had received either HFNC or early intubation on ICU admission.68 HFNC use was associated with increased ventilator-free days and reduced ICU length of stay, without a significant difference in all-cause in-hospital mortality.68
Moreover, data on patients with COVID-19 and with do-not-intubate/do-not-resuscitate status are available. Franco et al23 reported nearly 7% of subjects (12/163) with do-not-intubate orders treated with HFNC outside the ICU. However, in a large cohort study of 900 subjects with cancer and COVID-19, 132 subjects (14%) were still admitted to the ICU and 116 (12%) required invasive mechanical ventilation.71 We can conclude that HFNC may offer several advantages, reduce the intubation risk compared with conventional oxygen therapy, and represent a valid alternative to NIV in patients with severe COVID-19. However, RCTs are needed to effectively address the role of HFNC in this type of acute hypoxemic respiratory failure. Because pulmonary embolism, acute kidney injury, and myocardial injury are reported in a much higher proportion than non–COVID-19 ARDS,72 the mortality rate may represent a more-complex outcome in this disease, not entirely affected by different noninvasive respiratory support strategies.
Advantages of HFNC Combined With Prone Positioning
By following the strong evidence on the application of prone positioning in patients with typical ARDS who are undergoing invasive mechanical ventilation,83 awake prone positioning in patients with COVID-19 has been increasingly reported in the COVID-19 literature,74,75,80 which demonstrate improvements in oxygenation when used with HFNC or NIV.73 However, to date, evidence for prone positioning in this population includes a few small single-center cohort studies.74-82 The rationale for using prone positioning in patients who are not intubated is based on the redistribution of the ventilation-to-perfusion ratio to spared lung regions, which are better ventilated.83 Therefore, improved oxygenation by lung recruitment of previously dependent areas reduces the shunt,84,85 which decreases hypoxemic vasoconstriction and improves pulmonary vascular resistance and right-ventricular function.86 In addition, during the prone position, chest wall compliance decreases, which explains, in part, a more homogeneous distribution of ventilation and regional lung stress. It also reduces the risk of ventilation-induced lung injury and, possibly, pendelluft.87
Based on the physiologic benefits and available data, some investigators83,84,85 hypothesized that patients with COVID-19 and with respiratory distress at high risk for intubation might benefit from prone positioning. Ding et al88 reported the use of prone positioning in awake patients for the first time, which showed a reduction in the intubation rate in subjects with moderate-to-severe ARDS when treated with prone positioning combined with NIV or HFNC. In a retrospective study of 610 subjects from China, 10% of subjects with COVID-19 were managed with early HFNC and awake prone positioning, which achieved a lower need for invasive mechanical ventilation (<1% vs a national average of 2.3%) and lower mortality (3.83% vs 4.34%, respectively).73
Xu et al75 reported the effects of early awake prone positioning combined with HFNC in 10 subjects with COVID-19 who showed an improvement in 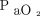 /
/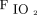 after prone positioning and avoiding intubation in all the subjects. In contrast, Elharrar et al74 found that oxygenation improved during prone positioning only in 6 of 24 study participants (25%). Furthermore, self-proning was not well tolerated in nearly 13%–25% of the subjects, with most reports using helmet CPAP or NIV. Therefore, we can speculate that HFNC may be more comfortable and feasible for the mobility of patients during self-proning. Recently, a large prospective multi-center observational cohort study that analyzed prone positioning in 55 of 199 awake subjects with COVID-19-associated acute hypoxemic respiratory failure who received HFNC showed that the use of awake prone positioning as an add-on therapy to HFNC did not reduce the risk of intubation or affect 28-d mortality, which showed a trend toward delayed intubation compared with HFNC alone.82 These data are similar to reports on prone positioning during helmet CPAP or conventional oxygen therapy in which the patients who responded to prone positioning treatment had no significant difference in intubation rate compared with non-responders.89
after prone positioning and avoiding intubation in all the subjects. In contrast, Elharrar et al74 found that oxygenation improved during prone positioning only in 6 of 24 study participants (25%). Furthermore, self-proning was not well tolerated in nearly 13%–25% of the subjects, with most reports using helmet CPAP or NIV. Therefore, we can speculate that HFNC may be more comfortable and feasible for the mobility of patients during self-proning. Recently, a large prospective multi-center observational cohort study that analyzed prone positioning in 55 of 199 awake subjects with COVID-19-associated acute hypoxemic respiratory failure who received HFNC showed that the use of awake prone positioning as an add-on therapy to HFNC did not reduce the risk of intubation or affect 28-d mortality, which showed a trend toward delayed intubation compared with HFNC alone.82 These data are similar to reports on prone positioning during helmet CPAP or conventional oxygen therapy in which the patients who responded to prone positioning treatment had no significant difference in intubation rate compared with non-responders.89
In conclusion, prone positioning may significantly improve gas exchange in patients with COVID-19 who were treated with noninvasive respiratory support devices. However, how this may affect the final outcome is not yet well established. Moreover, the use of prone positioning in patients with COVID-19 who are not intubated and treated with HNFC is yet to be addressed, and some RCTs are still ongoing (Table 2).
Aerosol-Generating Risk
Aerosol production by the patient’s airways contains particles that range in size from 0.1 to 100 μm; the smaller the droplet is, the longer the air dispersion lasts. Droplets (particles >5 μm) are produced by the upper airway90 and are at a higher risk of dispersion during conventional oxygen therapy and noninvasive respiratory support. Hence, supplemental low-flow conventional oxygen therapy has been considered a risk factor for the spread and airborne transmission of SARS-CoV-2.91 In addition, the fear that higher flows will increase virus aerosolization and environmental contamination 92 has listed HFNC as an aerosol-generating procedure by health-care agencies, such as Public Health England 93 and the National Institutes of Health,94 despite the lack of robust scientific evidence.95 Consequently, the HFNC utilization rate was relatively low during the early stages of the COVID-19 pandemic.7
Dhand and Li96 recently pointed out the difference between aerosol-generating and aerosol-dispersing procedures. The investigators highlighted that coughing and procedures that provoke cough (ie, suctioning, intubation, bronchoscopy) generate a burst of bioaerosol, whereas HFNC, NIV, or conventional oxygen therapy are only dispersing the bioaerosol generated by patients to a greater distance.96 However, the aerosol generation and dispersion phenomenon has been studied predominantly through experimental studies7 that used marked smoke to simulate aerosols (particles < 1 μm) rather than measuring particle spread. Therefore, the actual risk of infection transmission has not been quantified.97
Hui et al98 investigated the direct visualization of exhaled smoke dispersion (with particle sizes of ∼1 μm) on a human patient simulator in a negative pressure room during a normal breathing pattern and mild respiratory distress. The investigators detected a higher smoke dispersion with HFNC at 60 L/min compared with 10 L/min.98 However, they did not find considerable differences in the level of smoke between HFNC at 60 L/min, CPAP via nasal pillow at 20 cm H2O, or via oronasal mask at 20 cm H2O.98 Indeed, they found a significant increase in exhaled smoke dispersion (up to 620 mm) laterally when the connection between HFNC nasal prongs and the simulated nares were loose.98
More recently, studies have investigated the dispersion of contaminated aerosols during quiet and forced breathing or coughing, with or without HFNC, and reported different findings. Roberts et al99 studied healthy adults breathing spontaneously, with or without HFNC, at flows of 30 and 60 L/min at rest and after violent exhalation (snorting) and showed no increase in aerosol dispersion of intermediate size particles of 25 − 250 μm with HFNC at rest compared with snorting. Loh et al100 studied healthy adults breathing spontaneously with or without HFNC at 60 L/min and found that it did not impact the distance of droplet spread, regardless of HFNC use.
Jermy et al101 assessed airborne particle dispersion in human volunteers breathing at rest, coughing, snorting, or sneezing, with or without HFNC. They found a significant protective effect from sneezing during HFNC, which suggested that the high flow generated by HFNC may prevent the exit of infected air.101 They also showed that it would take 86 h of quiet breathing with HFNC at 60 L/min to release the same quantity of nasal fluid as a minute of coughing with no therapy.101 Kotoda et al102 assessed potential pathogen dispersion with or without HFNC at 60 L/min in a static non-breathing model by using thickened water or fresh yeast solution to mimic saliva and nasal mucus secretion and showed no increase in the risk of droplet dispersion with HFNC.
The only evidence in vivo on actual patients in the ICU is a recent RCT that showed that HFNC at 60 L/min did not generate a significantly different level of airborne bacterial contamination in the air sampled than did oxygen therapy delivered via a face mask.103 Nevertheless, because the study analyzed bacteria rather than viruses, the evidence did not clarify this divisive issue. More recent studies compared all devices applicable to the patient’s face (conventional oxygen therapy, HFNC, NIV). Li et al104 summarized all published studies by using an experimental model that looks at the exhaled smoke dispersion distances with different devices: HFNC at different flows (10, 30, 60 L/min), simple mask (10, 15 L/min), non-rebreather mask (10 L/min), and air-entrainment mask (6 L/min with 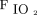 of 0.4 or 0.5). The smoke dispersion distance with the higher flow of HFNC (17.2 ± 3.3 cm) was lower than that of the non-rebreather (24.6 ± 2.2 cm) or the air-entrainment mask (39.7 ± 1.6 cm), which indicated that HFNC had the lowest risk of bioaerosol dispersion.104
of 0.4 or 0.5). The smoke dispersion distance with the higher flow of HFNC (17.2 ± 3.3 cm) was lower than that of the non-rebreather (24.6 ± 2.2 cm) or the air-entrainment mask (39.7 ± 1.6 cm), which indicated that HFNC had the lowest risk of bioaerosol dispersion.104
Gaeckle et al105 studied healthy adults and compared non-humidified nasal cannula at 4 L/min; facemask at 15 L/min; HFNC at 10, 30, and 50 L/min; and NIV with an inspiratory positive airway pressure/expiratory positive airway pressure of 12/5 and 20/10 cm H2O. They measured particles of sizes 0.37 and 20 μm during normal breathing, talking, deep breathing, and coughing, and found an increased number of particles during coughing; however, the investigators found no significant differences between HFNC and/or NIV and conventional oxygen therapy in different testing conditions.105 A proposed way to mitigate the aerosol spread when using HFNC is to apply a surgical mask on top of the patient’s face, as promoted in the early course of the pandemic in China.106 Moreover, it has recently been shown that this measure further reduces the velocity of exhaled gas flow, droplet deposition,107-109 and concentration of 0.5−5 µm-sized particles, particularly 30.5 cm from the patient’s face.110
Overall, the available evidence shows that HFNC is no worse than conventional oxygen delivery devices or NIV in terms of dispersion of a patient-generated bioaerosol. A recent observational study of 28 subjects treated with HFNC showed none of the ICU staff were infected during the study period and the following 14 d.63 HFNC, with its interface characterized by soft nasal prongs with large bores that fill up approximately half of the area of patients’ nostrils, minimizes the aerosol-generation risk. Placing a surgical mask over the patient’s face during HFNC treatment may further decrease the bioaerosol dispersion distance. Real-world assessment of droplets and aerosols spread in genuine clinical environments is urgently needed, along with testing measures to mitigate these risks.
HFNC Use in COVID-19
The role of noninvasive respiratory support and HFNC in suspected or confirmed SARS-CoV-2 infection has not yet been definitively clarified. Furthermore, no large RCTs exist on the use of HFNC or CPAP and/or NIV in patients with acute hypoxemic respiratory failure caused by a pandemic viral illness.7 In the early stages of the current pandemic, initial concerns about the risk of bioaerosol dispersion and delayed intubation led some scientific societies to limit or not recommend the application of HFNC and other noninvasive respiratory support devices with different and sometimes opposite recommendations between national and international organizations.111 Societies’ recommendations on the use of HFNC in COVID-19-associated acute hypoxemic respiratory failure are shown in Table S1 (see the supplementary materials at http://www.rcjournal.com).
Clinical Implications, Critical Issues, and Limitations
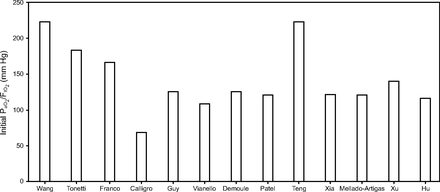
HFNC usage during the current pandemic varied across studies and countries (Table 2).7,112,113 The mean rate of HFNC usage reported was 22.8% in China and ranged from 4.8% to 42% in the United States,5,56,112-114 whereas data from other countries are not available. The criteria to start HFNC were also heterogeneous among the published studies with different initial 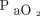 /
/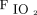 values (Table 2, Fig. 1) and outcomes (Table 2, Fig. 2). The actual suggestions on HFNC use are mainly based on expert opinion and emerging retrospective data in patients with COVID-19 from countries where outbreaks have occurred.111
values (Table 2, Fig. 1) and outcomes (Table 2, Fig. 2). The actual suggestions on HFNC use are mainly based on expert opinion and emerging retrospective data in patients with COVID-19 from countries where outbreaks have occurred.111
Initial 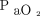 /
/ before starting high-flow nasal cannula in published studies on coronavirus disease 2019 (COVID-19). Studies not listed did not report data on initial
before starting high-flow nasal cannula in published studies on coronavirus disease 2019 (COVID-19). Studies not listed did not report data on initial 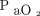 /
/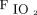 .
.
Main outcomes of high flow nasal cannula in published studies on coronavirus disease 2019 (COVID-19).
Some physiologic studies showed a heterogeneous pattern of respiratory mechanics patterns that ranged from almost normal compliance to very stiff lungs, similar to classic ARDS.115 This may explain the variable severity of hypoxia associated with hypocapnia, with or, more frequently, without dyspnea and increased breathing frequency.116 In fact, hypocapnia is almost always a consequence of increased alveolar ventilation and, consequently, incremented transpulmonary pressure, which all patients experience to a different extent.117 Therefore, the breathing frequency and clinical evidence of respiratory distress are not sensitive in identifying patients with increased transpulmonary pressure, predictive of self-inflicted lung injury.118 Meanwhile, these data give HFNC a critical role because it effectively reduces the transpulmonary pressure by the same amount as NIV,38,42 without the harmful effects of delivering a large tidal volume35 as frequently seen during NIV due to the joint action of the patient’s respiratory drive to breathe and the pressure support provided.
However, all the above-mentioned physiologic considerations raised some crucial points in applying HFNC to COVID-19 pneumonia. First, HFNC cannot generate or ensure an increased and stable continuous alveolar pressure; this is an important issue because lung recruitment is more likely to be achieved in COVID-19 pneumonia than in “usual” ARDS.115 Moreover, it is unclear whether the favorable effects of HFNC may be extended to patients with a predominantly vascular mechanism of respiratory failure due to pulmonary thromboembolism, even if we can assume that the ability to keep  stable and guarantee better oxygenation than conventional oxygen therapy could allow time for pharmacologic treatment to take effect. Moreover, compared with NIV or CPAP, HFNC can be more easily applied for patients during prone positioning, and the application of the combined treatments in the early phase of COVID-19 pneumonia may reduce the intubation rate.88
stable and guarantee better oxygenation than conventional oxygen therapy could allow time for pharmacologic treatment to take effect. Moreover, compared with NIV or CPAP, HFNC can be more easily applied for patients during prone positioning, and the application of the combined treatments in the early phase of COVID-19 pneumonia may reduce the intubation rate.88
Another major concern is how and what we need to monitor to test the efficacy of HFNC and to avoid delaying intubation.103,119 In many patients, respiratory failure may be associated with a normal breathing frequency or a blunted perception of dyspnea, likely related to a dysfunction of cortical structures linked to viral neuroinvasiveness120; this may affect the sensitivity of parameters commonly proposed to monitor the efficacy of HFNC and to predict its success or the timing of intubation.121 For example, the ROX index, defined as  /
/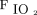 /breathing frequency, was shown to predict a high risk of intubation in non-COVID-19 acute hypoxemic respiratory failures treated with HFNC if < 4.88 at 12 h.122
/breathing frequency, was shown to predict a high risk of intubation in non-COVID-19 acute hypoxemic respiratory failures treated with HFNC if < 4.88 at 12 h.122
Hu et al70 showed that the ROX index at 6, 12, and 24 h of HFNC initiation was closely related to patient prognosis, and Xia et al67 confirmed the ROX index as a good predictive capacity of HFNC outcomes. Furthermore, in a retrospective study by Chandel et al,123 the ROX index was sensitive in identifying subjects with COVID-19 successfully weaned from HFNC. Nevertheless, slightly different cutoff values than that reported for subjects without COVID-19 were observed.121 Ricard et al2 suggested an algorithm that uses the ROX index to avoid delayed intubation in patients with COVID-19. Patients with a ROX index of <2.85, 3.47, and 3.85 after 2, 6, and 12 h of HFNC therapy, respectively, were more likely to fail, which highlights the importance of a dynamic assessment of the ROX index. Measurement of the alveolar-to-arterial oxygen gradient may be more precise in interpreting arterial oxygenation and more helpful in monitoring the effect of HFNC because it considers alveolar ventilation.124 The same 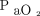 value may be an expression of different levels of alveolar ventilation (and transpulmonary pressure as a surrogate) when associated with varying values of
value may be an expression of different levels of alveolar ventilation (and transpulmonary pressure as a surrogate) when associated with varying values of 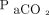 ; therefore, similar
; therefore, similar 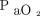 /
/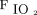 might be associated with a different alveolar-to-arterial oxygen gradient value.125
might be associated with a different alveolar-to-arterial oxygen gradient value.125
Summary
HFNC may play a role in managing patients with COVID-19 pneumonia with a low risk of bioaerosol dispersion into the environment. Early application, together with prone positioning, may significantly improve gas exchange and the outcome in these patients. However, the persistence of high breathing frequency and/or respiratory distress 1 h after application is associated with an increased risk of failure. Therefore, meticulous monitoring of patients on HFNC is crucial to avoid prolonged treatment and delay intubation.
Footnotes
- Correspondence: Claudia Crimi MD PhD, Respiratory Medicine Unit, A.O.U. “Policlinico-Vittorio Emanuele-San Marco,” Via S. Sofia, 78, 95123, Catania, Italy. E-mail: dott.claudiacrimi{at}gmail.com
The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2022 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.
- 11.
- 12.↵
- 13.↵
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.
- 30.
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.
- 52.
- 53.
- 54.
- 55.↵
- 56.↵
- 57.
- 58.
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.
- 77.
- 78.
- 79.
- 80.↵
- 81.
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵